- Department of Psychology and Collaborative Neuroscience Graduate Program, University of Guelph, Guelph, ON, Canada
Despite the advent of classic anti-emetics, chemotherapy-induced nausea is still problematic, with vomiting being somewhat better managed in the clinic. If post-treatment nausea and vomiting are not properly controlled, anticipatory nausea—a conditioned response to the contextual cues associated with illness-inducing chemotherapy—can develop. Once it develops, anticipatory nausea is refractive to current anti-emetics, highlighting the need for alternative treatment options. One of the first documented medicinal uses of Δ9-tetrahydrocannabinol (Δ9-THC) was for the treatment of chemotherapy-induced nausea and vomiting (CINV), and recent evidence is accumulating to suggest a role for the endocannabinoid system in modulating CINV. Here, we review studies assessing the therapeutic potential of cannabinoids and manipulations of the endocannabinoid system in human patients and pre-clinical animal models of nausea and vomiting.
Introduction
 Cannabis sativa has been used as a medicine for centuries (see Hanus and Mechoulam, 2005; Iversen, 2008). It was not until the 1970’s that oncologists demonstrated that smoked cannabis attenuated chemotherapy-induced nausea and vomiting (CINV). Few clinical trials have compared the efficacy of cannabis-based medicines with the currently recommended anti-emetic regimen, or as an adjunct to this treatment. We review findings on the potential of exogenous cannabinoids and manipulations of the endogenous cannabinoid system to reduce acute and anticipatory CINV.
Cannabis sativa has been used as a medicine for centuries (see Hanus and Mechoulam, 2005; Iversen, 2008). It was not until the 1970’s that oncologists demonstrated that smoked cannabis attenuated chemotherapy-induced nausea and vomiting (CINV). Few clinical trials have compared the efficacy of cannabis-based medicines with the currently recommended anti-emetic regimen, or as an adjunct to this treatment. We review findings on the potential of exogenous cannabinoids and manipulations of the endogenous cannabinoid system to reduce acute and anticipatory CINV.
Chemotherapy-Induced Nausea and Vomiting (CINV)
Chemotherapy patients experience acute nausea and vomiting (occurring up to 24 h post-treatment; Fiore and Gralla, 1984). If improperly managed, this post-treatment CINV can lead to anticipatory nausea and vomiting; a conditioned nausea response upon re-exposure to the chemotherapy clinic (Morrow, 1982). Current guidelines to manage highly emetogenic acute CINV recommend a three-drug regimen of the 5-hydroxytryptamine 3 (5-HT3) receptor antagonist (such as ondansetron), along with dexamethasone, and a neurokinin 1 (NK1) receptor antagonist (such as aprepitant) before beginning chemotherapy (Roila et al., 2010). Even with this standard treatment acute nausea is still problematic (no acute nausea reported in 66% of patients; Kim et al., 2015). None of these treatments are effective in reducing anticipatory nausea (e.g., Roscoe et al., 2000), with sedating benzodiazepines currently prescribed (Razavi et al., 1993; Malik et al., 1995). Therefore, nausea (acute and anticipatory) continues to be problematic.
Cannabinoids in Human CINV
Because current treatments cannot properly manage CINV, alternatives including constituents of the cannabis plant and modulation of the endogenous cannabinoid system, have been investigated.
Effect of Δ9-THC and Δ9-THC-Like Synthetics
One of the few recognized medicinal effects of the cannabis plant is the control of CINV, by Δ9-THC, the psychoactive compound in cannabis (Gaoni and Mechoulam, 1964). Synthetic Δ9-THC is available for treatment of CINV in capsule form as dronabinol (Marinol®), or nabilone (Cesamet®). Each of these compounds acts as a partial agonist of the cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptors. In comparison to placebo or the dopamine 2 (D2) receptor antagonists (anti-emetics which predated the 5-HT3 receptor antagonists), Δ9-THC or Δ9-THC-like synthetics are more effective in reducing acute CINV (Sallan et al., 1975; Chang et al., 1979; Ekert et al., 1979; Frytak et al., 1979; Herman et al., 1979; Kluin-Neleman et al., 1979; Orr et al., 1980; Steele et al., 1980; Einhorn et al., 1981; Orr and McKernan, 1981; Johansson et al., 1982; Jones et al., 1982; Levitt, 1982; Wada et al., 1982; Ahmedzai et al., 1983; Niamatali et al., 1984; Niiranen and Mattson, 1985; Dalzell et al., 1986; Niederle et al., 1986; Pomeroy et al., 1986; Chan et al., 1987; McCabe et al., 1988; Lane et al., 1990).
The only published clinical trial assessing the effect of dronabinol on anticipatory nausea showed that dronabinol was ineffective, although most patients were receiving highly emetogenic chemotherapy regimens (Lane et al., 1991). Therefore, dronabinol may be effective in reducing anticipatory nausea developing from less emetogenic chemotherapy regimens.
Pre-clinical Animal Models of Vomiting
Since rats and mice cannot vomit, species capable of vomiting are used in emesis research. Suncus murinus (house musk shrew) or Cryptotis parva(least shrew) vomit to toxins such as nicotine (Matsuki et al., 1988, 1990; Torii et al., 1991; Nakayama et al., 2005; Parker et al., 2009; Rock et al., 2012), the chemotherapeutic agent cisplatin (Matsuki et al., 1988, 1990; Torii et al., 1991; Darmani, 1998, 2001b; Sam et al., 2003; Lau et al., 2005; Parker et al., 2009; Ray et al., 2009; Rock et al., 2012), or lithium chloride (LiCl; e.g., Parker et al., 2004). Ferrets also vomit following cisplatin or morphine 6 glucuronide (M6G; Van Sickle et al., 2001, 2003; Sharkey et al., 2007). These species have therefore been used to study emesis. Please refer to Table 1 for details regarding the findings of exogenous cannabinoids and manipulations of the endogenous cannabinoid system on vomiting in animal models.
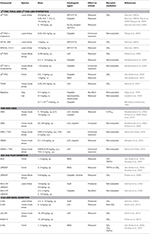 Table 1. Effect of exogenous cannabinoids and manipulations of the endogenous cannabinoid system on vomiting in animal models.
Table 1. Effect of exogenous cannabinoids and manipulations of the endogenous cannabinoid system on vomiting in animal models.
Effect of Δ9-THC, Tetrahydrocannabinolic Acid (THCA), and Δ9-THC-Like Synthetics on Vomiting
In the least shrew, CB1 receptor agonists such as Δ9-THC (20 mg/kg, i.p.) reduced vomiting induced by the CB1 receptor antagonist/inverse agonist, SR141716 (20 mg/kg, intraperitoneal, i.p.; Darmani, 2001a). As well, Δ9-THC (20 mg/kg, i.p.) reduced cisplatin-induced vomiting, and this effect was reversed by SR141716 [10 mg/kg, subcutaneous (s.c.) or 2 mg/kg, i.p.] in the least shrew (Darmani, 2001b; Ray et al., 2009; Wang et al., 2009). In the house musk shrew, Δ9-THC (2.5–20 mg/kg, i.p.) also reduced LiCl- and cisplatin-induced vomiting, these effects were blocked by SR141716 (2.5 mg/kg, i.p.) (Kwiatkowska et al., 2004; Parker et al., 2004). In ferrets, Δ9-THC (0.5, 1 mg/kg, i.p.) reduced cisplatin-, or M6G-induced vomiting, these effects were blocked by SR141716 (5 mg/kg, i.p.; Van Sickle et al., 2003) or AM251 (5 mg/kg, i.p.; Van Sickle et al., 2001). In addition, Δ9-THC’s precursor tetrahydrocannabinolic acid (THCA), present in fresh cannabis and decarboxylated upon heating or drying of the plant, (0.05, 0.5 mg/kg, i.p.) reduced LiCl-induced vomiting, an effect reversed by SR141716 (2.5 mg/kg, i.p.; Rock et al., 2013). These results complement human findings that Δ9-THC is anti-emetic, exerting its effect via the CB1receptor.
Effect of Cannabidiol (CBD) and Cannabidiolic Acid (CBDA) on Vomiting
For another non-psychoactive cannabinoid, cannabidiol (CBD) low doses (5, 10 mg/kg, i.p) reduced, but high doses (20–40 mg/kg, i.p.) potentiated LiCl-, nicotine-, and cisplatin-induced vomiting in house musk shrews (Kwiatkowska et al., 2004; Parker et al., 2004). Suppression of vomiting by CBD at low doses (5, 10 mg/kg, s.c.) was blocked by a 5-hydroxytryptamine 1A (5-HT1A) receptor antagonist (Rock et al., 2012). CBD’s precursor cannabidiolic acid (CBDA), is decarboxylated when the fresh cannabis plant is heated or dried. In house musk shrews, CBDA (0.1, 0.5 mg/kg, i.p.) reduced LiCl-, and cisplatin-induced emesis (Bolognini et al., 2013). These findings suggest that CBD and CBDA are anti-emetic in a dose-dependent manner, with CBDA being more potent.
Effect of Anandamide (AEA) and FAAH Inhibition on Vomiting
The endogenous cannabinoid, anandamide (AEA), produced and released on-demand, is rapidly degraded by fatty acid amide hydrolase (FAAH). As well, FAAH degrades other fatty acids including oleoylethanolamide (OEA) and palmitoylethanolamine (PEA), which act on peroxisome proliferator-activated receptor alpha (PPARα), instead of CB1 or CB2receptors. Interestingly, Venkatesan et al. (2016) reported increased levels of serum OEA and PEA (with a trend toward increased AEA and 2-AG) while patients were experiencing cyclic vomiting. On the other hand, no differences in plasma AEA, OEA or PEA were detected in pregnant women experiencing hyperemesis gravidarum—severe nausea and vomiting (Gebeh et al., 2014).
In animal models, AEA (1, 2 mg/kg, i.p.) reduced M6G-induced emesis in ferrets, an effect blocked by a transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor antagonist (Sharkey et al., 2007) or AM251 (5 mg/kg, i.p.; Van Sickle et al., 2005). The FAAH inhibitor, URB597 (3, 5 mg/kg, i.p.) also reduced M6G-induced emesis in ferrets, an effect blocked by AM251 (5 mg/kg, i.p.) or a TRPV1 receptor antagonist (Van Sickle et al., 2005; Sharkey et al., 2007) but a PPARα antagonist was not evaluated. URB597 (0.9 mg/kg, i.p.) also reduced nicotine-induced vomiting in house musk shrews, an effect blocked by SR141716 (2.5 mg/kg, i.p.; Parker et al., 2009). These results suggest the anti-emetic effects of AEA and FAAH inhibition are mediated by activation of the CB1receptor. In ferrets, the TRPV1 receptor also plays a role, an effect not yet been evaluated in house musk shrews.
In comparison, administration of the FAAH inhibitors AA-5-HT (10 mg/kg, i.p.) or URB597 (20 mg/kg, i.p.) themselves induced emesis (Darmani et al., 2005); however 20 mg/kg of URB597 is a much higher dose than is typically given. These species-dependent effects of AEA in the modulation of emesis are puzzling, warranting further investigation.
Effect of 2-AG and MAGL Inhibition on Vomiting
The endogenous cannabinoid 2-Arachidonoylglycerol (2-AG), produced and released on-demand, is rapidly degraded by monoacylglycerol lipase (MAGL). In least shrews, 2-AG (2.5, 5, 10 mg/kg, i.p.) produced emesis (Darmani, 2001c). Indeed, in response to cisplatin in least shrews, brain 2-AG levels increased, while gut 2-AG levels decreased (Darmani et al., 2005). This is interesting, as Choukèr et al. (2010) reported lower blood endocannabinoid levels among those experiencing motion sickness, and higher blood endocannabinoid levels among those not.
In contrast, in house musk shrews, 2-AG (1–10 mg/kg, i.p.) did not induce emesis. Instead, 2-AG (2, 5 mg/kg, i.p.) reduced LiCl-induced vomiting (Sticht et al., 2013). Furthermore, 2-AG (1, 2 mg/kg, i.p.) reduced M6G-induced emesis in ferrets, effects blocked by a TRPV1 receptor antagonist (Sharkey et al., 2007) or AM251 (5 mg/kg, i.p.; Van Sickle et al., 2005) or the CB2 receptor antagonist AM630 (5 mg/kg, i.p.; Van Sickle et al., 2005). Although AM630 did not block vomiting produced by M6G in ferrets, Rock et al. (2016) found that the CB2 receptor agonist, HU308 (2.5 and 5 mg/kg, i.p.) reduced LiCl-induced vomiting in house musk shrews, an effect that was reversed by the CB2 receptor antagonist, AM630 (3 mg/kg, i.p.). These results together suggest that CB1, CB2 and TRPV1 receptors play a role in the emetic response depending on species and emetic agent employed.
The selective MAGL inhibitor MJN110 (10, 20 mg/kg, i.p.) suppressed LiCl-induced vomiting in house musk shrews; an effect reversed by SR141716 (2.5 mg/kg, i.p.; Parker et al., 2015). These results suggest CB1receptor activation for 2-AG’s anti-emetic effect, but also suggest TRPV1 or CB2 receptor mediation in ferrets, effects not yet investigated in house musk shrews. Overall, these species-dependent effects involving 2-AG and AEA warrant further investigation.
Conditioned Gaping re-clinical Models of Nausea in Rats
Use of pre-clinical animal models has led to a good understanding of emesis neurobiology (Hornby, 2001), but the brain circuits mediating nausea are still not well characterized (Andrews and Horn, 2006). Such nausea circuitry may be more complex than that of emesis (see Kenward et al., 2015). Emesis is a gastrointestinal event controlled by structures within the brainstem (Hornby, 2001), whereas nausea is thought to require forebrain activation (Sanger and Andrews, 2006; Horn, 2008; Holmes et al., 2009). Although the visceral inputs from the gastrointestinal tract to the brain have been identified (Cechetto and Saper, 1987), it is unclear how these inputs are processed in the forebrain to produce nausea, largely due to the lack of reliable animal models of nausea. Here we describe current animal models of nausea. For a complete review of these models please refer to Sharkey et al. (2014).
To evaluate potential anti-nausea compounds, selective pre-clinical animal models are necessary. One such model is conditioned gaping in rats. Please refer to Table 2 for details regarding the effects of exogenous cannabinoids and manipulations of the endogenous cannabinoid system in rat models of conditioned gaping.
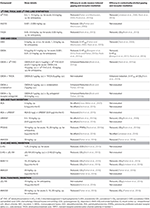 Table 2. Effect of exogenous cannabinoids and manipulations of the endogenous cannabinoid system on models of acute and anticipatory nausea in rats.
Table 2. Effect of exogenous cannabinoids and manipulations of the endogenous cannabinoid system on models of acute and anticipatory nausea in rats.
Acute Nausea-Induced Conditioned Gaping
Although rats cannot vomit, they display conditioned gaping reactions to a taste previously paired with an illness-inducing agent such as LiCl (Grill and Norgren, 1978). Only emetic drugs produce, and anti-emetic treatments (including cannabinoids) block conditioned gaping (see Parker, 2014 for review). Therefore, acute nausea-induced conditioned gaping is a reliable model of acute nausea in rats.
Contextually Elicited Conditioned Gaping, A Preclinical Model of Anticipatory Nausea
Rats also display conditioned gaping upon re-exposure to a nausea-paired context; this model is similar to the development of anticipatory nausea in humans (Limebeer et al., 2008). Furthermore, much like with human anticipatory nausea, a 5-HT3 receptor antagonist does not reduce contextually elicited conditioned gaping (Limebeer et al., 2006; Rock et al., 2014). Humans are treated with nonspecific benzodiazepines for anticipatory nausea, similarly, benzodiazepines reduce contextually elicited conditioned gaping in rats (Rock et al., 2014). Therefore, there is face validity for contextually elicited gaping as a preclinical model of anticipatory nausea.
The Role of the Interoceptive Insular Cortex in Conditioned Gaping
Because the specific brain region(s) critical for nausea are still unclear, we are investigating the role of the endogenous cannabinoid system in nausea using the conditioned gaping model. One region of interest is the interoceptive insular cortex (IC), an area involved in the sensation of nausea in humans (Penfield and Faulk, 1955), as stimulation of the interoceptive IC (Ostrowsky et al., 2000; Isnard et al., 2004; Catenoix et al., 2008) and functional neuroimaging studies in humans (Napadow et al., 2013; Sclocco et al., 2014), pinpoint the interoceptive IC as a region critical for nausea.
Effect of Δ9-THC, THCA, and Δ9-THC-Like Synthetics on Nausea
Acute nausea
Δ9-THC (0.5, 1, 10 mg/kg, i.p.), HU210 (0.001, 0.005 mg/kg, i.p.), and THCA (0.05, 0.5 mg/kg, i.p.) reduced acute nausea-induced conditioned gaping; an effect blocked by SR141716 (2.5 mg/kg, i.p.) (Parker and Mechoulam, 2003; Rock et al., 2013, 2015a).
Anticipatory nausea
Δ9-THC (0.5 mg/kg, i.p.) also reduced contextually elicited conditioned gaping (Limebeer et al., 2006; Rock et al., 2014), as did THCA (0.05 mg/kg, i.p), these effects were blocked by SR141716 (2.5 mg/kg, i.p.; Rock et al., 2013). These results suggest that CB1 receptor agonism reduces acute and anticipatory nausea in rats. However, the potential of CB2receptor, TRPV1 receptor and PPARα antagonism to reduce the anti-nausea effects of THC or THCA have not been evaluated.
Effect of CBD and CBDA on Nausea
Acute nausea
CBD (5 mg/kg, i.p. or s.c.) or CBDA (0.5 μg/kg–0.1 mg/kg, i.p.) reduced acute nausea-induced conditioned gaping (Parker and Mechoulam, 2003; Rock et al., 2012), these effects were blocked by a 5-HT1A receptor antagonist (Rock et al., 2012, 2015a; Bolognini et al., 2013; Rock and Parker, 2013a). When combined with a low dose of ondansetron (1 μg/kg, i.p.), a subthreshold dose of CBDA (0.1 μg/kg, i.p.) enhanced the suppression of nausea-induced conditioned gaping (Rock and Parker, 2013a).
Anticipatory nausea
CBD (1, 5 mg/kg, i.p.) or CBDA (0.001, 0.01, 0.1 mg/kg, i.p.) suppressed contextually elicited gaping in the absence of any locomotor impairments (Rock et al., 2008, 2014; Bolognini et al., 2013), these effects were all reversed by a 5-HT1A receptor antagonist (Bolognini et al., 2013). These results suggest a 5-HT1A receptor mediated effect for CBD and CBDA in acute and anticipatory nausea and also a synergistic potential when combined with other anti-emetic agents.
Effect of AEA and FAAH Inhibition on Nausea
Acute nausea
FAAH inhibition (by PF3845, but not URB597) reduces acute nausea by a PPARα mechanism of action, not a CB1 receptor mechanism (Rock et al., 2015b). Previous work suggested that URB597 in combination with AEA also reduced LiCl-induced aversive responding, but not gaping per se(Cross-Mellor et al., 2007). The potential of TRPV1 or CB2 receptor antagonists to reverse the anti-nausea effects of FAAH inhibition has not yet been evaluated. It is interesting that elevated OEA and PEA occur in serum of patients when they are experiencing cyclical vomiting (Venkatesan et al., 2016), suggesting that they may be playing a homeostatic protective role. Current investigations are underway to determine if the anti-nausea effects of FAAH inhibition (possibly by a PPARα mechanism of action) are peripherally or centrally mediated.
Anticipatory nausea
In the preclinical model of anticipatory nausea, both URB597 (0.3, 10 but not 0.1 mg/kg, i.p.) and PF3845 (10 and 20 mg/kg, i.p.) suppressed the expression of previously established contextually elicited gaping, with both effects blocked by CB1 receptor antagonism, but not PPARα antagonism (Rock et al., 2008, 2015b). In addition, the selective FAAH inhibitor, AM4303 (20 mg/kg, i.p.), also reduced contextually-elicited conditioned gaping, with an increase in interoceptive IC AEA levels (Parker et al., 2016). These results suggest that FAAH inhibition may reduce anticipatory nausea through a CB1 receptor mediated effect; however, the potential of TRPV1 receptor antagonists and CB2 receptor agonists to reverse LiCl-induced anticipatory nausea expression has not yet been evaluated.
Effect of 2-AG and MAGL Inhibition on Nausea
Acute nausea
Exogenous 2-AG (1.25, 2 mg/kg, i.p.) suppressed acute nausea-induced conditioned gaping; this effect was blocked by cyclooxygenase (COX) inhibition (but not CB1 or CB2 antagonism; Sticht et al., 2012). When combined with the MAGL inhibitor JZL184 (40 mg/kg, i.p.), 2-AG (2 mg/kg, i.p.) suppressed acute nausea. Since this effect was reversed by AM251 (Sticht et al., 2012), prolonging 2-AG’s duration of action (by MAGL inhibition) prevents the nausea produced by longer acting LiCl by acting at the CB1 receptor. In addition, the MAGL inhibitors MJN110 (10, 20 mg/kg, i.p.) or AM4301 (20 mg/kg, i.p.) reduced acute nausea-induced conditioned gaping, both effects were blocked by SR141716 (1 or 2.5 mg/kg, i.p.; Parker et al., 2015, 2016).
Intracranial administration of MAGL inhibitors (MJN110 [2 μg] or AM4301 [2 μg]), but not FAAH inhibitors (URB597 [0.01 μg] or PF3845 [2 μg]) into the interoceptive IC reduced acute nausea-induced conditioned gaping (Parker et al., 2016; Sticht et al., 2016) by a CB1 receptor mechansim of action (Sticht et al., 2016). Furthermore, selective increases in interoceptive IC 2-AG levels were detected following systemic (20 mg/kg, i.p.) or intra-interoceptive IC infusions of MJN110 (2 μg; Sticht et al., 2016). Interestingly, MJN110 (10 mg/kg, i.p.) reduced LiCl-induced increased c-Fos immunoreactivity in the interoceptive IC (Sticht et al., 2016). Finally, systemic injection of LiCl selectively elevated 2-AG levels, but not AEA, in the interoceptive IC. These data suggest that 2-AG acts as an endogenous anti-nausea compound in the interoceptive IC.
Anticipatory nausea
MJN110 (10, 20 mg/kg, i.p.) also reduced contextually-elicited conditioned gaping (with elevated interoceptive IC 2-AG levels), an effect blocked by SR141716 (1 mg/kg, i.p.; Parker et al., 2015). Furthermore, intra-interoceptive IC, MJN110 (2 μg, but not PF3845, nor ondansetron) suppressed contextually elicited conditioned gaping, blocked by CB1receptor antagonism (Limebeer et al., 2016). The MAGL inhibitor, AM4301 (10, 20 mg/kg, i.p.), also reduced contextually elicited conditioned gaping, with a selective increase in interoceptive IC 2-AG levels (Parker et al., 2016). These results suggest 2-AG (but not AEA) reduces anticipatory nausea in the interoceptive IC, as well as acute nausea.
Effect of Dual FAAH/MAGL Inhibition on Nausea
Acute nausea
The dual FAAH/MAGL inhibitor AM4302 (20 mg/kg, i.p.) suppressed acute nausea-induced conditioned gaping (Parker et al., 2016). Intra-interoceptive IC administration of the dual inhibitor JZL195 (10 μg) also suppressed acute nausea-induced conditioned gaping (Sticht et al., 2016).
Anticipatory nausea
Systemic administration of JZL195 (10 mg/kg, i.p.) also suppressed contextually elicited gaping, an effect blocked by SR141716 (2.5 mg/kg, i.p.; Limebeer et al., 2014). The dual inhibitor AM4302 (5, 10, 20 mg/kg, i.p.) was more effective than a FAAH (AM4303) or MAGL inhibitor (AM4301) in reducing contextually elicited gaping, an effect blocked by SR141716 (2.5 mg/kg, i.p), with a concomitant increase in 2-AG and AEA in the interoceptive IC (Parker et al., 2016). Therefore, dual FAAH/MAGL inhibition may boost the anti-nausea effects of elevation of 2-AG or AEA on their own for the treatment of anticipatory nausea.
Conclusions
Animal models suggest that, in general, Δ9-THC, THCA, CBD, and CBDA, and manipulations of the endogenous cannabinoid system, have anti-emetic and anti-nausea properties. However, 2-AG and AEA’s role in emesis is inconsistent across species. Further investigation is needed regarding the potential role of TRPV1 receptors in the anti-nausea effects produced by treatments that elevate AEA. It is time to take some of the preclinical findings (in particular CBDA, FAAH, and MAGL inhibition) into clinical trials for the treatment of acute and anticipatory nausea.
Author Contributions
ER wrote the article; LP edited the article.
Funding
This research was funded by research grants from the Natural Sciences and Engineering Research Council of Canada (92057) and Canadian Institutes of Health Research (137122) to LP and Dr. Keith Sharkey.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ahmedzai, S., Carlyle, D. L., Calder, I. T., and Moran, F. (1983). Anti-emetic efficacy and toxicity of nabilone, a synthetic cannabinoid, in lung cancer chemotherapy. Br. J. Cancer 48, 657–663. doi: 10.1038/bjc.1983.247
Andrews, P. L., and Horn, C. C. (2006). Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton. Neurosci. 125, 100–115. doi: 10.1016/j.autneu.2006.01.008
Bolognini, D., Rock, E. M., Cluny, N. L., Cascio, M. G., Limebeer, C. L., Duncan, M., et al. (2013). Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br. J. Pharmacol. 168, 1456–1470. doi: 10.1111/bph.12043
Catenoix, H., Isnard, J., Guénot, M., Petit, J., Remy, C., and Mauguière, F. (2008). The role of the anterior insular cortex in ictal vomiting: a stereotaxtic electroencephalography study. Epilepsy Behav. 13, 560–563. doi: 10.1016/j.yebeh.2008.06.019
Cechetto, D. F., and Saper, C. B. (1987). Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J. Comp. Neurol. 262, 27–45. doi: 10.1002/cne.902620104
Chan, H. S., Correia, J. A., and MacLeod, S. M. (1987). Nabilone versus prochlorperazine for control of cancer chemotherapy-induced emesis in children: a double-blind, crossover trial. Pediatrics 79, 946–952.
Chang, A. E., Shiling, D. J., Stillman, R. C., Goldberg, N. H., Seipp, C. A., Barofsky, I., et al. (1979). Delta-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann. Intern. Med. 91, 819–824. doi: 10.7326/0003-4819-91-6-819
Choukèr, A., Kaufmann, I., Kreth, S., Hauer, D., Feuerecker, M., Thieme, D., et al. (2010). Motion sickness, stress and the endocannabinoid system. PLoS ONE 5:e10752. doi: 10.1371/journal.pone.0010752
Cross-Mellor, S. K., Ossenkopp, K. P., Piomelli, D., and Parker, L. A. (2007). Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacology (Berl). 190, 135–143. doi: 10.1007/s00213-006-0589-7
Dalzell, A. M., Bartlett, H., and Lilleyman, J. S. (1986). Nabilone: an alternative antiemetic for cancer chemotherapy. Arch. Dis. Child. 61, 502–505. doi: 10.1136/adc.61.5.502
Darmani, N. A. (1998). Serotonin 5-HT3 receptor antagonists prevent cisplatin-induced emesis in Cryptotis parva: a new experimental model of emesis. J. Neural Transm. (Vienna). 105, 1143–1154. doi: 10.1007/s007020050118
Darmani, N. A. (2001a). Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology 24, 198–203. doi: 10.1016/S0893-133X(00)00197-4
Darmani, N. A. (2001b). Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol. Biochem. Behav. 69, 239–249. doi: 10.1016/S0091-3057(01)00531-7
Darmani, N. A. (2001c). The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by 9-tetrahydrocannabinol and other cannnabinoids. J. Pharmacol. Exp. Ther. 300, 34–42. doi: 10.1124/jpet.300.1.34
Darmani, N. A., and Crim, J. L. (2005). Delta-9-tetrahydrocannabinol differentially suppresses emesis versus enhanced locomotor activity produced by chemically diverse dopamine D2/D3 receptor agonists in the least shrew (Cryptotis parva). Pharmacol. Biochem. Behav. 80, 35–44. doi: 10.1016/j.pbb.2004.10.019
Darmani, N. A., McClanahan, B. A., Trinh, C., Petrosino, S., Valenti, M., and Di Marzo, V. (2005). Cisplatin increases brain 2-arachidonoylglycerol (2-AG) and concomitantly reduces intestinal 2-AG and anandamide levels in the least shrew. Neuropharmacology 9, 502–513. doi: 10.1016/j.neuropharm.2005.04.007
Einhorn, L. H., Nagy, C., Fumas, B., and Williams, S. D. (1981). Nabilone: an effective antiemetic in patients receiving cancer chemotherapy. J. Clin. Pharmacol. 21, 64S–69S. doi: 10.1002/j.1552-4604.1981.tb02576.x
Ekert, H., Waters, K. D., Jurk, I. H., Mobilia, J., and Loughnan, P. (1979). Amelioration of cancer chemotherapy-induced nausea and vomiting by delta-9-tetrahydrocanninol. Med. J. Aust. 2, 657–659.
Fiore, J. J., and Gralla, R. J. (1984). Pharmacologic treatment of chemotherapy-induced nausea and vomiting. Cancer Invest. 2, 351–361. doi: 10.3109/07357908409040312
Frytak, S., Moertel, C. G., O’Fallon, J. R., Rubin, J., Creagan, E. T., O’Connell, M. J., et al. (1979). Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A comparison with prochlorperazine and a placebo. Ann. Intern. Med. 91, 825–830. doi: 10.7326/0003-4819-91-6-825
Gaoni, Y., and Mechoulam, R. (1964). Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86, 1646–1647. doi: 10.1021/ja01062a046
Gebeh, A. K., Willets, J. M., Marczylo, T. H., and Konje, J. C. (2014). Plasma anandamide and related n-acylethanolamide levels are not elevated in pregnancies complicated by hyperemesis gravidarum. J. Matern. Fetal Neonatal Med. 27, 954–959. doi: 10.3109/14767058.2013.847413
Grill, H. J., and Norgren, R. (1978). The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 143, 263–279. doi: 10.1016/0006-8993(78)90568-1
Gylys, J. A., Doran, K. M., and Buyniski, J. P. (1979). Antagonism of cisplatin induced emesis in the dog. Res. Commun. Chem. Pathol. Pharmacol. 23, 61–68.
Hanus, L. O., and Mechoulam, R. (2005). “Cannabinoid chemistry: an overview,” in Cannabinoids as Therapeutics, ed R. Mechoulam (Basel: Birkhäuser), 23–46.
Herman, T. S., Einhorn, L. H., Jones, S. E., Nagy, C., Chester, A. B., Dean, J. C., et al. (1979). Superiority of nabilone over prochlorperazine as an antiemetic in patients receiving cancer chemotherapy. N. Engl. J. Med. 300, 1295–1297. doi: 10.1056/NEJM197906073002302
Holmes, A. M., Rudd, J. A., Tattersall, F. D., Aziz, Q., and Andrews, P. L. (2009). Opportunities for the replacement of animals in the study of nausea and vomiting. Br. J. Pharmacol. 157, 865–880. doi: 10.1111/j.1476-5381.2009.00176.x
Horn, C. C. (2008). Why is the neurobiology of nausea and vomiting so important? Appetite 50, 430–434. doi: 10.1016/j.appet.2007.09.015
Hornby, P. J. (2001). Central neurocircuitry associated with emesis. Am. J. Med. 111, 106S–112S. doi: 10.1016/s0002-9343(01)00849-x
Isnard, J., Guénot, M., Sindou, M., and Mauguière, F. (2004). Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia 45, 1079–1090. doi: 10.1111/j.0013-9580.2004.68903.x
Johansson, R., Kilkku, P., and Groenroos, M. (1982). A double-blind, controlled trial of nabilone vs. prochlorperazine for refractory emesis induced by cancer chemotherapy. Cancer Treat. Rev. 9B, 25–33. doi: 10.1016/S0305-7372(82)80032-7
Jones, S. E., Durant, J. R., Greco, A., and Robertone, A. (1982). A multi-institutional Phase III study of nabilone vs. placebo in chemotherapy-induced nausea and vomiting. Cancer Treat Rev. 9B, 45–48. doi: 10.1016/S0305-7372(82)80035-2
Kenward, H., Pelliganda, L., Savary-Bataillec, K., and Elliotta, J. (2015). Nausea: current knowledge of mechanisms, measurement and clinical impact. Vet. J. 203, 36–43. doi: 10.1016/j.tvjl.2014.10.007
Kim, H. J., Shin, S. W., Song, E. K., Lee, N. R., Kim, J. S., Ahn, J. S., et al. (2015). Ramosetron versus ondansetron in combination with aprepitant and dexamethasone for the prevention of highly emetogenic chemotherapy-induced nausea and vomiting: a multicenter, randomized phase III trial, KCSG PC10-21. Oncologist 20, 1440–1447. doi: 10.1634/theoncologist.2015-0128
Kluin-Neleman, J. C., Neleman, F. A., Meuwissen, O. J., and Maes, R. A. (1979). delta 9-Tetrahydrocannabinol (THC) as an antiemetic in patients treated with cancerchemotherapy; a double-blind cross-over trial against placebo. Vet. Hum. Toxicol. 21, 338–340.
Kwiatkowska, M., Parker, L. A., Burton, P., and Mechoulam, R. (2004). A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology (Berl). 174, 254–259. doi: 10.1007/s00213-003-1739-9
Lane, M., Smith, F. E., Sullivan, R. A., and Plasse, T. F. (1990). Dronabinol and prochlorperazine alone and in combination as antiemetic agents for cancer chemotherapy. Am. J. Clin. Oncol. 13, 480–484. doi: 10.1097/00000421-199012000-00006
Lane, M., Vogel, C. L., Ferguson, J., Krasnow, S., Saiers, J. L., Hamm, J., et al. (1991). Dronabinol and prochlorperazine in combination for treatment of cancer chemotherapy-induced nausea and vomiting. J. Pain Symptom Manage. 6, 352–359. doi: 10.1016/0885-3924(91)90026-Z
Lau, A. H., Rudd, J. A., and Yew, D. T. (2005). Action of ondansetron and CP-99,994 on cisplatin- induced emesis and locomotor activity in Suncus murinus (house musk shrew). Behav. Pharmacol. 16, 605–612. doi: 10.1097/00008877-200512000-00002
Levitt, M. (1982). Nabilone vs. placebo in the treatment of chemotherapy-induced nausea and vomiting in cancer patients. Cancer Treat. Rev. 9, 49–53. doi: 10.1016/S0305-7372(82)80036-4
Limebeer, C. L., Abdullah, R. A., Rock, E. M., Imhof, E., Wang, K., Lichtman, A. H., et al. (2014). Attenuation of anticipatory nausea in a rat model of contextually elicited conditioned gaping by enhancement of the endocannabinoid system. Psychopharmacology (Berl). 231, 603–612. doi: 10.1007/s00213-013-3282-7
Limebeer, C. L., Hall, G., and Parker, L. A. (2006). Exposure to a lithium-paired context elicits gaping in rats: a model of anticipatory nausea. Physiol. Behav. 88, 398–403. doi: 10.1016/j.physbeh.2006.04.014
Limebeer, C. L., Krohn, J. P., Cross-Mellor, S., Litt, D. E., Ossenkopp, K. P., and Parker, L. A. (2008). Exposure to a context previously associated with nausea elicits conditioned gaping in rats: a model of anticipatory nausea. Behav. Brain Res. 187, 33–40. doi: 10.1016/j.bbr.2007.08.024
Limebeer, C. L., Rock, E. M., Puvanenthirarajah, N., Niphakis, M. J., Cravatt, B. F., and Parker, L. A. (2016). Elevation of 2-AG by monoacylglycerol lipase inhibition in the visceral insular cortex interferes with anticipatory nausea in a rat model. Behav. Neurosci. 130, 261–266. doi: 10.1037/bne0000132
London, S. W., McCarthy, L. E., and Borison, H. L. (1979). Suppression of cancer chemotherapy-induced vomiting in the cat by nabilone, a synthetic cannabinoid. Proc. Soc. Exp. Biol. Med. 160, 437–440. doi: 10.3181/00379727-160-40465
Malik, I. A., Khan, W. A., Qazilbash, M., Ata, E., Butt, A., and Khan, M. A. (1995). Clinical efficacy of lorazepam in prophylaxis of anticipatory, acute, and delayed nausea and vomiting induced by high doses of cisplatin. A prospective randomized trial. Am. J. Clin. Oncol. 18, 170–175. doi: 10.1097/00000421-199504000-00017
Matsuki, N., Torii, Y., Muto, M., Ueno, S., and Saito, H. (1990). Emesis induced by cisplatin and its block by serotonergic (5HT3) antagonists in Suncus murinus. Eur. J. Pharmacol. 183, 1968–1969. doi: 10.1016/0014-2999(90)92319-E
Matsuki, N., Ueno, S., Kaji, T., Ishihara, A., Wang, C. H., and Saito, H. (1988). Emesis induced by cancer chemotherapeutic agents in the Suncus murinus: a new experimental model. Jpn. J. Pharmacol. 48, 303–306. doi: 10.1254/jjp.48.303
McCabe, M., Smith, F. P., MacDonald, J. S., Woolley, P. V., Goldberg, D., and Schein, P. S. (1988). Efficacy of tetrahydrocannabinol in patients refractory to standard antiemetic therapy. Invest. New Drugs 6, 243–246. doi: 10.1007/BF00175407
McCarthy, L. E., and Borison, H. L. (1981). Antiemetic activity of N-methyllevonantradol and nabilone in cisplatin-treated cats. J. Clin. Pharmacol. 21, 30S–37S. doi: 10.1002/j.1552-4604.1981.tb02570.x
Morrow, G. R. (1982). Prevalence and correlates of anticipatory nausea and vomiting in chemotherapy patients. J. Natl. Cancer Inst. 68, 585–588.
Nakayama, H., Yamakuni, H., Higaki, M., Ishikawa, H., Imazumi, K., Matsuo, M., et al. (2005). Antiemetic activity of FK1052, a 5-HT3- and 5-HT4-receptor antagonist, in Suncus murinusand ferrets. J. Pharmacol. Sci. 98, 396–403. doi: 10.1254/jphs.FPJ05001X
Napadow, V., Sheehan, J. D., Kim, J., Lacount, L. T., Park, K., Kaptchuk, T. J., et al. (2013). The brain circuitry underlying the temporal evolution of nausea in humans. Cereb. Cortex 23, 806–813. doi: 10.1093/cercor/bhs073
Niamatali, C., Fallon, S. D., and Egan, E. L. (1984). Nabilone in the management of prochlorperazine resistant cancer chemotherapy induced emesis. Ir. Med. J. 77, 276–277.
Niederle, N., Schütte, J., and Schmidt, C. G. (1986). Crossover comparison of the antiemetic efficacy of nabilone and alizapride in patients with nonseminomatous testicular cancer receiving cisplatin therapy. Klin. Wochenschr. 64, 362–365. doi: 10.1007/BF01728184
Niiranen, A., and Mattson, K. (1985). A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy. Am. J. Clin. Oncol. 8, 336–340. doi: 10.1097/00000421-198508000-00013
Orr, L. E., and McKernan, J. F. (1981). Antiemetic effect of delta 9-tetrahydrocannabinol in chemotherapy-associated nausea and emesis as compared to placebo and compazine. J. Clin. Pharmacol. 21, 76S–80S.
Orr, L. E., McKernan, J. F., and Bloome, B. (1980). Antiemetic effect of tetrahydrocannabinol. Compared with placebo and prochlorperazine in chemotherapy-associated nausea and emesis. Arch. Intern. Med. 140, 1431–1433.
Ostrowsky, K., Isnard, J., Ryvlin, P., Guénot, M., Fischer, C., and Mauguière, F. (2000). Functional mapping of the insular cortex: clinical implication in temporal lobe epilepsy. Epilepsia 41, 681–686. doi: 10.1111/j.1528-1157.2000.tb00228.x
Parker, L. A. (2014). Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. Eur. J. Pharmacol. 722, 122–133. doi: 10.1016/j.ejphar.2013.09.070
Parker, L. A., Kwiatkowska, M., Burton, P., and Mechoulam, R. (2004). Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology (Berl). 171, 156–161. doi: 10.1007/s00213-003-1571-2
Parker, L. A., Limebeer, C. L., Rock, E. M., Litt, D. L., Kwiatkowska, M., and Piomelli, D. (2009). The FAAH inhibitor URB-597 interferes with cisplatin- and nicotine-induced vomiting in the Suncus murinus (house musk shrew). Physiol. Behav. 97, 121–124. doi: 10.1016/j.physbeh.2009.02.014
Parker, L. A., Limebeer, C. L., Rock, E. M., Sticht, M. A., Ward, J., Turvey, G., et al. (2016). A comparison of novel, selective fatty acid amide hydrolase (FAAH), monoacyglycerol lipase (MAGL) or dual FAAH/MAGL inhibitors to suppress acute and anticipatory nausea in rat models. Psychopharmacology (Berl) 233, 2265–2275. doi: 10.1007/s00213-016-4277-y
Parker, L. A., and Mechoulam, R. (2003). Cannabinoid agonists and antagonists modulate lithium-induced conditioned gaping in rats. Integr. Physiol. Behav. Sci. 38, 133–145. doi: 10.1007/BF02688831
Parker, L. A., Niphakis, M. J., Downey, R., Limebeer, C. L., Rock, E. M., Sticht, M. A., et al. (2015). Effect of selective inhibition of monoacylglycerol lipase (MAGL) on acute nausea, anticipatory nausea, and vomiting in rats and Suncus murinus. Psychopharmacology (Berl).232, 583–593. doi: 10.1007/s00213-014-3696-x
Penfield, W., and Faulk, M. E. (1955). The insula: further observations on its function. Brain 78, 445–470. doi: 10.1093/brain/78.4.445
Pomeroy, M., Fennelly, J. J., and Towers, M. (1986). Prospective randomized double-blind trial of nabilone versus domperidone in the treatment of cytotoxic-induced emesis. Cancer Chemother. Pharmacol. 17, 285–288. doi: 10.1007/BF00256701
Ray, A. P., Griggs, L., and Darmani, N. A. (2009). Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew. Behav. Brain Res. 196, 30–36. doi: 10.1016/j.bbr.2008.07.028
Razavi, D., Delvaux, N., Farvacques, C., De Brier, F., Van Heer, C., Kaufman, L., et al. (1993). Prevention of adjustment disorders and anticipatory nausea secondary to adjuvant chemotherapy: a double-blind, placebo-controlled study assessing the usefulness of alprazolam. J. Clin. Oncol. 11, 1384–1390.
Rock, E. M., Bolognini, D., Limebeer, C. L., Cascio, M. G., Anavi-Goffer, S., Fletcher, P. J., et al. (2012). Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 165, 2620–2634. doi: 10.1111/j.1476-5381.2011.01621.x
Rock, E. M., Boulet, N., Limebeer, C. L., Mechoulam, R., and Parker, L. A. (2016). Cannabinoid 2 (CB2) receptor agonism reduces lithium chloride-induced vomiting in Suncus murinus and nausea-induced conditioned gaping in rats. Eur. J. Pharmacol. 786, 94–99. doi: 10.1016/j.ejphar.2016.06.001
Rock, E. M., Kopstick, R. L., Limebeer, C. L., and Parker, L. A. (2013). Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br. J. Pharmacol. 170, 641–648. doi: 10.1111/bph.12316
Rock, E. M., Limebeer, C. L., Mechoulam, R., Piomelli, D., and Parker, L. A. (2008). The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology (Berl). 196, 389–395. doi: 10.1007/s00213-007-0970-1
Rock, E. M., Limebeer, C. L., Navaratnam, R., Sticht, M. A., Bonner, N., Engeland, K., et al. (2014). A comparison of cannabidiolic acid with other treatments for anticipatory nausea using a rat model of contextually elicited conditioned gaping. Psychopharmacology (Berl). 231, 3207–3215. doi: 10.1007/s00213-014-3498-1
Rock, E. M., Limebeer, C. L., and Parker, L. A. (2015a). Effect of combined doses of Δ(9)-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea using rat (Sprague- Dawley) models of conditioned gaping. Psychopharmacology (Berl). 232, 4445–4454. doi: 10.1007/s00213-015-4080-1
Rock, E. M., Limebeer, C. L., Ward, J. M., Cohen, A., Grove, K., Niphakis, M. J., et al. (2015b). Interference with acute nausea and anticipatory nausea in rats by fatty acid amide hydrolase (FAAH) inhibition through a PPARα and CB1 receptor mechanism, respectively: a double dissociation. Psychopharmacology (Berl). 232, 3841–3848. doi: 10.1007/s00213-015-4050-7
Rock, E. M., and Parker, L. A. (2013a). Effect of low doses of cannabidiolic acid and ondansetron on LiCl-induced conditioned gaping (a model of nausea-induced behaviour) in rats. Br. J. Pharmacol. 169, 685–692. doi: 10.1111/bph.12162
Rock, E. M., and Parker, L. A. (2013b). Suppression of lithium chloride-induced conditioned gaping (a model of nausea-induced behaviour) in rats (using the taste reactivity test) with metoclopramide is enhanced by cannabidiolic acid. Pharmacol. Biochem. Behav. 111, 84–89. doi: 10.1016/j.pbb.2013.08.012
Rock, E. M., and Parker, L. A. (2015). Synergy between cannabidiol, cannabidiolic acid, and Δ(9)-tetrahydrocannabinol in the regulation of emesis in the Suncus murinus (house musk shrew). Behav. Neurosci. 129, 368–370. doi: 10.1037/bne0000057
Roila, F., Herrstedt, J., Aapro, M., Gralla, R. J., Einhorn, L. H., Ballatori, E., et al. (2010). Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann. Oncol. 21, 232–243. doi: 10.1093/annonc/mdq194
Roscoe, J. A., Morrow, G. R., Hickok, J. T., and Stern, R. M. (2000). Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J. Pain Symptom Manage. 20, 113–121. doi: 10.1016/S0885-3924(00)00159-7
Sallan, S. E., Zinberg, N. E., and Frei, E. III. (1975). Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N. Engl. J. Med. 293, 795–797. doi: 10.1056/NEJM197510162931603
Sam, T. S., Cheng, J. T., Johnston, K. D., Kan, K. K., Ngan, M. P., Rudd, J. A., et al. (2003). Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew). Eur. J. Pharmacol. 472, 135–145. doi: 10.1016/S0014-2999(03)01863-6
Sanger, G. J., and Andrews, P. L. (2006). Treatment of nausea and vomiting: gaps in our knowledge. Auton. Neurosci. 129, 3–16. doi: 10.1016/j.autneu.2006.07.009
Sclocco, R., Kim, J., Garcia, R. G., Sheehan, J. D., Beissner, F., Bianchi, A. M., et al. (2014). Brain circuitry supporting multi-organ autonomic outflow in response to nausea. Cereb. Cortex26, 485–497. doi: 10.1093/cercor/bhu172
Sharkey, K. A., Cristino, L., Oland, L. D., Van Sickle, M. D., Starowicz, K., Pittman, Q. J., et al. (2007). Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur. J. Neurosci. 25, 2773–2782. doi: 10.1111/j.1460-9568.2007.05521.x
Sharkey, K. A., Darmani, N. A., and Parker, L. A. (2014). Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur. J. Pharmacol. 722, 134–146. doi: 10.1016/j.ejphar.2013.09.068
Steele, N., Gralla, R. J., and Braun, D. W. Jr., Young, C. W. (1980). Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on chemotherapy-induced emesis. Cancer Treat. Rep. 64, 219–224.
Sticht, M. A., Limebeer, C. L., Rafla, B. R., Abdullah, R. A., Poklis, J. L., Ho, W., et al. (2016). Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology 102, 92–102. doi: 10.1016/j.neuropharm.2015.10.039
Sticht, M. A., Limebeer, C. L., Rafla, B. R., and Parker, L. A. (2015). Intra-visceral insular cortex 2-arachidonoylglycerol, but not N-arachidonoylethanolamide, suppresses acute nausea-induced conditioned gaping in rats. Neuroscience 286, 338–344. doi: 10.1016/j.neuroscience.2014.11.058
Sticht, M. A., Long, J. Z., Rock, E. M., Limebeer, C. L., Mechoulam, R., Cravatt, B. F., et al. (2012). Inhibition of monoacylglycerol lipase attenuates vomiting in Suncus murinus and 2-arachidonoyl glycerol attenuates nausea in rats. Br. J. Pharmacol. 165, 2425–2435. doi: 10.1111/j.1476-5381.2011.01407.x
Sticht, M. A., Rock, E. M., and Parker, L. A. (2013). 2-arachidonoylglycerol interferes with lithium-induced vomiting in the house musk shrew, Suncus murinus. Physiol. Behav. 120, 228–232. doi: 10.1016/j.physbeh.2013.08.015
Torii, Y., Saito, H., and Matsuki, N. (1991). 5-Hydroxytryptamine is emetogenic in the house musk shrew, Suncus murinus. Naunyn Schmiedebergs Arch. Pharmacol. 344, 564–567.
Van Sickle, M. D., Duncan, M., Kingsley, P. J., Mouihate, A., Urbani, P., Mackie, K., et al. (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332. doi: 10.1126/science.1115740
Van Sickle, M. D., Oland, L. D., Ho, W., Hillard, C. J., Mackie, K., Davison, J. S., et al. (2001). Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology 121, 767–774. doi: 10.1053/gast.2001.28466
Van Sickle, M. D., Oland, L. D., Mackie, K., Davison, J. S., and Sharkey, K. A. (2003). Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G566–G576. doi: 10.1152/ajpgi.00113.2003
Venkatesan, T., Zadvornova, Y., Raff, H., and Hillard, C. J. (2016). Endocannabinoid-related lipids are increased during an episode of cyclic vomiting syndrome. Neurogastroenterol. Motil.doi: 10.1111/nmo.12843. [Epub ahead of print].
Wada, J. K., Bogdon, D. L., Gunnell, J. C., Hum, G. J., Gota, C. H., and Rieth, T. E. (1982). Double-blind, randomized, crossover trial of nabilone vs. placebo in cancer chemotherapy. Cancer Treat. Rev. 9B, 39–44. doi: 10.1016/S0305-7372(82)80034-0
Citation: Rock EM and Parker LA (2016) Cannabinoids As Potential Treatment for Chemotherapy-Induced Nausea and Vomiting. Front. Pharmacol. 7:221. doi: 10.3389/fphar.2016.00221
Received: 05 May 2016; Accepted: 11 July 2016;
Published: 26 July 2016.
Allyn C. Howlett, Wake Forest School of Medicine, USA
Maria Grazia Morgese, University of Foggia, Italy
Francesco Rossi, Seconda Università Degli Studi di Napoli, Italy
Copyright © 2016 Rock and Parker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda A. Parker, parkerl@uoguelph.ca



 Erin M. Rock
Erin M. Rock Linda A. Parker
Linda A. Parker