Vegetation History and Archaeobotany
Abstract
Conventional wisdom states Cannabis sativa originated in Asia and its dispersal to Europe depended upon human transport. Various Neolithic or Bronze age groups have been named as pioneer cultivators. These theses were tested by examining fossil pollen studies (FPSs), obtained from the European Pollen Database. Many FPSs report Cannabis or Humulus (C/H) with collective names (e.g. Cannabis/Humulus or Cannabaceae). To dissect these aggregate data, we used ecological proxies to differentiate C/H pollen, as follows: unknown C/H pollen that appeared in a pollen assemblage suggestive of steppe (Poaceae, Artemisia, Chenopodiaceae) we interpreted as wild-type Cannabis. C/H pollen in a mesophytic forest assemblage (Alnus, Salix, Populus) we interpreted as Humulus. C/H pollen curves that upsurged and appeared de novo alongside crop pollen grains we interpreted as cultivated hemp. FPSs were mapped and compared to the territories of archaeological cultures. We analysed 479 FPSs from the Holocene/Late Glacial, plus 36 FPSs from older strata. The results showed C/H pollen consistent with wild-type C. sativa in steppe and dry tundra landscapes throughout Europe during the early Holocene, Late Glacial, and previous glaciations. During the warm and wet Holocene Climactic Optimum, forests replaced steppe, and Humulusdominated. Cannabis retreated to steppe refugia. C/H pollen consistent with cultivated hemp first appeared in the Pontic-Caspian steppe refugium. GIS mapping linked cultivation with the Copper age Varna/Gumelniţa culture, and the Bronze age Yamnaya and Terramara cultures. An Iron age steppe culture, the Scythians, likely introduced hemp cultivation to Celtic and Proto-Slavic cultures.
Keywords
Cannabis sativa Humulus lupulus European Pollen Database Europe GIS Pleistocene Holocene
Introduction
 Linnaeus (1737) knew Cannabis sativa as a cultivated plant in Europe, so he assumed its centre of origin (CoO) was elsewhere. He suggested a CoO in India Orientali (encompassing the Indian subcontinent, southeastern Asia, and the Malay Archipelago), Japonia (Japan), or Malabaria (the Malabar coast of southwest India). Most scholars concur with de Candolle (1883) who offered Central Asia as the CoO of C. sativa. He collated linguistic, historical, archaeological, and palaeontological data, as well as “in what country it grows spontaneously and without the help of man”. He proposed that C. sativa expanded to Europe under the aegis of human transport around 1500 bce, and he implicated the Scythians.
Linnaeus (1737) knew Cannabis sativa as a cultivated plant in Europe, so he assumed its centre of origin (CoO) was elsewhere. He suggested a CoO in India Orientali (encompassing the Indian subcontinent, southeastern Asia, and the Malay Archipelago), Japonia (Japan), or Malabaria (the Malabar coast of southwest India). Most scholars concur with de Candolle (1883) who offered Central Asia as the CoO of C. sativa. He collated linguistic, historical, archaeological, and palaeontological data, as well as “in what country it grows spontaneously and without the help of man”. He proposed that C. sativa expanded to Europe under the aegis of human transport around 1500 bce, and he implicated the Scythians.
Some botanists placed the CoO of C. sativa in Europe (Thiébault de Berneaud 1835; Keppen 1886), or a CoO spanning Asia and Europe (Herder 1892; Vavilov 1926). Lamarck (1785) recognized two species, C. sativa and C. indica. He suggested that C. sativa grew croît naturellement in Persia and presque naturalisée in Europe, whereas C. indica originated in India. Many botanists treat these taxa as subspecies, C. sativa ssp. sativa, and C. sativa ssp. indica (Small and Cronquist 1976). Winterschmidt (1818) recognized two species, C. sativaand C. chinensis, with their CoOs in Russia and Ostindien, respectively.
De Candolle limited his palaeontological evidence to print fossils (impressions of leaves or fruits in rocks), and not fossil pollen (accurately, subfossil pollen). Reports of print fossils of C. sativa have not been convincing (four reported world-wide), with one exception (Palamarev 1982). Conversely, hundreds of fossil pollen studies (FPSs) across Eurasia have identified Cannabis pollen. Researchers have utilized this database to track the cultivation history of C. sativa in Europe and Asia. Dörfler (1990) analysed 77 European FPSs conducted by himself and others. He concluded that hemp cultivation began in west-central Europe by the Iron age. Clarke and Merlin (2013) reviewed 133 European FPSs. They concluded that C. sativa diffused from Asia to Europe during the Bronze age. Long et al. (2017) synthesized 46 FPSs in their pan-Eurasian study. They also concluded that C. sativa diffused from Asia to Europe during the Bronze age.
All three meta-analyses (Dörfler, Clarke and Merlin, Long et al.) corroborated FPS data with archaeobotanical evidence—seed, fiber, cordage, textiles, and pottery impressions or pseudoliths of the same. We have also examined archaeobotanical evidence, published separately (McPartland and Hegman 2017). Robust archaeobotanical evidence identified the Bronze age Yamnaya culture as an early adopter of C. sativa, in southeastern Europe.
The purpose of this study is to revisit European FPSs, by accessing an expanded database, and using a different pollen identification method. Unlike previous studies (Dörfler 1990; Clarke and Merlin 2013; Long et al. 2017), we included FPSs that analysed strata anterior to the Holocene epoch. As de Candolle (1883) stated, “conditions anterior to our epoch determined the greater number of the facts of the actual distribution of plants”. Examining pollen from earlier epochs will address the question of C. sativa endemicity in Europe, prior to its transport by humans.
The previous three meta-analyses utilized pollen grain morphology for pollen identification. Dörfler (1990) encountered problems separating Cannabis from Humulus pollen. Many palynologists, confronted with the morphological similarities between Cannabis and Humulus, resort to collective names, e.g. Cannabis/Humulus, or Cannabaceae. Hereafter we abbreviate these lumped data as C–H pollen. Clarke and Merlin (2013) were flummoxed by FPSs that lumped data as C–H pollen; they understood the complexities regarding grain morphology (Fleming and Clarke 1998). Long et al. (2017) resolved the C–H dilemma by limiting FPSs to studies that explicitly identified pollen as Cannabis—a strategy that excluded a lot of C–H data.
Methods
Search strategy and data analysis
We accessed a larger database by using several internet search engines. The European Pollen Database (EPD, http://www.europeanpollendatabase.net) was searched in April 2013 (McPartland et al. 2013) and again in December 2015. We retrieved fossil pollen studies that included pollen identified as Cannabis/Humulus, Humulus/Cannabis, Cannabis-type, Humulus-type, or Cannabaceae. The FPSs deposited in the EPD are typically linked to publications, but some FPSs are unpublished “grey literature.” Additional FPSs were identified via Web of Science, PubMed, and Google Scholar. The keywords and Boolean operators were: palynology AND (Cannabis OR Humulus). We also used citation tracking—references in retrieved publications were searched for antecedent sources, and these were retrieved.
We excluded FPSs that analysed strata limited to the historic era, because those data did not inform our research questions: the presence of pre-Neolithic endemicity, and the identification of prehistoric hemp cultivators. To map pollen in space and time, retrieved publications had to meet three inclusion criteria: (1) precise geographical coordinates; (2) accurate chronology; (3) a minimal amount of pollen grains.
- 1.
Precise geographical coordinates were localized to within a hundredth degree of latitude and longitude. Some studies not provide geographical coordinates. We obtained coordinates of those sites via Google Earth, which uses the World Geodetic System of 1984 (WGS84) datum. The boundaries of our data collection correspond to the definition of European territory by Flora Europaea (Akeroyd 1993). The problematic eastern border of Europe is delimited by the crest of the Greater Caucasus Mountains, the north western shoreline of the Caspian Sea, the Ural River, and the crest of the Ural Mountains.
- 2.
Accurate chronology was achieved by restricting data to FPSs with calibrated radiocarbon (14C) dates. Many studies in the EPD have been retroactively calibrated, using control points (relative and independent dates) and CLAM software (Giesecke et al. 2014). A scarcity of FPSs from the Pontic-Caspian steppe—a critical region—impelled us to include several uncalibrated studies from that region. Possible dating errors arising from retroactively calibrated data or from uncalibrated data (as well as anomalously old 14C dates due to hard water effects) were minimized by our binning methodology—temporal gaps of 500 years were placed between each time slice (see below).
- 3.
Establishing the minimal amount of C–H pollen grains as an inclusion criterion proved difficult. Palynologists debate the minimal amount of pollen required to determine the presence of a plant species at a study site (versus pollen that arrived there via long-distance transport). The absolute minimum—one pollen grain—may result in “utilization fallacy” (Fuller 2006). Wild-type C. sativa colonizes ephemeral niches, such as flood water-disturbed alluvial soil. This ephemeral niche is consistent with its sporadic appearance in the fossil record. We adopted a unique inclusion criterion: for a retrieved publication to be included in our study, C–Hpollen had to appear in a minimum of five strata within a stratigraphic core. For an elaboration regarding the use of this metric, please read Online Resource 1, Extended Methods.
Pollen identification
Palynologists usually identify Cannabis and Humulus pollen by morphological characters, including grain diameter, exine thickness, and pore protrusion or pore aperture diameter. For example, Mercuri et al. (2002) identified grains ≤ 23 µm diameter as Humulus, ≥ 28 µm as Cannabis, and between 23 and 28 µm as undetermined. Others who tried to differentiate Cannabis and Humulus by grain diameter, even in comparison with reference slides, have considered it “most unreliable” (Punt and Malotaux 1984), “not possible… a suspect activity” (Whittington and Gordon 1987), “a dubious procedure” (Whittington and Edwards 1989), and “not cleanly achieved” (Hauschild 1991). The use of exine thickness, pore aperture diameter, and pore protrusion has also been criticized and contested (Engelmark 1976; French and Moore 1986; Whittington and Gordon 1987; Tweddle 2000).
Discerning Cannabis from Humulus is an old problem. “The pollen in question consists of spherical, structureless, and almost completely transparent vesicles with thin walls” (Fröman 1939). Purkyně (1830) provided the first illustration of C. sativa pollen, but it was the sole species whose identification he questioned, out of 284 species in his book. This old problem remains intractable despite new and improved microscopy. Simply: C. sativa and Humulus lupulus are highly plastic yet homologous species, whose morphological and phytochemical characters are variable and overlap (McPartland and Guy 2017). Botanists have synonymized H. lupulus under Cannabis (Scopoli 1772), and herbarium specimens of C. sativa have been misidentified as H. lupulus.
We collated 12 reference texts or methods papers, and their data regarding mean grain diameters show great variability. For C. sativa they range from 19.0 to > 28 µm, for H. lupulusthey range from 16.2 to < 25 µm (see Online Resource 1). Confronted with these inconsistencies, many palynologists transform their identification into a larger class, e.g. Cannabis/Humulus or Humulus/Cannabis. Or they apply the collective names “Humulus-type,” “Cannabis-type”, or Cannabaceae (sic, which now includes Celtidaceae).
To dissect these aggregate data, we used ecological proxies as an inferential, probabilistic method to differentiate Cannabis and Humulus pollen. Wild-type C. sativa flourishes in steppes, an open, treeless habitat (de Candolle 1883; Herder 1892; Vavilov 1926). Temperate steppe is dominated by Poaceae and Artemisia species. Desert steppe is dominated by Artemisia spp. and Chenopodiaceae. Phytosociologists and other field botanists report wild-type C. sativa cohabitating with Poaceae, Artemisia, and Chenopodiaceae; hereafter abbreviated PAC (for attestations see Online Resources 1).
Palynologists have extended these associations into the past. The BIOME Project designates Cannabis fossil pollen as a “botanical marker” of the “grassland biome”, along with PAC pollen and the relative absence of tree pollen (Tarasov et al. 1998, 2000). Cannabis fossil pollen may indicate a xerophytic steppe—hot and arid (van Geel et al. 2004; Kuneš 2008) or a mesophytic steppe—moderately warm and wet (Riehl and Pustovoytov 2006). “Dry tundra” describes a Pleistocene-early Holocene biome that was cooler than steppe, and drier than contemporary tundra. In this biome assembly, Cannabis and Artemisia pollen are categorized as “arctic forbs” (Binney et al. 2017) or cryophytes/xerophytes (Bolikhovskaya 2007). Dirksen and van Geel (2004) commented, “The synchronous fluctuations of Artemisia, Chenopodiaceae and Cannabis ruderalis pollen curves are remarkable”.
In contrast, H. lupulus requires trees to climb. Wild H. lupulus flourishes in mesophytic deciduous forests. Phytosociologists and other field botanists report H. lupulus associating with alder (Alnus spp.), willow (Salix spp.) and poplar (Populus spp.), hereafter abbreviated ASP. Palynologists have extended these associations into the past. Fries (1958, 1962) first noted synchrony in pollen counts of Humulus and Alnus. He said C–H fossil pollen did not appear in Sweden until Alnus trees migrated into the region during the early Holocene. BIOME project palynologists characterize Humulus as a drought-intolerant climber of trees (Ni et al. 2010), and assign Humulus pollen as a botanical marker of deciduous broadleaved forests (Zhou et al. 2007), or tropical evergreen forests (Lee and Liew 2010).
Arboreal/nonarboreal ratio
Firbas (1937) reasoned that Neolithic humans had to clear forests to grow crops. He detected this as a decrease in tree pollen (arboreal pollen, AP) and an increase in pollen of grasses, sedges, and forbs (nonarboreal pollen, NAP). Some palynologists argue that the AP-to-NAP ratio has limited predictive value as an indicator of landscape openness. Others find significant correlation between NAP percentages and percentage cover of open herb vegetation. AP and NAP percentages are oppositional—when one goes down, the other goes up. Similarly, palynologists have shown that Alnus and PAC demonstrate oppositional characters in studies using multivariate analyses, such as PCA, RDA, and NJ methods.
Pollen of cultivated C. sativa
Scholz (1957) distinguished between wild-type and cultivated Cannabis by grain diameter, 15–25 µm and 25–35 µm, respectively. No palynologists have adopted grain diameter as a criterion to distinguish between wild-type and cultivated Cannabis. Many palynologists interpret C–Hpollen as that of cultivated hemp when its pollen count surges or becomes a continuous curve in synchrony with pollen from other crop plants (e.g. Fries 1958, 1962; Godwin 1967; Wilson 1975; Maher 1977; van Zant et al. 1979; Whittington and Edwards 1989; Dörfler 1990; Peglar 1993; Hall et al. 1995; Fleming and Clarke 1998).
The other crop plants in these studies include Avena (oats), Hordeum (barley), Secale (rye), Triticum (wheat), and Cerealia-type (undifferentiated cereal pollen). Pollen curves of two weed species, Centaurea cyanus and Scleranthus annuus, have also been used to interpret C–Hpollen as that of cultivated hemp (Whittington and Jarvis 1986; Gaillard and Berglund 1988; Edwards and Whittington 1990).
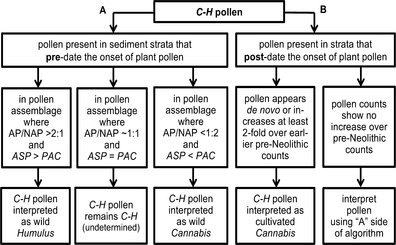
An algorithm was developed to differentiate C–H pollen (Fig. 1). We interpreted C–H pollen as that of cultivated Cannabis when it appeared de novo along with crop pollen, or increased at least twofold over earlier pre-Neolithic counts. To differentiate C–H pollen in pre-agricultural strata, we used ecological proxies. When C–H occurred in a pollen assemblage where the AP-to-NAP ratio ≤ 2 (i.e. ≤ 33%/66%), dominated by steppe vegetation (PAC), we interpreted it as wild-type Cannabis. When C–H occurred in a pollen assemblage where the AP/NAP ratio ≥ 2 (i.e. ≥ 66%/33%), dominated by ASP, we inferred it to be Humulus. In some ambiguous FPSs, pollen counts of PAC and ASP rise and fall in near-synchrony, and the AP/NAP ratio approaches 1:1 (i.e. 50%/50%). At these sites, we classified C–H pollen as unresolved C/H.
Algorithm for the identification of C–H pollen
Readers are invited to explore Online Resource 1 for: (1) the morphological identification of Cannabis and Humulus pollen; (2) phytosociological studies linking C. sativa with PAC spp., and H. lupulus with ASP spp.; (3) debates regarding AP/NAP ratios and pollen signals indicative of crop cultivation; (4) methods of data extraction and binning for algorithm application.
- 1.
18,500–15,000 cal bp, including the latter half of the Last Glacial Maximum and the onset of deglaciation, when dry tundra and steppe plants colonized northern Europe, and mesophytic tree species were limited to glacial refugia, mostly in southern peninsulas.
- 2.
14,500–10,500 cal bp, including the Late Glacial and onset of the Holocene, an unstable period of deglaciation, punctuated by short-lived warming and cooling episodes, such as the Bølling, Older Dryas, Allerød, and Younger Dryas.
- 3.
10,000–7,500 cal bp, including most of the Early Holocene (Walker et al. 2012), a period of improved climate, re-emerging forests, and increased anthropogenic impact on landscapes, prior to Neolithic cultures penetrating Europe beyond the Mediterranean coastline and the lower reaches of the Danube River.
- 4.
7,000–5,000 cal bp, including most of the Middle Holocene, a period including the Mid-Holocene Climatic Optimum, when agriculture spread across much of low-altitude Europe, and when copper smelting began.
- 5.
4,500–2,300 cal bp, onset of the Late Holocene, a period that began with the Bronze age, spanned the Iron age, and ended with the earliest recorded European history. This period includes archaeological evidence of hemp cultivation.
- 6.
2,000–800 cal bp, a period that began when Europe beyond the Roman Empire was still in the prehistoric Iron age. The period ends with the beginning of hop cultivation. Our analysis stops there, in agreement with Wilson (1975), who hypothesized that pollen from cultivated hops may impact pollen diagrams (and thereby conflict with part “B” of the pollen identification algorithm).
GIS mapping
Latitude and longitude of each FPS was plotted, using geographic information system (GIS) software (ArcGIS 10.3). FPS sites were plotted on six maps, corresponding to the six binned time slices. Each FPS site was marked with a symbol indicating pollen interpretation—either wild-type C. sativa, cultivated C. sativa, H. lupulus, or unresolved C/H pollen. To better illustrate data trends over time, the six bins are grouped together as separate maps with the same scale and extent (aka presented as small multiples). Some authors of FPSs did not discuss the archaeological contexts of their sites. The locations and time slices of FPSs were compared with eight maps that delineated territories of archaeological cultures: Early Neolithic (ca. 6800–3500 bce), Late Neolithic and Copper age (ca. 5500–2700 bce), Early Bronze age (ca. 3200–1800 bce), Middle Bronze age (ca. 3000–1500 bce), Late Bronze age (ca. 1700–500 bce), Early Iron age (ca. 1100–400 bce), Middle-to-Late Iron age (ca. 800 bce–100 ce), and End-stage Iron age cultures beyond the Roman Empire (ca. 100–500 ce). These time intervals overlapped because the Neolithic, Copper, Bronze, and Iron ages spread at different rates across Europe. See Online Resource 2 for maps and methodology.
Results
The search strategy identified 603 FPSs that included C–H pollen from the Late Glacial/Holocene. Eighty studies did not meet our inclusion criteria, and another 44 studies reported duplicate data. The remaining 479 FPSs were tabulated, each with an accession number, study location, details regarding application of the algorithm, and duplicate reports. Excluded studies were also tabulated. Included and excluded FPSs appear in Online Resource 3, Table S1 and Table S2, respectively.
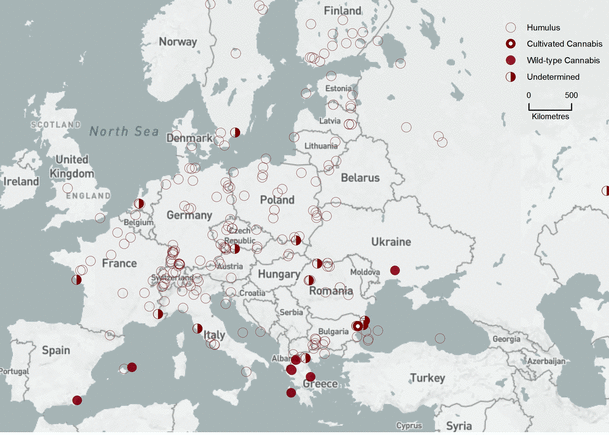
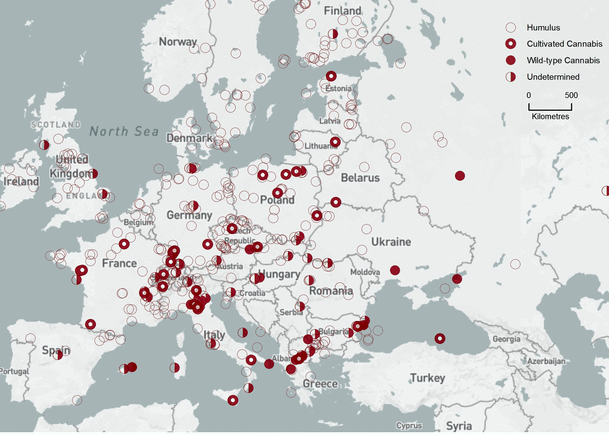
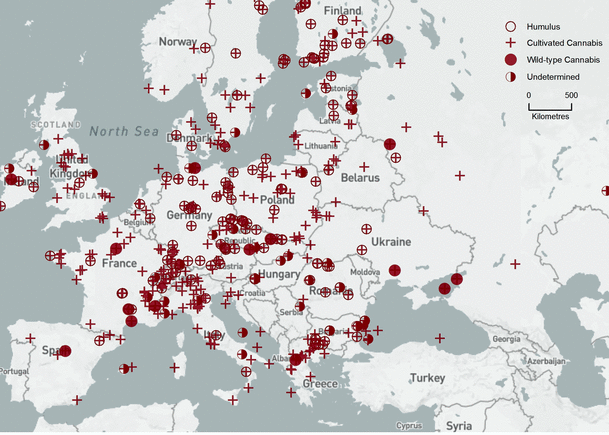
Geographical locations of FPSs, with pollen interpretations, distributed over six times slices, are presented in Figs. 2, 3, 4, 5, 6, 7. Locations of all the 479 FPSs, plotted on a map of Europe, are provided in an interactive map, available at http://arcg.is/2jK9uAv (also see Online Resource 2, Map S1, for a non-interactive version). Interactive functionalities include FPS site queries (click on each individual site to obtain its accession number and other data), pan and zoom, and changing the basemap (for topography, vegetation type, etc.). Note that some FPSs are deep-water cores, so they are located in the Black, Adriatic, and North Seas. Data from the 479 FPSs were binned and mapped:
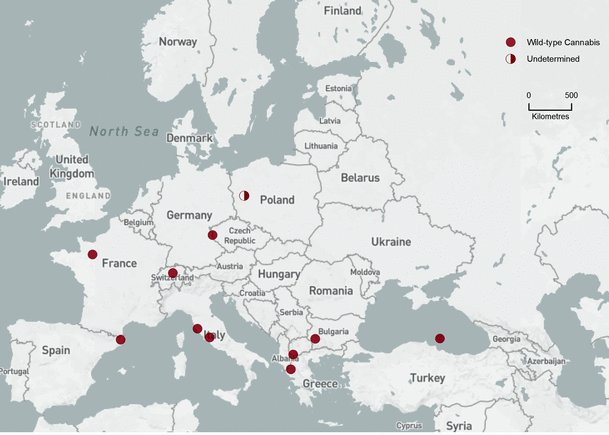
Bin 1 (18,500–15,000 cal bp, Fig. 2). Only eleven FPSs in this time slice reflect a paucity of pollen sediment during the Late Glacial Maximum. In many areas of Europe, the landscape was dominated by nonarboreal (NAP) pollen and steppe species (PAC). The algorithm interprets C–H pollen in this assemblage as wild-type Cannabis. However, pioneer trees, especially Betula, occasionally blanketed landscapes with up to 85% arboreal pollen (AP). C–H pollen in these landscapes was interpreted as unresolved C/H pollen.
Bin 1 (18,500–15,000 cal bp). Background base map by Natural Earth, free open-source map data, http://www.naturalearthdata.com
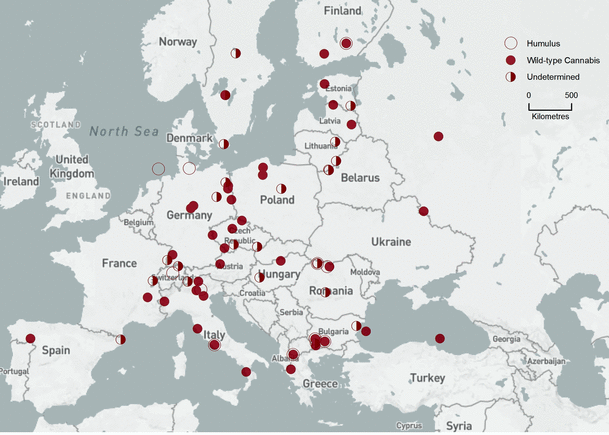
Bin 2 (14,500–10,500 cal bp, Fig. 3). NAP, PAC, and C–H pollen inferred as wild-type Cannabisoccurred throughout Europe. Pollen from all three Humulus associates—ASP—began spreading from glacial refugia. At some FPS sites, the percentage of ASP pollen equalled PAC pollen, which made the determination of C–H pollen uncertain. At other FPS sites, pollen counts of AP + ASP clearly overtook those of NAP + PAC that dominated in Bin 1; C–H pollen at those sites was interpreted as Humulus in forested landscapes.
Bin 2 (14,500–10,500 cal bp). Background base map by Natural Earth, free open-source map data, http://www.naturalearthdata.com
Bin 3 (10,000–7,500 cal bp, Fig. 4). More NAP-to-AP turnover occurred, including many FPSs where PAC = ASP, possibly representing the coexistence of H. lupulus and C. sativa in forest-steppe communities. Crop plant pollen was detected at several FPS sites, but these sites lacked C–H pollen in this time slice.
Bin 3 (10,000–7,500 cal bp). Background base map by Natural Earth, free open-source map data, http://www.naturalearthdata.com
Bin 4 (7,000–5,000 cal bp, Fig. 5). AP + ASP expansion peaked in this time slice, when C–Hpollen interpreted as Humulus blanketed Europe. Data consistent with wild-type Cannabispollen were limited to the Mediterranean coast (Greece, Spain) and the Pontic steppe (Ukraine, Bulgaria). FPS sites with crop pollen did not show signals interpreted as cultivated Cannabisaccording to the algorithm, with one exception (see discussion).
Bin 4 (7,000–5,000 cal bp). Background base map by Natural Earth, free open-source map data, http://www.naturalearthdata.com
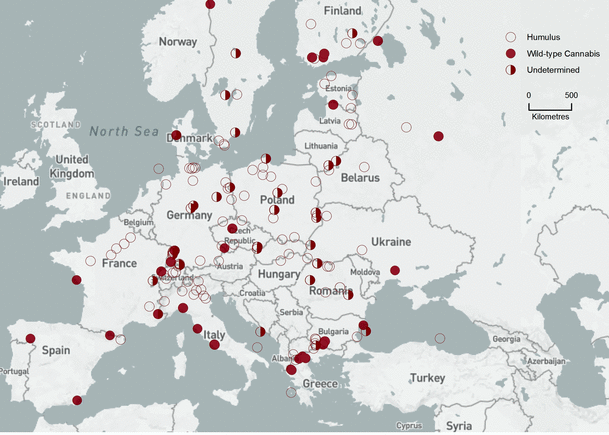
Bin 5 (4,500–2,300 cal bp, Fig. 6). Humulus flourished in heavily-forested Europe. Only 13 FPSs showed pollen signals consistent with wild-type Cannabis. Thirty-five FPSs showed C–Hpollen interpreted as cultivated Cannabis, mostly north of the Alps.
Bin 5 (4,500–2,300 cal bp). Background base map by Natural Earth, free open-source map data, http://www.naturalearthdata.com
Bin 6 (2,000–800 cal bp, Fig. 7). The pollen signal of Humulus was replaced by that of cultivated Cannabis across Europe. Fourteen FPSs showed high levels of NAP, PAC, and crop pollen, but no clear surge of C–H pollen. This pollen signal is not addressed by the algorithm. It may be interpreted as weedy C. sativa that escaped cultivation, or sampling sites that were too far away from cultivation sites to register a clear surge in pollen.
Bin 6 (2,000–800 cal bp). Background base map by Natural Earth, free open-source map data, http://www.naturalearthdata.com
In addition to the above, we identified 36 FPSs with C–H pollen that predated 18,500 cal bp. The studies are few in number, and span a large swath of uncalibrated time, from thousands (kya) to millions (mya) of years ago. They were tabulated rather than mapped, in Table 1.
Interpretation of C–H pollen in studies predating 18,500 cal bp
|
C–H pollen consistent with Cannabis sativa |
C–H pollen undetermined (Cannabis–Humulus) |
C–H pollen consistent with Humulus lupulus |
|---|---|---|
|
Last Glacial Maximum, OIS 4–2, 70–18.5 kya |
||
|
Bulgaria (n = 2), Russia (2), Estonia, Greece, Ukraine |
||
|
Weichselian cooling trend, OIS 5d-a, 115–70 kya |
||
|
Italy, Poland |
Poland, Ukraine |
|
|
Eemian/Mikulino interglacial, OIS 5e, 130–115 kya |
||
|
Russia |
France, Italy, Netherlands, Poland (5), Russia, Sweden |
|
|
Penultimate glaciation, OIS 6, 191–130 kya |
||
|
Russia (2), Italy |
||
|
Zhizdra cooling event (OIS 7, 243–191 kya), Sanian 2 glaciation (OIS 12, 420–400 kya), or Gremyache interglacial (OIS 19, 770 kya) |
||
|
Poland (2), Russia (2) |
||
|
Eopleistocene (1.8–1.2 mya) or Early Pliocene (5.32–3.6 mya) |
||
|
Russia, Romania, Kazakhstan |
||
|
Late Miocene (11.6–5.8 mya) or Oligocene (33.9–23.0) |
||
|
Bulgaria |
Russia, Georgia, Bulgaria, Italy, Czech Republic |
|
Discussion
This study has several limitations. As with any pollen-based method, the algorithm (Fig. 1) is challenged by inherent assumptions and biases. It assumes that ecological niches of modern Cannabis, Humulus, PAC, and ASP can be extrapolated to past populations (the “nearest living relative” paradigm). To wit, natural plant communities break down in landscapes altered by anthropogenic activities. Synanthropic communities, however, often include C. sativa and PACspecies; we cite examples in Online Resource 1. The results generated by our algorithm are best characterized as “probabilities, not proofs.”
The dispersal of FPS datapoints in Figs. 2, 3, 4, 5, 6 and 7 reflects the clumped distribution of palynologists, not the distribution of pollen. Some regions of Europe are dense with FPSs (e.g. UK, Switzerland, Czech Republic), whereas few studies have been conducted in Ukraine and southeast Russia. The paucity of studies in southeast Russia is unfortunate, because Flora Europaea states that “native” populations grow there (Akeroyd 1993).
In Fig. 7, copious pollen from cultivated Cannabis no doubt swamped some of the signal coming from wild Humulus. Conversely, woodland clearance would have enhanced potential habitats for Humulus. Today, cultural landscapes provide artificial surfaces for wild Humulusgrowth—forest edges, hedges, walls, and fences (Lisci and Pacini 1993). These situations could result in a surge of Humulus pollen, potentially misinterpreted as cultivated Cannabis. See Online Resource 1 for elaborations on these and other criticisms of our methods.
In general, when extracting data from FPSs, our dichotomizing proxies were quite robust: NAP vs. AP, and PAC vs. ASP. This made the inference of C–H pollen as either C. sativa or H. lupulus rather straightforward. Other authors have noted that pollen counts of Alnus and PACdemonstrate an oppositional character (Hicks and Birks 1996; Kuneš et al. 2008; Conner 2011).
Indigenous European C. sativa
Global studies of climate change indicate that climate variability began to cycle into stadials (glacial periods) and interstadials (warm periods) during the Pliocene Epoch (Ehlers and Gibbard 2007). The oldest C–H pollen consistent with Cannabis in Europe appeared during the Olduvai cold stage, beginning 1.8 mya (Danukalova 2010). Older C–H pollen from the late Miocene, 6.1–5.3 mya (Ivanov et al. 2007), appeared in an ambiguous milieu (AP/NAP ~ 1:1, PAC = ASP), which the algorithm classified as unresolved C/H. However, another site in Bulgaria, also from the late Miocene, reported a similar mixed flora—and yielded a fossil fruit of Cannabis: carefully analysed, illustrated, and convincing (Palamarev 1982).
Studies that predate 18,500 cal bp (Table 1) and Bin 1 (18,500–15,000 cal bp, Fig. 2) support our hypothesis that Cannabis expanded from Asia to Europe prior to human agency. This should not be surprising, because her sister genus—Humulus—also expanded from Asia to Europe prior to human agency. European print fossils of Humulus date back to the Miocene (Collinson 1989). Several FPSs in Fig. 2 fall within regions traditionally considered glacial refugia of tree species—in Iberia, Italy and Greece. Nevertheless, the pollen in these tree refugia was dominated by NAP, PAC and C. sativa; they may represent “refugia within refugia” (Feliner 2011).
It may seem like a climactic improbability that northern Europe served as C. sativa habitat during the Last Glacial Maximum (Fig. 2) and subsequent deglaciation (Fig. 3). But recall, C. sativa has been classified an “arctic forb” (Bolikhovskaya 2007; Binney et al. 2017). In fact, C. sativa can tolerate climatic conditions north of 68°N (Schübeler 1875). In perspective, our maps here extend up to 63.5°N. Furthermore, northern Europe underwent post-glacial isostatic rebound. This caused land uplift, nearly 300 m in places (Berglund 2004). Uplift coupled with glacial melt created alluvial ravines cutting through dry tundra and steppe—a disturbed landscape perfect for C. sativa.
The dominance of NAP + PAC pollen in Bin 1 is gradually replaced by AP + ASP pollen in Bins 2 through 5. Thus by proxy, C–H pollen interpreted as Cannabis in Bin 1 is gradually replaced by Humulus in Bins 2 through 5. Our estimates of PAC and ASP distribution and abundance across time and space broadly agree with isopollen maps by Huntley and Birks (1983). They present separate maps of P, A, C, and A, S, P pollen across Europe, in time slices corresponding to Bins 2 through 5, in a meta-analysis of 423 FPS. Our estimates also broadly agree with an updated study by Brewer et al. (2017), who used pollen percentage symbols for spatial visualization in a meta-analysis of 828 FPSs. Their maps show the distribution and abundance of P, A, (no C), and A, S, P pollen. Our estimates of Alnus and Salix expansion during the Holocene parallel results in other studies (King and Ferris 1998; Alsos et al. 2009; Douda et al. 2014). Also in agreement with our results, BIOME studies show steppe landscapes contracting from 18 to 6 kya, and rebounding at 0 kya (Prentice et al. 1996; Tarasov et al. 2000).
Aggregating many FPSs enables the separation of collective signal from the noise within individual FPSs. A trend emerges from the collective data: C. sativa colonized Europe during stadials, and was largely replaced by H. lupulus during interstadials. This trend parallels cycles reported for Artemisia (Blyakharchuk and Amel’chenko 2012; Liu et al. 2013; Cao et al. 2013). Thus C. sativa and Artemisia retreated to “interstadial refugia” (sensu López-García et al. 2010), diametrically opposite to tree species, which retreated to refugia during stadials.
Our study shows a nadir of C–H pollen interpreted as Cannabis pollen in Bin 4 (Fig. 5), following the Mid-Holocene Climatic Optimum (MHCO). Cannabis was limited to two interstadial refugia: the Pontic steppe (Ukraine, Bulgaria) and the Mediterranean coast (Greece, Spain). Akeroyd (1993) reports a single modern refugium of “native” C. sativapersisting in the Pontic steppe (southeastern Russia). Consistent with our MHCO findings, Pokorný et al. (2015) demonstrated that steppe landscapes underwent a MHCO bottleneck in the Czech Republic. Their pollen diagram showed Poaceae and Artemisia persisted through the MHCO, but no Cannabis pollen appeared during that interval. We hypothesize that this bottleneck contributed to allopatric segregation between two recognized subspecies—the European C. sativa ssp. sativa, and the Asian C. sativa ssp. indica (Small and Cronquist 1976).
No cultivated C. sativa in Neolithic Europe
C–H pollen consistent with Cannabis largely disappeared from Europe by the time farmers and/or agricultural technology arrived from the Fertile Crescent. West Asian “founder crops” diffused into Europe. This crop package included flax, Linum usitatissimum L., a fiber and seed oil crop (functionally analogous to C. sativa). We hypothesize that people from three Neolithic cultures had the potential to domesticate wild-type C. sativa. This hypothesis was generating by constructing maps that delineated the territories of Neolithic cultures (Online Resource 2, Maps S2, S3). We compared these culture maps with locations of C–H pollen consistent with wild-type Cannabis in Figs. 4 and 5. Those locations fell into the territories of Neolithic Greece, the Cardium Pottery culture, and the Bug-Dniester culture. For scholars interested in citations, see Online Resource 3, Table S1 (Neolithic Greece studies #89, 282, 284, 288, 289; Cardium Pottery: #94, 196; Bug-Dniester culture: #237).
However, no pollen signals consistent with cultivated Cannabis arise within these cultures, or any early Neolithic cultures (e.g. Starčevo-Körös-Criş, Linearbandkeramik, early Cucuteni-Tripolye, see Map S2). This agrees with archaeological studies of these cultures, which lack evidence of C. sativa (Cârciumaru 1996; Bogaard 2004; Kreuz et al. 2005; Conolly et al. 2008). When it comes to Cannabis, these farmers either “missed the boat,” or their cultivation of hemp was a “false start” (meaning its cultivation was quickly abandoned).
Rimantienė (1979) identified Canabinaceae (sic) pollen at Šventoji in Lithuania, a Narva culture site with an uncalibrated 14C date of 4,190 bp. She identified it as C. sativa, alongside a grain of “millet” pollen. Our algorithm interpreted the pollen as Humulus. Consistent with this, Rimantienė (1992) stated that Alnus glutinosa surrounded the Šventoji lagoon of 4,190 bp. We analysed 24 fossil pollen studies in the Baltic region (Table S1, studies #12–30, 246–250). All C–H pollen from the Baltic Neolithic was interpreted as Humulus or C/H (undetermined). The earliest C–H pollen suggestive of cultivated Cannabis in the Baltic region appeared 500 bce(study #12). Piličiauskas et al. (2017) recently deconstructed Rimantienė’s identification of Cannabis and the entire concept of Subneolithic farming in the Baltic region.
C. sativa during the Copper age
We compared the territories of Copper age cultures (Map S3) with locations of C–H pollen consistent with Cannabis in Fig. 5. This suggests that two Copper age cultures had the potential to domesticate wild-type C. sativa: the Greek Chalcolithic (Table S1, studies #89, 94, 284, 288), and the Cucuteni-Tripolye culture (study #237). C–H pollen consistent with cultivated Cannabis occurred at one site in Bulgaria (#274). This site may correspond to the Varna culture or Gumelniţa culture. However, pollen at five other Varna and Gumelniţa sites was interpreted as Humulus (#276), or undetermined C/H (#70, 71, 273, 275). Archaeological studies of Gumelniţa and Cucuteni-Tripolye sites have found C. sativa seeds and less-robust evidence—pottery seed impressions (Clarke and Merlin 2013; Long et al. 2017; McPartland and Hegman 2017).
C. sativa during the Bronze age
Eight Bronze age cultures had potential: C–H pollen consistent with wild-type Cannabis in Fig. 6 appeared within the boundaries of several Bronze age cultures (Maps S4–S6). These include the Netted Ware culture (Table S1, study #6), Ezero culture (#271), Yamnaya culture (#237, 427), Corded Ware culture (#305), Bell-Beaker culture (#198), Terramara culture (#347), Aegean Bronze age (#282, 284, 288), and Mycenaean Greece (#282, 284, 288).
C–H pollen interpreted as cultivated C. sativa appeared in four studies: One study in Yamnaya territory (#274) agrees with archaeological studies, which have recovered C. sativa seeds or pottery seed impressions (Clarke and Merlin 2013; Long et al. 2017; McPartland and Hegman 2017). Two study sites are associated with the Terramara culture (#344, 349). However, pollen in 11 other studies at Terramara sites suggested Humulus or indeterminate C/H pollen. One FPS in France (#436) was likely contaminated by taphonomic processes, as admitted by its authors.
Collectively, FPSs show evidence of hemp cultivation during the Copper and Bronze ages. This poses a larger question: was European C. sativa domesticated autochthonously, separate from its domestication in eastern China? This complex issue is discussed in our sister publication (McPartland and Hegman 2017). Basically, we agree with Vavilov (1926), “It is probable that the cultivation of hemp arose simultaneously and independently in several places”.
C. sativa during the Iron age
C–H pollen interpreted as cultivated Cannabis appeared within territories and time slices occupied by several Iron age cultures (Maps S7–S9). By the Iron age, the Proto-Indo-European language diverged into several language groups. We organized this section by language groups, including Iranian, Greek, Celtic, Slavic, and Baltic languages.
The Iranian-speaking Scythians migrated to the Pontic steppe from Central Asia. Three FPSs in Scythian territory showed C–H pollen consistent with wild-type or cultivated Cannabis (Table S1, studies #243, 244, 427). At the dawn of European history (ca. 440 bce), Herodotus said Open image in new window “grows both wild and cultivated” in the land of Scythia (Herodotus 2007). No less than a dozen Scythian sites in Europe have recovered seeds, pottery seed impressions, cordage, or textiles (McPartland and Hegman 2017).
“grows both wild and cultivated” in the land of Scythia (Herodotus 2007). No less than a dozen Scythian sites in Europe have recovered seeds, pottery seed impressions, cordage, or textiles (McPartland and Hegman 2017).
Herodotus introduced Open image in new window with Open image in new window
with Open image in new window “there is, there exists”, a verb he joined to a noun he assumed was unknown to the reader. Pollen suggestive of wild-type Cannabis occurred in Greece prior to Herodotus’ time, but no archaeological evidence suggests the Iron age Greeks recognized its utility. After Herodotus’ time, pollen consistent with cultivated Cannabisappeared in Greece or Greek colonies (studies #84, 361, 362). Pollen consistent with cultivated Cannabis appeared at one FPS site prior to Herodotus, ca. 525 bce (#287). However, that site borders Thrace. Herodotus described the Thracians making textiles from Open image in new window
“there is, there exists”, a verb he joined to a noun he assumed was unknown to the reader. Pollen suggestive of wild-type Cannabis occurred in Greece prior to Herodotus’ time, but no archaeological evidence suggests the Iron age Greeks recognized its utility. After Herodotus’ time, pollen consistent with cultivated Cannabisappeared in Greece or Greek colonies (studies #84, 361, 362). Pollen consistent with cultivated Cannabis appeared at one FPS site prior to Herodotus, ca. 525 bce (#287). However, that site borders Thrace. Herodotus described the Thracians making textiles from Open image in new window fiber. Surprisingly, no other studies within Thracian territory showed pollen signals of cultivated C. sativa in the bce era.
fiber. Surprisingly, no other studies within Thracian territory showed pollen signals of cultivated C. sativa in the bce era.
The Iron age Celts (Hallstatt phases C and D, and La Tène culture) grew hemp, as attested by plentiful macroscopic evidence (Clarke and Merlin 2013; Long et al. 2017; McPartland and Hegman 2017). C–H pollen consistent with cultivated Cannabis was found in France (#184, 189, 190, 191, 193, 366, 369, 371–374, 380, 436), Germany (#126, 128, 130, 132, 134, 135), Switzerland (#334, 336, 442, 443), Great Britain (#201, 390), Hungary (#95, 97), Czech Republic (#114, 451), northern Italy (#340), and Spain (#386). This plethora of evidence partially reflects the density of palynologists in these countries.
Studies in Proto-Slavic territories showed pollen signals consistent with hemp cultivation, associated with the Pomeranian culture (#43, 45, 265), Przeworsk culture (#38, 39, 44, 47, 48, 51, 262, 263, 266), and Zarubintsy culture (#239). Three Proto-Baltic cultures also showed C–H pollen consistent with cultivated Cannabis: the West Baltic Barrow Culture (#12, 13, 28, 256, 474), Milograd culture (#245), and Bogaczewo culture (#12, 13, 16, 28, 253, 256). Proto-Germanic cultures showed evidence of hemp cultivation: the Jastorf culture (#317), Nordic Pre-Roman Iron age (#230), and Nordic Roman Iron age (#232, 233, 299, 397, 457), as well as the Proto-Germanic-Slavic Wielbark culture (#34, 259, 260).
The question arises whether Celtic, Proto-Slavic, and Proto-Baltic cultures began cultivating C. sativa autochthonously, or as a result of interacting with Scythians. The evidence of Scythian cultivation is old. In Central Asia, Cannabis pollen at Proto-Scythian Tagar culture sites arises 900 bce (McPartland and Guy 2016). After 900 bce the Scythians moved to the Pontic steppe, where signals of hemp cultivation preceded their arrival, back to the Bronze age.
The Scythians impacted deeply on the Celts, in the realms of art, animal husbandry, military strategy, language, and even clothing. The oldest evidence of Scythian–Celtic interactions that we could find was a 7th century bce burial in Bulgaria, which combined elements of Scythian culture along with a Hallstatt vessel (Braund 2015). Scythian artifacts in Hallstatt-occupied Hungary first appear around 550 bce (Bartosiewicz and Gál 2010). A Hallstatt burial at Vix in France from 525 bce contains items and motifs inspired by Scythian culture (Megaw 1966).
These data collectively suggest a conservative date of 550 bce as the terminus post quem for Scythian contact with the Celts. Only three sites in Celtic territory showed pollen signals consistent with hemp cultivation prior to 550 bce (#114, 135, 340). To wit, the oldest ones (#135, 340) had problems with dating. In contrast, 28 FPSs in Celtic territory showed pollen signals of hemp cultivation arising post-550 bce, after their contact with the Scythians.
The Scythians also impacted Proto-Slavic cultures. The Scythians left a trail of burned-out settlements built by the Proto-Slavic Lusatian culture around 600 bce (Bukowski 1977). A horde of Scythian artifacts found at Witaszkowo in Lusatia dates to 550 bce (Furtwängler 1883). Only two pre-550 bce sites in Slavic/Baltic territory showed signals consistent with hemp cultivation, and they occurred in the southeast, towards the Scythian homeland (#245, 580 bce; #265, 570 bce). Ralska-Jasiewiczowa and van Geel (1998) linked the appearance of Cannabispollen in Poland with Scythian incursions. The Scythians appear to be responsible for the spread of Cannabis amongst several Iron age European cultures.
Moving into Bin 6 (Fig. 7), C–H pollen interpreted as cultivated Cannabis blankets much of Europe. Dörfler (1990) cites macroscopic and pollen findings of hemp cultivation spreading into new regions in synchrony with Roman invasions.
Pollen evidence suggestive of hemp cultivation appears in new places after the Romans arrived (Map S9), such as the Italian Alps (#171, 173, 174), Spain (#197, 386), Switzerland (#169), Austria (#309), Great Britain (#201, 203, 207), and Greece (#282, 286).
We plan to compare these results with linguistic data, by examining European cognates for hemp in Indo-European, Finno-Ugric, Caucasus, and Semitic language families. We also plan to extend our fossil pollen research into Asia.
Notes
Acknowledgements
Felix Bittmann, Anna Maria Mercuri, Mark Merlin, and two anonymous reviewers greatly improved this manuscript with their suggestions. Funding was provided by GW Pharmaceuticals.
Supplementary material
References
-
Akeroyd JR (1993) Cannabis L. In: Tutin TG et al (eds) Flora Europaea, vol 1: Psilotaceae to Platanaceae. Cambridge University Press, Cambridge, p 78Google Scholar
-
Alsos IG, Alm T, Norman S, Brochmann C (2009) Past and future range shifts and loss of diversity in dwarf willow (Salix herbacea L.) inferred from genetics, fossils and modeling. Glob Ecol Biogeogr 18:223–239CrossRefGoogle Scholar
-
Bartosiewicz L, Gál E (2010) Living on the frontier: “Scythian” and “Celtic” animal exploitation in Iron age northeastern Hungary. In: Campana DV et al (eds) Anthropological approaches to zooarchaeology. Oxbow Books, Oxford, pp 115–127Google Scholar
-
Berglund M (2004) Holocene shore displacement and chronology in Angermanland, eastern Sweden, the Scandinavian glacio-isostatic uplift centre. Boreas 33:48–60CrossRefGoogle Scholar
-
Binney H, Edwards M, Macias-Fauria M et al (2017) Vegetation of Eurasia from the Last Glacial Maximum to the present: key biogeographic patterns. Quat Sci Rev 157:80–97CrossRefGoogle Scholar
-
Blyakharchuk TA, Amel’chenko VP (2012) Dynamics of the range of Artemisia genus over the territory of Western Siberia and adjacent regions in Holocene in connection with climate change on the basis of pollen data. Contemp Probl Ecol 5:136–145CrossRefGoogle Scholar
-
Bogaard A (2004) Neolithic farming in central Europe. Routledge, LondonGoogle Scholar
-
Bolikhovskaya NS (2007) Spatial and temporal regularities in the evolution of vegetation and climate of North Eurasia in the Neopleistocene. Archaeol Ethnol Anthropol Eurasia 4(32):2–28CrossRefGoogle Scholar
-
Braund D (2015) Thracians and Scythians: tensions, interactions, and osmosis. In: Valena J, Nankov E, Graninger D (eds) A companion to ancient Thrace. Wiley Blackwell, Chichester, pp 352–365Google Scholar
-
Brewer S, Giesecke T, Davis BAS et al (2017) Late-glacial and Holocene European pollen data. J Maps 13:921–928CrossRefGoogle Scholar
-
Bukowski Z (1977) The Scythian influence in the area of Lusatian culture. Zakład Narodowy imienia Ossolińskich, WrocławGoogle Scholar
-
Cao XY, Ni JA, Herzschuh U et al (2013) A late quaternary pollen dataset from eastern continental Asia for vegetation and climate reconstructions: set up and evaluation. Rev Palaeobot Palynol 194:21–27CrossRefGoogle Scholar
-
Cârciumaru M (1996) Paleoetnobotanica. Studii în preistoria şi protoistoria României. Editura Glasul Bucovinei, IaşiGoogle Scholar
-
Clarke RC, Merlin MD (2013) Cannabis evolution and ethnobotany. University of California Press, BerkeleyGoogle Scholar
-
Collinson ME (1989) The fossil history of the Moraceae, Urticaceae (including Cecropiaceae), and Cannabaceae. In: Crane PR, Blackmore S (eds) Evolution, systematics, and fossil history of the hamamelidae, vol 2. Clarendon Press, Oxford, pp 319–339Google Scholar
-
Conner SE (2011) A promethean legacy: late quaternary vegetation history of southern Georgia, Caucasus. Walpole, LeuvenGoogle Scholar
-
Conolly J, Colledge S, Shennan S (2008) Founder effect, drift, and adaptive change in domestic crop use in early Neolithic Europe. J Archaeol Sci 35:2,797–2,804CrossRefGoogle Scholar
-
Danukalova GA (2010) The refined Quaternary Stratigraphic Scale of the Fore-Urals and main events in southern Urals Region. Stratigr Geol Correl 18:331–348CrossRefGoogle Scholar
-
De Candolle AP (1883) Origine des Plantes Cultivées. Baillière, ParisGoogle Scholar
-
Dirksen VG, van Geel B (2004) Mid to late Holocene climate change and its influence on cultural development in south central Siberia. In: Scott EM et al (eds) Impact of the Environment on Human Migration in Eurasia. Kluwer Academic Publishers, Dordrecht, pp 291–307CrossRefGoogle Scholar
-
Dörfler W (1990) Die Geschichte des Hanfanbaus in Mitteleuropa aufgrund palynologischer Untersuchungen und von Großrestnachweisen. Prähistorische Zeitschrift 65:218–244CrossRefGoogle Scholar
-
Douda J, Doudová J, Drašnarová A et al (2014) Migration patterns of subgenus Alnus in Europe since the Last Glacial Maximum: a systematic review. PLoS One 9(2):e88709CrossRefGoogle Scholar
-
Edwards KJ, Whittington G (1990) Palynological evidence for the growing of Cannabis sativa L. (hemp) in medieval and historical Scotland. Trans Inst Br Geogr 15:60–69CrossRefGoogle Scholar
-
Ehlers J, Gibbard PL (2007) The extent and chronology of Cenozoic global glaciation. Quat Int 164–165:6–20CrossRefGoogle Scholar
-
Engelmark R (1976) The vegetation history of the Umeå area during the past 4000 years. Early Norrl 9:73–111Google Scholar
-
Feliner GN (2011) Southern European glacial refugia: a tale of tales. Taxon 60:365–372Google Scholar
-
Firbas F (1937) Der pollenanalytische Nachweis des Getreideanbaus. Zeitschrift für Botanik 31:447–478Google Scholar
-
Fleming MP, Clarke RC (1998) Physical evidence for the antiquity of Cannabis sativa L. J Int Hemp Assoc 5:80–92Google Scholar
-
French CN, Moore PD (1986) Deforestation, Cannabis cultivation and schwingmoor formation at Cors Llyn (Llyn Mire), Central Wales. New Phytol 102:469–482CrossRefGoogle Scholar
-
Fries M (1958) Vegetationsutveckling och odlingshistoria i Varnhemstrakten: en pollenanalytisk undersökning i Västergötland. Acta Phytogeographica Suecica 39:1–63Google Scholar
-
Fries M (1962) Studies of the sediments and the vegetational history in the Ösbysjön basin, north of Stockholm. Oikos 13:76–96CrossRefGoogle Scholar
-
Fröman I (1939) Die Hölzer des Rades und der Hopfenfund. In: von Post L, Oldeberg A, Fröman I (eds) Ein eisenzeitliches Rad aus dem Filaren-See in Södermanland, Schweden. Wahlström & Widstrand, Stockholm, pp 89–98Google Scholar
-
Fuller DQ (2006) Agricultural origins and frontiers in South Asia: a working synthesis. J World Prehist 20:1–86CrossRefGoogle Scholar
-
Furtwängler A (1883) Der Goldfund von Vettersfelde. Reimer, BerlinGoogle Scholar
-
Gaillard MJ, Berglund BE (1988) Land-use history during the last 2700 years in the area of Bjäresjö, Sweden. In: Berks HH et al (eds) The cultural landscape—past, present and future. Cambridge University Press, Cambridge, pp 409–425Google Scholar
-
Giesecke T, Davis B, Brewer S et al (2014) Towards mapping the late quaternary vegetation change of Europe. Veget Hist Archaeobot 14:75–86CrossRefGoogle Scholar
-
Godwin B (1967) Pollen analytic evidence for the cultivation of Cannabis in England. Rev Paleobot Palynol 4:71–80CrossRefGoogle Scholar
-
Hall A, Kenward H, Large F (1995) Biological remains from a medieval ‘pond’ at Higher Lane, Fazakerley, north Liverpool, Merseyside (site code FAZ94). Rep Environ Archaeol Unit York 96/5:1–29Google Scholar
-
Hauschild S (1991) Pollenanalytische Untersuchungen zur Vegetations- und Siedlungsgeschichte am Höherer See in Oberösterreich. Doctoral thesis, University of Göttingen, GöttingenGoogle Scholar
-
Herder FG (1892) Plantae Raddeanae apetalae V. Acta Horti Petropolitani 12:31–132Google Scholar
-
Herodotus (2007) The landmark Herodotus: the histories In: Strassler RB, Purvis AL (eds) Pantheon Books, New YorkGoogle Scholar
-
Hicks S, Birks HJB (1996) Numerical analysis of modern and fossil pollen spectra as a tool for elucidating the nature of fine-scale human activities in boreal areas. Veget Hist Archaeobot 5:257–272CrossRefGoogle Scholar
-
Huntley B, Birks HJB (1983) An atlas of past and present pollen maps for Europe: 0–13,000 years ago. Cambridge University Press, CambridgeGoogle Scholar
-
Ivanov DA, Bozukov VS, Koleva-Rekalova EK (2007) Late Miocene flora from SE Bulgaria: vegetation, landscape and climate reconstruction. Phytologia Balcanica 13:281–292Google Scholar
-
Keppen T (1886) Догадка о происхожденіи большинства индоевропейскихъ названій конопли. Журнал Министерства народнаго просвěщенія 245:73–86Google Scholar
-
King RA, Ferris C (1998) Chloroplast DNA phylogeography of Alnus glutinosa (L.) Gaertn. Mol Ecol 7:1,151–1,161CrossRefGoogle Scholar
-
Kreuz A, Marinova E, Schäfer E, Wiethold J (2005) A comparison of early Neolithic crop and weed assemblages from the Linearbandkeramik and the Bulgarian Neolithic cultures: differences and similarities. Veget Hist Archaeobot 14:237–258CrossRefGoogle Scholar
-
Kuneš P (2008) Human-driven and natural vegetation changes of the last glacial and early Holocene. Doctoral thesis, Charles University Prague, PragueGoogle Scholar
-
Kuneš P, Pokorný P, Šída P (2008) Detection of the impact of early Holocene hunter-gatherers on vegetation in the Czech Republic using multivariate analysis of pollen data. Veget Hist Archaeobot 17:269–287CrossRefGoogle Scholar
-
Lamarck JB (1785) Encyclopédie Méthodique. Botanique Tome premier 2:345–752Google Scholar
-
Lee CY, Liew PM (2010) Late quaternary vegetation and climate changes inferred from a pollen record of Dongyuan Lake in southern Taiwan. Palaeogeogr Palaeoclimatol Palaeoecol 287:58–66CrossRefGoogle Scholar
-
Linnaeus C (1737) Hortus Cliffortianus. AmsterdamGoogle Scholar
-
Lisci M, Pacini E (1993) Plants growing on the walls of Italian towns. 2: Reproductive ecology. Giornale Botanico Italiano 127:1,053–1,078CrossRefGoogle Scholar
-
Liu HY, Liu K, Wei FL (2013) Artemisia pollen-indicated steppe distribution in southern China during the Last Glacial Maximum. J Palaeogeogr 2:297–305Google Scholar
-
Long TW, Wagner M, Demske D et al (2017) Cannabis in Eurasia: origin of human use and Bronze age trans-continental connections. Veget Hist Archaeobot 26:245–258CrossRefGoogle Scholar
-
López-García JM, Blain HA, Allué E et al (2010) First fossil evidence of an “interglacial refugium” in the Pyrenean region. Naturwissenshaften 97:753–761CrossRefGoogle Scholar
-
Maher LJ (1977) Palynological studies in the western arm of lake superior. Quat Res 7:14–44CrossRefGoogle Scholar
-
McPartland JM, Guy GW (2016) Cannabis may have evolved in the northeastern Tibetan Plateau, based on an interdisciplinary study of genetics, fossil pollen, and ecology. In: Proceedings of the 26th annual symposium on the cannabinoids. International Cannabinoid Research Society, Research Triangle Park, p 61Google Scholar
-
McPartland JM, Guy GW (2017) Models of Cannabis taxonomy, cultural bias, and conflicts between scientific and vernacular names. Bot Rev 83:327–381CrossRefGoogle Scholar
-
McPartland JM, Hegman W (2017) Cannabis utilization and diffusion patterns in prehistoric Europe: a critical analysis of archaeological evidence. Veget Hist Archaeobot. https://doi.org/10.1007/s00334-017-0646-7Google Scholar
-
McPartland JM, Guy GW, Hegman W (2013) Distribution of Cannabis sativa in Europe based on fossil pollen and ecological analyses. In: Proceedings of the 23th annual symposium on the cannabinoids. International Cannabinoid Research Society, Research Triangle Park, p 1Google Scholar
-
Megaw JVS (1966) The vix burial. Antiquity 40(157):38–44CrossRefGoogle Scholar
-
Mercuri AM, Accorsi CA, Mazzanti MB (2002) The long history of Cannabis and its cultivation by the Romans in central Italy, shown by pollen records from Lago Albano and Lago di Nemi. Veget Hist Archaeobot 11:263–276CrossRefGoogle Scholar
-
Ni J, Yu G, Harrison SP, Prentice IC (2010) Palaeovegetation in China during the late quaternary: Biome reconstructions based on a global scheme of plant functional types. Palaeogeogr Palaeoclimatol Palaeoecol 289:44–61CrossRefGoogle Scholar
-
Palamarev E (1982) Неогенската карпофлора на Мелнишкия басейн. Paleontol Stratigr Lithol 16:3–43Google Scholar
-
Peglar SM (1993) The development of the cultural landscape around Diss Mere, Norfolk, UK, during the past 7000 years. Rev Palaeobot Palynol 76:1–47CrossRefGoogle Scholar
-
Piličiauskas G. Kisielienė D, Piličiauskas G (2017) Deconstructing the concept of Subneolithic farming in the southeastern Baltic. Veget Hist Archaeobot 26:183–193CrossRefGoogle Scholar
-
Pokorný P, Chytrý M, Juřičková L et al (2015) Mid-Holocene bottleneck for central European dry grasslands: did steppe survive the forest optimum in northern Bohemia. Czech Republic? Holocene 25:716–726CrossRefGoogle Scholar
-
Prentice IC, Guiot J, Huntley B et al (1996) Reconstructing biomes from palaeoecological data: a general method and its application to European pollen data at 0 and 6 ka. Clim Dyn 12:185–194CrossRefGoogle Scholar
-
Punt W, Malotaux M (1984) Cannabaceae, Moraceae and Urticaceae. Rev Palaeobot Palynol 42:23–44CrossRefGoogle Scholar
-
Purkyně JE (1830) De cellulis antherarum fibrosis, nec non de granorum pollinarium formis commentatio phytotomico. J. D. Gruesonius, VratislaviaeGoogle Scholar
-
Ralska-Jasiewiczowa M, van Geel B (1998) Human impact on the vegetation of the Lake Gościąż surroundings in prehistoric and early-historic times. In: Ralska-Jasiewiczowa M et al (eds) Lake Gościąż, central Poland. A monographic study, Part 1. Polish Academy of Sciences, Kraków, pp 267–294Google Scholar
-
Riehl S, Pustovoytov K (2006) Comment on van Geel et al. J Archaeol Sci 31 (2004) “Climate change and the expansion of the Scythian culture after 850 bc: a hypothesis”. J Archaeol Sci 33:143–145CrossRefGoogle Scholar
-
Rimantienė R (1979) Šventoji I: Narvos kultūros gyvenvietės. Mokslas, VilniusGoogle Scholar
-
Rimantienė R (1992) Neolithic hunter-gathers at Šventoji in Lithuania. Antiquity 66:367–376CrossRefGoogle Scholar
-
Scholz H (1957) Der wilde Hanf als Ruderalpflanze Mitteleuropas. Verhandlungen des Botanischen Vereins für die Provinz Brandenburg 83(97):61–64Google Scholar
-
Schübeler FC (1875) Die Pflanzenwelt Norwegens. Ein Beitrag zur Natur-und Culturgeschichte Nord-Europas. Brøgger, ChristianiaGoogle Scholar
-
Scopoli GA (1772) Flora Carniolica, Tom. II, 2 edn. Krauss, Vienna ndGoogle Scholar
-
Small E, Cronquist A (1976) A practical and natural taxonomy for Cannabis. Taxon 25:405–435CrossRefGoogle Scholar
-
Tarasov P, Webb T, Andreev AA et al. (1998). Present-day and mid-Holocene biomes reconstructed from pollen and plant macrofossil data from the former Soviet Union and Mongolia. J Biogeogr 25:1,029–1,053Google Scholar
-
Tarasov PE, Volkova VS, Webb T et al (2000) Last Glacial Maximum biomes reconstructed from pollen and plant macrofossil data from northern Eurasia. J Biogeogr 27:609–620CrossRefGoogle Scholar
-
Thiébaut de Berneaud A (1835) Chanvre. In: Guérin-Méneville FE (ed) Dictionnaire pittorosque d’histoire naturelle et des phénomènes de la nature, Tome 2. De Cosson, Paris, pp 87–89Google Scholar
-
Tweddle JC (2000) A high resolution palynological study of the Holocene vegetational development of central Holderness, Eastern Yorkshire, with particular emphasis on the detection of prehistoric human activity. Doctoral thesis, University of Sheffield, SheffieldGoogle Scholar
-
Van Geel B, Bokovenko NA, Burova ND et al (2004) Climate change and the expansion of the Scythian culture after 850 bc: a hypothesis. J Archaeol Sci 31:1,735–1,742CrossRefGoogle Scholar
-
Van Zant KL, Webb T, Peterson GM et al (1979) Increased Cannabis/Humulus pollen, an indicator of European agriculture in Iowa. Palynology 3:227–233CrossRefGoogle Scholar
-
Vavilov NI (1926) The origin of the cultivation of “primary” crops, in particular cultivated hemp. Труды по прикладной ботанике, генетике и селекции 16:221–233Google Scholar
-
Walker MJC, Berkelhammer M, Björck S et al (2012) Formal subdivision of the Holocene Series/Epoch: a discussion paper by a working group of INTIMATE (Integration of ice-core, marine and terrestrial records) and the subcommission on quaternary stratigraphy. J Quat Sci 27:649–659CrossRefGoogle Scholar
-
Whittington G, Edwards KJ (1989) Problems in the interpretation of Cannabaceae pollen in the stratigraphic record. Pollen Spores 31:79–96Google Scholar
-
Whittington G, Gordon AD (1987) The differentiation of the pollen of Cannabis sativa L. from that of Humulus lupulus L. Pollen Spores 29:111–120Google Scholar
-
Whittington G, Jarvis J (1986) Kilconquhar Loch, Fife: an historical and palynological investigation. Proc Soc Antiqu Scotl 116:413–428Google Scholar
-
Wilson DG (1975) Plant remains from the Graveney boat and the early history of Humulus lupulus L. in W. Europe. New Phytol 75:627–648CrossRefGoogle Scholar
-
Winterschmidt JS (1818) Naturgetreue Darstellung aller inn- und ausländischen Material-Samen und getrockneten Früchte. NürnbergGoogle Scholar
-
Zhou B, Shen CD, Sun WD et al (2007) Elemental carbon record of paleofire history on the Chinese Loess Plateau during the last 420 ka and its response to environmental and climate changes. Palaeogeogr Palaeoclimatol Palaeoecol 252:617–625CrossRefGoogle Scholar