- Scientific Reports 7, Article number: 4858(2017)
- doi:10.1038/s41598-017-05017-5
- Received:Accepted:Published online:
Abstract
The Cannabinoid Receptor Interacting Protein 1 (Cnrip1) was discovered as an interactor with the intracellular region of Cannabinoid Receptor 1 (CB1R, also known as Cnr1 or CB1). Functional assays in mouse show cannabinoid sensitivity changes and Cnrip1 has recently been suggested to control eye development in Xenopus laevis. Two Cnrip1 genes are described in zebrafish, cnrip1a and cnrip1b. In situ mRNA hybridisation revealed accumulation of mRNA encoding each gene primarily in brain and spinal cord, but also elsewhere. For example, cnrip1b is expressed in forming skeletal muscle. CRISPR/Cas9 genome editing generated predicted null mutations in cnrip1a and cnrip1b. Each mutation triggered nonsense-mediated decay of the respective mRNA transcript. No morphological or behavioural phenotype was observed in either mutant. Moreover, fish lacking both Cnrip1a and Cnrip1b both maternally and zygotically are viable and fertile and no phenotype has so far been detected despite strong evolutionary conservation over at least 400 Myr.
Introduction
 The discovery of (−)-trans-Δ9-tetrahydrocannabinol (THC) as the major psychoactive component of marijuana (Cannabis sativa)1 and subsequent cloning of the THC receptor2 helped to identify the endogenous cannabinoid system (eCS). The eCS is a signalling network responding to endogenous lipid transmitters (endocannabinoids) that bind to the G-protein coupled cannabinoid receptors CB1R and CB2R3. These receptors act via Gαi/o binding to their second and third intracellular loops and C-terminal 8th helix to initiate signalling events typical of this class of transducing proteins, such as inhibition of adenylyl cyclase and activation of MAPK and FAK4, 5. CB1R has also been suggested to affect PI3K and PKB signalling and a variety of ion channels3, 6. Endocannabinoids are frequently produced post-synaptically in response to neurotransmitter signalling and act retrogradely on pre-synaptic terminals to attenuate pre-synaptic depolarisation and diminish further neurotransmitter release, forming a negative feedback loop3, 7. The eCS is involved in numerous pathological conditions, ranging from neurological disorders, such as Parkinson’s, Huntington’s and Alzheimer’s diseases8, to gastrointestinal, cardiovascular and reproductive disorders9 and is also potentially involved in relief of pain, chemotherapy-induced nausea and vomiting, and anorexia10. It thus has potential for therapeutic drug development11.
The discovery of (−)-trans-Δ9-tetrahydrocannabinol (THC) as the major psychoactive component of marijuana (Cannabis sativa)1 and subsequent cloning of the THC receptor2 helped to identify the endogenous cannabinoid system (eCS). The eCS is a signalling network responding to endogenous lipid transmitters (endocannabinoids) that bind to the G-protein coupled cannabinoid receptors CB1R and CB2R3. These receptors act via Gαi/o binding to their second and third intracellular loops and C-terminal 8th helix to initiate signalling events typical of this class of transducing proteins, such as inhibition of adenylyl cyclase and activation of MAPK and FAK4, 5. CB1R has also been suggested to affect PI3K and PKB signalling and a variety of ion channels3, 6. Endocannabinoids are frequently produced post-synaptically in response to neurotransmitter signalling and act retrogradely on pre-synaptic terminals to attenuate pre-synaptic depolarisation and diminish further neurotransmitter release, forming a negative feedback loop3, 7. The eCS is involved in numerous pathological conditions, ranging from neurological disorders, such as Parkinson’s, Huntington’s and Alzheimer’s diseases8, to gastrointestinal, cardiovascular and reproductive disorders9 and is also potentially involved in relief of pain, chemotherapy-induced nausea and vomiting, and anorexia10. It thus has potential for therapeutic drug development11.
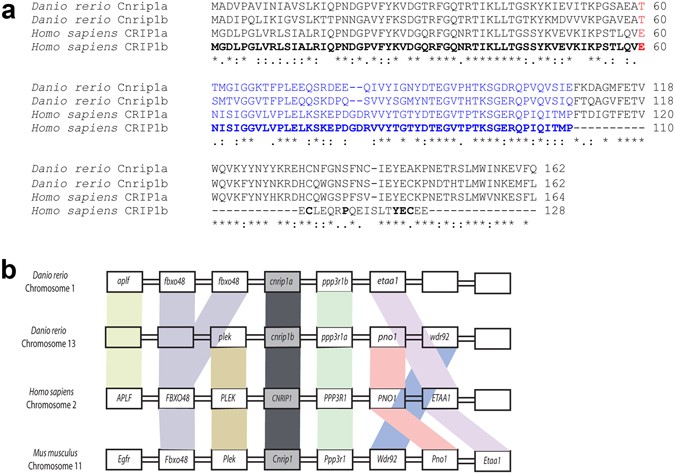
To understand the biochemical mechanism of endocannabinoid action and find possible drug targets, screens have been performed for proteins interacting with CB1R12,13,14. Two alternative splicing variant proteins, named CRIP1a and CRIP1b, deriving from the human Cannabinoid Receptor Interacting Protein 1 gene (CNRIP1)12 have been reported to bind to the conserved C-terminal intracellular portion of CB1R, a region thought to be critical for receptor activation, sensitivity and recycling15, 16. Human CRIP1a and CRIP1b share identical N-termini, but diverge after residue 110 (Fig. 1a). The longer CRIP1a isoform (164 amino acids in human) is conserved across vertebrates and invertebrates, whereas CRIP1b (128 amino acids in human) is restricted to certain primates17. Functionally, CRIP1a, but not CRIP1b, has been reported to modulate CB1R-mediated tonic inhibition of voltage-gated Ca2 + channels12 and recent evidence has indicated that CRIP1a can compete with β-arrestins for the C-terminal region of CB1R, suggesting a role of internalization of the receptor18.
Figure 1
Discussion
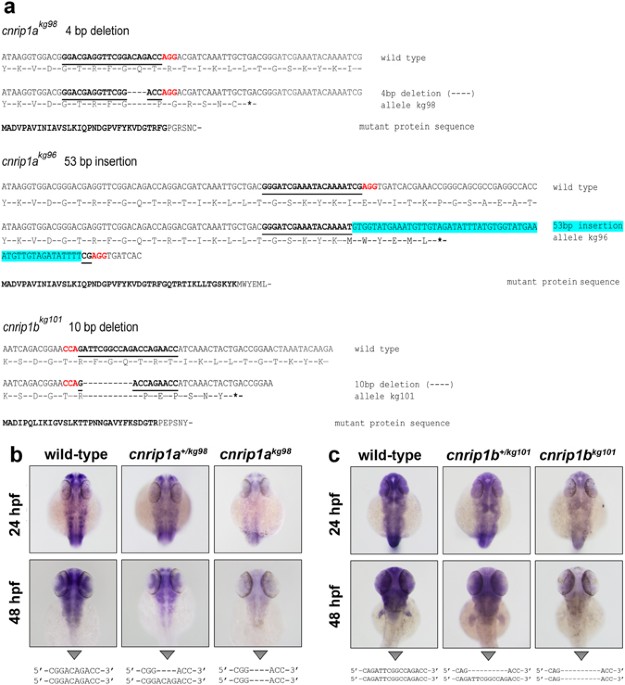
There remains a formal possibility that our mutations, despite creating early stop codons in the first exon, are not null, either because the short truncated polypeptides can play some role or because of some kind of ribosomal re-initiation or stop codon read-through permits synthesis of a long protein. In the absence of functional antibodies recognizing the zebrafish proteins, we cannot be certain that no functional folded Cnrip1 protein is synthesised. However, we think this unlikely for several reasons. Firstly, nonsense mediated mRNA decay for each gene suggests that exon splice junction complexes are not removed downstream of the in-frame stop codons, indicating that ribosomes do not progress normally to the end of the three exon transcripts from each gene. Consistent with this view, there are no in-frame methionine codons nearby downstream of the novel stop codons that might encourage re-initiation by a stalled ribosome before it dissociates from the mRNA. Secondly, the reduced mRNA levels will mean that fewer ribosomal initiation events occur on each transcript and thus the absolute number of truncated polypeptides synthesised per unit time is likely lower than that of the full length proteins in wild types. Thirdly, the truncated polypeptides are under 40 amino acids in length (compared with 162 amino acids for each full length protein) and thus are unlikely to create a stable folded domain and will therefore be more rapidly degraded than the full length proteins. For these reasons, we suspect our mutants are functionally null.
Genomic analysis reveals that the genomes of zebrafish and most other fish contain duplicated copies of a clear homologue of the human CNRIP1 gene. This strongly suggests that (a) CNRIP1 is a conserved gene that has had an important function in vertebrates for at least 400 Myr, (b) that after the extra teleost genome duplication the duplicates developed essential functions sufficiently rapidly for both genes to be retained and well conserved. An invertebrate homologue of CNRIP1, F29A7.4, is present in C. elegans, has an ~80 amino acid N-terminal extension compared to vertebrates but is expressed in the ventral nerve cord and various neurons40. Akin to our findings in zebrafish, F29A7.4has not been observed to have a loss of function phenotype in two independent RNAi screens (http://www.wormbase.org/species/c_elegans/gene/WBGene00017914).
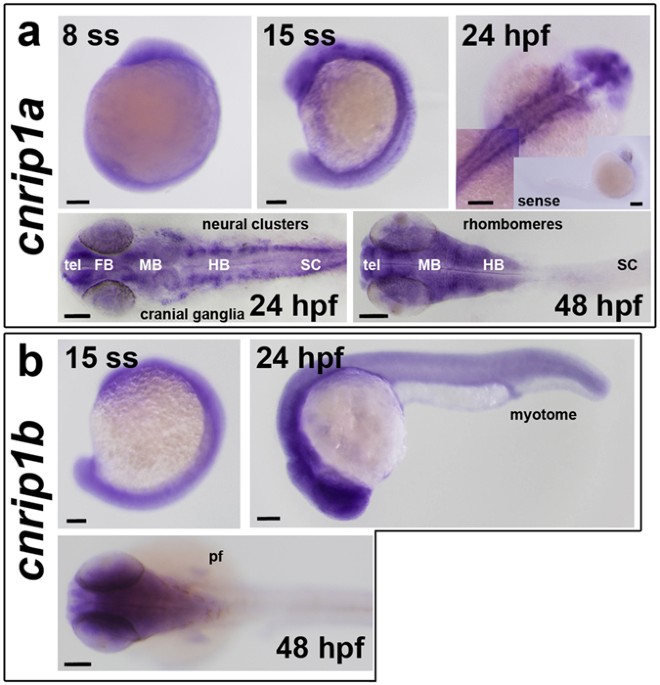
A consistent characteristic of CNRIP1 family genes, also observed in zebrafish, is strong expression in neural tissue (recently reviewed41). Both cnrip1a and cnrip1b are expressed in the zebrafish nervous system, with cnrip1a mRNA sometimes concentrated in particular regions of neuronal terminal differentiation. Cells in specific regions of the brain and in placode-derived cranial ganglia express highly. This conserved location of expression is consistent with the conserved synteny of the chromosomal region around CNRIP1 coding sequence across vertebrates. Expression in neurons is consistent with the suggested ability of CNRIP1 to bind to the intracellular region of CB1R, but does not exclude other functions in neurons. Cnrip1b is expressed more broadly than cnrip1a, appearing in muscle and fin tissue in the late embryo. As CB1R is expressed in many tissues, this finding could indicate functions beyond neuronal action of endocannabinoids. In future, it will be essential to assess the response of cnrip1a and cnrip1bmutant fish to gain and loss of function of endogenous and exogenous cannabinoids, as well as explore alternative functional avenues.
Methods
Zebrafish lines and maintenance
Genetically-altered Danio rerio on a primarily AB background were created and reared at King’s College London on a 14/10 hr light/dark cycle at 28.5 °C42. All experiments were performed in accordance with licences held under the UK Animals (Scientific Procedures) Act 1986 and later modifications and conforming to all relevant guidelines and regulations.
In situ mRNA hybridisation
In situ mRNA hybridisation (ISH) was performed as described previously43, 44. IMAGE clones for both cnrip1a (clone ID: 7149263) and cnrip1b (clone ID: 8760081) genes (Thermo Scientific) were grown as in Table S2 and confirmed by sequencing. A PCR reaction was then performed to add T3 and T7 RNA polymerase sites to create antisense and sense probes. Generic pDNR-LIB primers were used for cnrip1a, while specific primers for cnrip1b were designed. Primers (Integrated DNA Technologies; Table S3) were used to amplify template DNA (Table S4), purified using the QIAquick PCR Purification Kit (QIAGEN) and digoxigenin-labelled RNA probes synthesised, treated with DNase and purified with the Illustra Microspin G-50 kit (GE Healthcare).
CRISPR/Cas9 genome editing
Genome editing was adapted from ref. 45. CRISPR target sites in the first coding exon were selected with ZiFiT46 and potential off-targets minimized with the specific ZiFiT tool. Optimised flanking primers creating a ~120 bp PCR product for High Resolution Melt Analysis (HRM; 20 bp each, Tm 60 °C) and 200–400 bp product for DNA sequencing were selected for each gRNA with Primer 3 software47 and oligos bought from MWG Eurofins. CRISPR oligos were annealed, ligated into BsaI-digested pDR274 (Addgene), plasmid DNA purified, sequenced, digested with DraI and the 284 bp fragment gel-purified and used to synthesise gRNA with T7 RiboMAX large scale RNA production kit (Promega) which was phenol/ethanol purified, quantified by gel and Qubit (Invitrogen), aliquoted in 5 µl samples at 200 ng/µl and stored at −80 °C. NotI-HF-linearised pCS2-Cas9 was transcribed using mMessage mMachine SP6 kit (Ambion) and product purified as for gRNA.
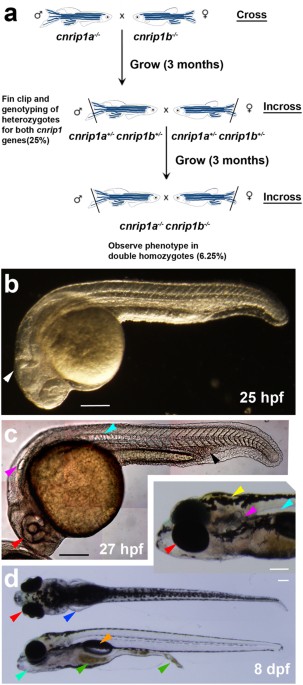
HRMA-selected AB wild-type fish were DNA sequenced over the target loci to avoid polymorphisms, crossed and the resulting embryos injected with 1 nl containing 60 ng/µl gRNA, 200 ng/µl Cas9 mRNA and phenol red and GFP mRNA to select injected embryos. Twenty morphologically-normal 48 hpf larvae were analysed by HRMA to verify mutagenesis, their F0 siblings grown to adulthood and backcross F1 progeny analysed for transmission by HRMA. Mutant loci of F1s were sequenced to identify F0s transmitting mutations of interest, F1 siblings grown to adulthood and F1 heterozygotes identified by HRMA and sequencing of fin-clip DNA. Subsequent generations were bred by outcross to non-polymorphic wild-type AB. To generate double mutants, cnrip1a and cnrip1b F2 heterozygotes were crossed to obtain F3 progeny in which 25% of fish were dual heterozygotes (cnrip1a+/−;cnrip1b+/−). These fish were in-crossed to generate a F4 progeny of which 6.25% were predicted to be homozygous for both genes (cnrip1a−/−;cnrip1b−/−).
High resolution melt analysis (HRMA)
Total genomic DNA was extracted from single embryos at 24 or 48 hpf or fin-clip tissue using 100 µl of PeqLab lysis solution (31-401-E) with 20 µg proteinase K incubated at 55 °C overnight and inactivated at 85 °C for 45 minutes. For the HRMA, 1 µl of each DNA lysate was mixed with 2× MeltDoctor HRM mix (Life Technologies), forward and reverse HRM primers (0.14 µM) to 32 µl and half loaded in duplicate wells on a MicroAmp Optical 384-well plate (Life Technologies), sealed with optical adhesive film, centrifuged at maximum speed for 30 seconds on a bench plate centrifuge to eliminate bubbles and HRMA performed on a ViiA7 real-time PCR system (Applied Biosystems).
Screening for phenotype
Multiple lays of in-crosses between fish carrying identical or distinct alleles were screened for phenotypes. In each lay, about a hundred F2–F4 embryos at 4–10 hpf were monitored for alterations in gastrulation, epiboly and formation of the embryonic shield. From 10–36 hpf, defects in somites, myogenesis, brain, spinal cord, notochord, eye and heart were screened. Subsequently, alteration in brain, spinal cord, eye, heart, cardiovascular system and fin morphology, pigmentation hatching, touch response and swimming patterns were analysed. At 96 hpf, larvae were screened again for touch response and motility, defects in pigmentation, pectoral fins, jaw, branchial arches, blood, ear, liver and gut. At 5 dpf, larvae were transferred to the nursery, where they were monitored on a weekly basis for later developmental defects or behaviour abnormalities.
Statistics
χ2 tests were performed in Excel taking p < 0.05 as significant.
Additional Information
References
- 1.
Gaoni, Y. & Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Amer Chem Soc86, 1646–1647, doi:10.1021/ja01062a046 (1964).
- 2.
Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C. & Bonner, T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature346, 561–564, doi:10.1038/346561a0 (1990).
- 3.
Di Marzo, V., Bifulco, M. & De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nature reviews. Drug discovery3, 771–784, doi:10.1038/nrd1495 (2004).
- 4.
Piomelli, D. The molecular logic of endocannabinoid signalling. Nature reviews. Neuroscience4, 873–884, doi:10.1038/nrn1247(2003).
- 5.
Anavi-Goffer, S. et al. Helix 8 Leu in the CB1 cannabinoid receptor contributes to selective signal transduction mechanisms. J Biol Chem282, 25100–25113, doi:10.1074/jbc.M703388200 (2007).
- 6.
De Petrocellis, L., Cascio, M. G. & Di Marzo, V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol141, 765–774, doi:10.1038/sj.bjp.0705666 (2004).
- 7.
Flores, Á., Maldonado, R. & Berrendero, F. Cannabinoid-hypocretin cross-talk in the central nervous system: what we know so far. Frontiers Neurosci7, doi:10.3389/fnins.2013.00256(2013).
- 8.
Fernández-Ruiz, J., Romero, J. & Ramos, J. In EndocannabinoidsVol. 231 Handbook of Experimental Pharmacology (ed Roger G. Pertwee) Ch. 8, 233–259 (Springer International Publishing, 2015).
- 9.
Maccarrone, M. et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends in Pharmacol Sci36, 277–296, doi:10.1016/j.tips.2015.02.008 (2015).
- 10.
Maida, V. & Daeninck, P. J. A user’s guide to cannabinoid therapies in oncology. Curr Oncol23, 398–406, doi:10.3747/co.23.3487(2016).
- 11.
Sharma, C. et al. Small molecules from nature targeting G-protein coupled cannabinoid receptors: potential leads for drug discovery and development. Evidence-based Complementary and Alternative Medicine: eCAM2015, 238482, doi:10.1155/2015/238482 (2015).
- 12.
Niehaus, J. L. et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Molecular pharmacology72, 1557–1566, doi:10.1124/mol.107.039263(2007).
- 13.
Sanchez, C. et al. The CB(1) cannabinoid receptor of astrocytes is coupled to sphingomyelin hydrolysis through the adaptor protein fan. Molecular pharmacology59, 955–959 (2001).
- 14.
Martini, L. et al. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. Faseb J21, 802–811, doi:10.1096/fj.06-7132com (2007).
- 15.
Nie, J. & Lewis, D. L. Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J Neuroscience21, 8758–8764 (2001).
- 16.
Tiburu, E. K. et al. Human cannabinoid 1 GPCR C-terminal domain interacts with bilayer phospholipids to modulate the structure of its membrane environment. The AAPS journal13, 92–98, doi:10.1208/s12248-010-9244-7 (2011).
- 17.
Elphick, M. R. The evolution and comparative neurobiology of endocannabinoid signalling. Philosophical transactions Royal Society London. Series B.367, 3201–3215, doi:10.1098/rstb.2011.0394 (2012).
- 18.
Blume, L. C. et al. Cannabinoid receptor interacting protein 1a competition with beta-Arrestin for CB1 receptor binding sites. Molecular pharmacology91, 75–86, doi:10.1124/mol.116.104638(2017).
- 19.
Ludanyi, A. et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neuroscience:28, 2976–2990, doi:10.1523/jneurosci.4465-07.2008 (2008).
- 20.
Bojnik, E. et al. Changes in the cannabinoid (CB1) receptor expression level and G-protein activation in kainic acid induced seizures. Epilepsy research99, 64–68, doi:10.1016/j.eplepsyres.2011.10.020 (2012).
- 21.
Blume, L. C. et al. Striatal CB1 and D2 receptors regulate expression of each other, CRIP1A and delta opioid systems. J Neurochemistry124, 808–820, doi:10.1111/jnc.12139 (2013).
- 22.
Guggenhuber, S. et al. Cannabinoid receptor-interacting protein Crip1a modulates CB1 receptor signaling in mouse hippocampus. Brain structure & function, doi:10.1007/s00429-015-1027-6 (2015).
- 23.
Smith, T. H. et al. Cannabinoid receptor-interacting protein 1a modulates CB1 receptor signaling and regulation. Molecular pharmacology87, 747–765, doi:10.1124/mol.114.096495 (2015).
- 24.
Blume, L. C. et al. Cannabinoid receptor interacting protein suppresses agonist-driven CB1 receptor internalization and regulates receptor replenishment in an agonist-biased manner. J Neurochemistry139, 396–407, doi:10.1111/jnc.13767 (2016).
- 25.
Blume, L. C., Eldeeb, K., Bass, C. E., Selley, D. E. & Howlett, A. C. Cannabinoid receptor interacting protein (CRIP1a) attenuates CB1R signaling in neuronal cells. Cellular signalling27, 716–726, doi:10.1016/j.cellsig.2014.11.006 (2015).
- 26.
Mailleux, P., Parmentier, M. & Vanderhaeghen, J. J. Distribution of cannabinoid receptor messenger RNA in the human brain: an in situ hybridization histochemistry with oligonucleotides. Neuroscience letters143, 200–204 (1992).
- 27.
Matsuda, L. A., Bonner, T. I. & Lolait, S. J. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol327, 535–550, doi:10.1002/cne.903270406 (1993).
- 28.
Tsou, K., Brown, S., Sanudo-Pena, M. C., Mackie, K. & Walker, J. M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience83, 393–411 (1998).
- 29.
Egertova, M. & Elphick, M. R. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol422, 159–171 (2000).
- 30.
Pertwee, R. G., Stevenson, L. A., Elrick, D. B., Mechoulam, R. & Corbett, A. D. Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. Br J Pharmacol105, 980–984 (1992).
- 31.
Andresen, K. et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology61, 1651–1659, doi:10.1002/hep.27707 (2015).
- 32.
Bethge, N. et al. Colorectal cancer DNA methylation marker panel validated with high performance in Non-Hodgkin lymphoma. Epigenetics9, 428–436, doi:10.4161/epi.27554 (2014).
- 33.
Oster, B. et al. Identification and validation of highly frequent CpG island hypermethylation in colorectal adenomas and carcinomas. Int J Cancer129, 2855–2866, doi:10.1002/ijc.25951 (2011).
- 34.
Lind, G. E. et al. Identification of an epigenetic biomarker panel with high sensitivity and specificity for colorectal cancer and adenomas. Mol Cancer10, 85, doi:10.1186/1476-4598-10-85 (2011).
- 35.
Zheng, X., Suzuki, T., Takahashi, C., Nishida, E. & Kusakabe, M. cnrip1 is a regulator of eye and neural development in Xenopus laevis. Genes to Cells20, 324–339, doi:10.1111/gtc.12225 (2015).
- 36.
Hu, S. S. et al. Architecture of cannabinoid signaling in mouse retina. J Comp Neurol518, 3848–3866, doi:10.1002/cne.22429(2010).
- 37.
Pasquier, J. et al. Gene evolution and gene expression after whole genome duplication in fish: the PhyloFish database. BMC Genomics17, 368, doi:10.1186/s12864-016-2709-z (2016).
- 38.
Thisse, B. & Thisse, C. Fast Release Clones: A High Throughput Expression Analysis (2004).
- 39.
Boehm, V. & Gehring, N. H. Exon Junction Complexes: Supervising the Gene Expression Assembly Line. Trends Genet32, 724–735, doi:10.1016/j.tig.2016.09.003 (2016).
- 40.
Shaye, D. D. & Greenwald, I. OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One6, e20085, doi:10.1371/journal.pone.0020085 (2011).
- 41.
Oltrabella, F. et al. Role of the endocannabinoid system in vertebrates: Emphasis on the zebrafish model. Dev Growth Differentiation, doi:10.1111/dgd.12351 [Epub ahead of print] (2017).
- 42.
Westerfield, M. The Zebrafish Book – A guide for the laboratory use of zebrafish (Danio rerio) (University of Oregon Press, 2000).
- 43.
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc3, 59–69, doi:nprot.2007.514 [pii] 10.1038/nprot.2007.514 (2008).
- 44.
Jin, L. & Lloyd, R. V. In situ hybridization: methods and applications. J Clinical Laboratory Analysis11, 2–9 (1997).
- 45.
Hwang, W. Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nature biotechnology31, 227–229, doi:10.1038/nbt.2501 (2013).
- 46.
Sander, J. D. et al. ZiFiT (Zinc Finger Targeter): an updated zinc finger engineering tool. Nucleic Acids Res38, W462–468, doi:10.1093/nar/gkq319 (2010).
- 47.
Koressaar, T. & Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics (Oxford, England)23, 1289–1291, doi:10.1093/bioinformatics/btm091 (2007).
Acknowledgements
We thank Vikki Williams for genotyping, Pat Doherty, Anthony Graham, Yaniv Hinits and members of the Hughes lab for advice and Bruno Correia da Silva and his staff for care of the fish. LF had an EPSRC PhD studentship in the laboratory of RAS. GB was funded by the Biotechnology and Biological Sciences Research Council (BB/K0101151) to SMH. SMH is a Medical Research Council (MRC) Scientist with Programme Grant G1001029 and MR/N021231/1 support.
Author information
Affiliations
-
Randall Division of Cell and Molecular Biophysics, New Hunt’s House, Guy’s Campus, King’s College London, London, SE1 1UL, UK
- Laura Fin
- , Giorgia Bergamin
- , Roberto A. Steiner
- & Simon M. Hughes
Contributions
Experiments were performed by L.F., G.B. and S.M.H. S.M.H. and G.B. guided the work. S.M.H. assembled the Figures and wrote the manuscript based on the PhD thesis of L.F. All authors edited the manuscript. R.A.S. and S.M.H. conceived the project.
Competing Interests
The authors declare that they have no competing interests.
Corresponding authors
Correspondence to Roberto A. Steiner or Simon M. Hughes.
Supplementary information