Open Access
Abstract
Introduction
 Since ancient times it has been known that some plants confer medical benefits beyond simply providing essential nutrients. Modern drug discovery relies extensively on cues from naturally existing compounds,1,2 and research has been able to show that the mechanisms involved in the natural synthesis of these medicinal compounds are in many cases significantly more efficient and potent than industrial methods can achieve.3 In an effort to enhance the pharmacological effects of synthetic drugs, there has been a push to understand these biosynthetic pathways and the synergies between the various natural compounds.4
Since ancient times it has been known that some plants confer medical benefits beyond simply providing essential nutrients. Modern drug discovery relies extensively on cues from naturally existing compounds,1,2 and research has been able to show that the mechanisms involved in the natural synthesis of these medicinal compounds are in many cases significantly more efficient and potent than industrial methods can achieve.3 In an effort to enhance the pharmacological effects of synthetic drugs, there has been a push to understand these biosynthetic pathways and the synergies between the various natural compounds.4
Materials and Methods
Cannabis sativa L. and Δ9-Tetrahydrocannabinolic Acid
Optical Setup
Results and Discussion
Δ9-Tetrahydrocannabinolic Acid
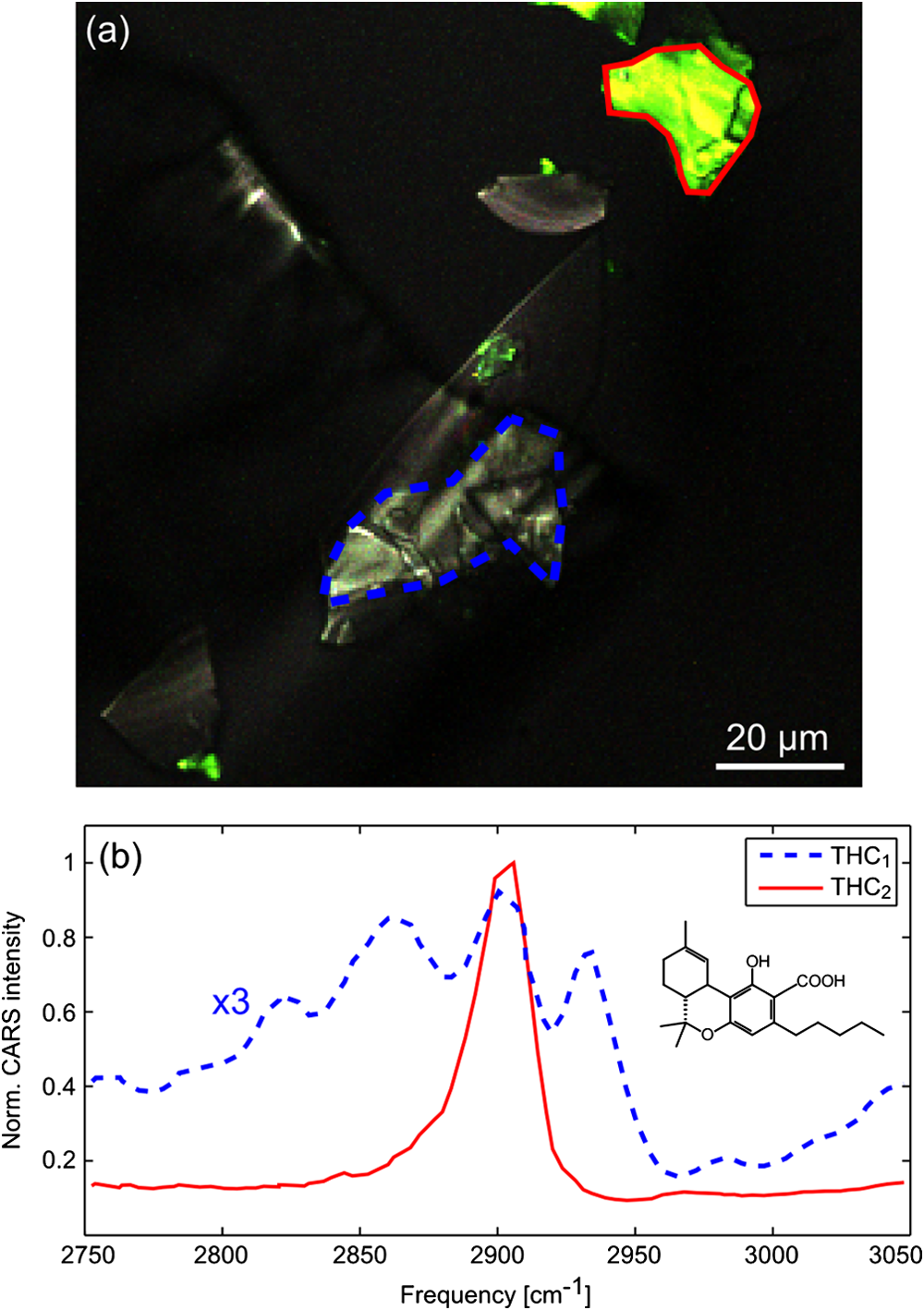
Fig. 1 (a) Hyperspectral CARS image of pure THCa crystals displaying two distinct colors. (b) Spectra extracted from the indicated regions of (a). Key marker frequencies for THC 1 THC 1 are 2825, 2860, 2905, and 2940 cm − 1 2940 cm − 1 , while THC 2 THC 2 has only a single strong peak at 2900 cm − 1 2900 cm − 1 . Small inclusions of THC 2 THC 2 within the blue region generate additional intensity at 2900 cm − 1 2900 cm − 1 in the THC 1 THC 1 spectrum. The THCa structure is shown in part (b). Note that both spectra in part (b) have been normalized to the maximum value of the THC 2 THC 2 spectrum, and that the THC 1 THC 1 spectrum has been scaled by an additional factor of three.
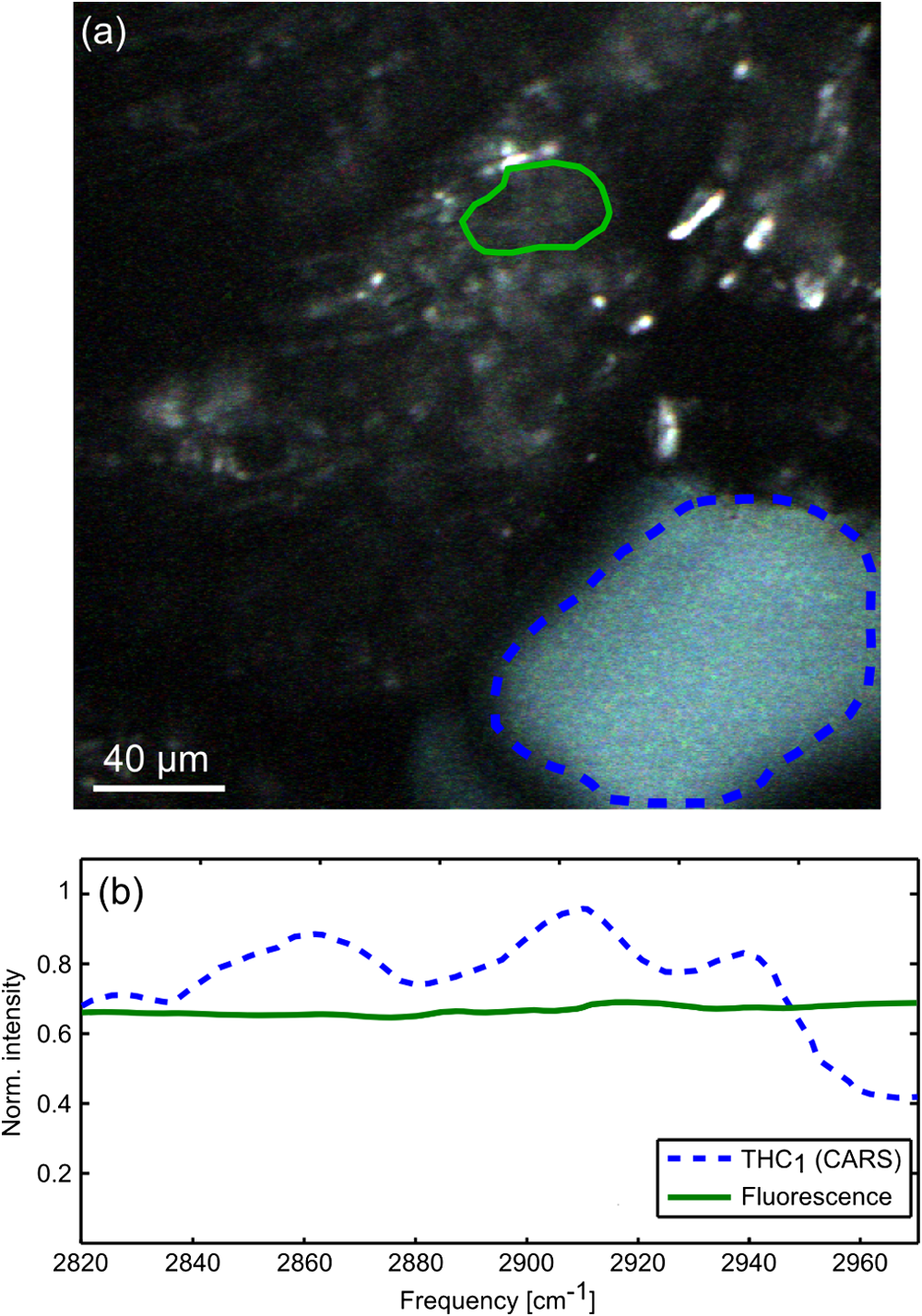
Cannabis sativa (Leaf)
Cannabis sativa (Pistil)
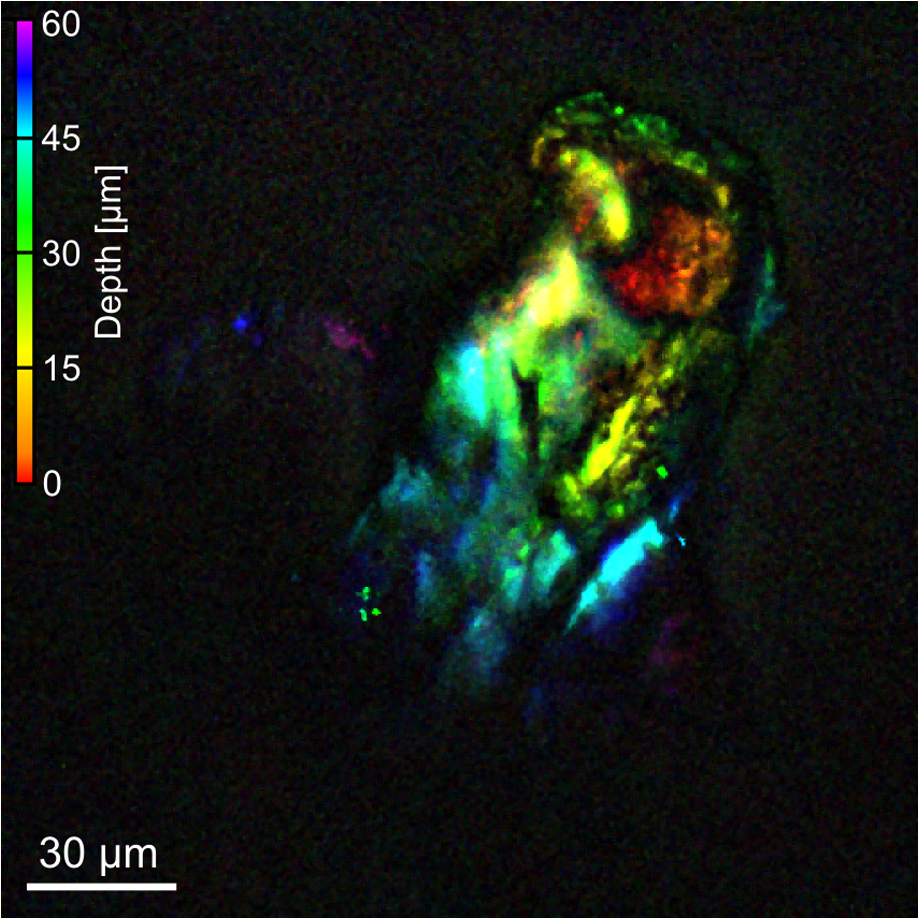
Fig. 3 Color-coded projection of a CARS z z -stack in a glandular trichome, measured at the THC 1 THC 1 peak at 2940 cm − 1 2940 cm − 1 . Reds and oranges indicate the bottom of the stack, while blues and purples are at the top of the stack. Many different physical morphologies are visible at different regions within this trichome.
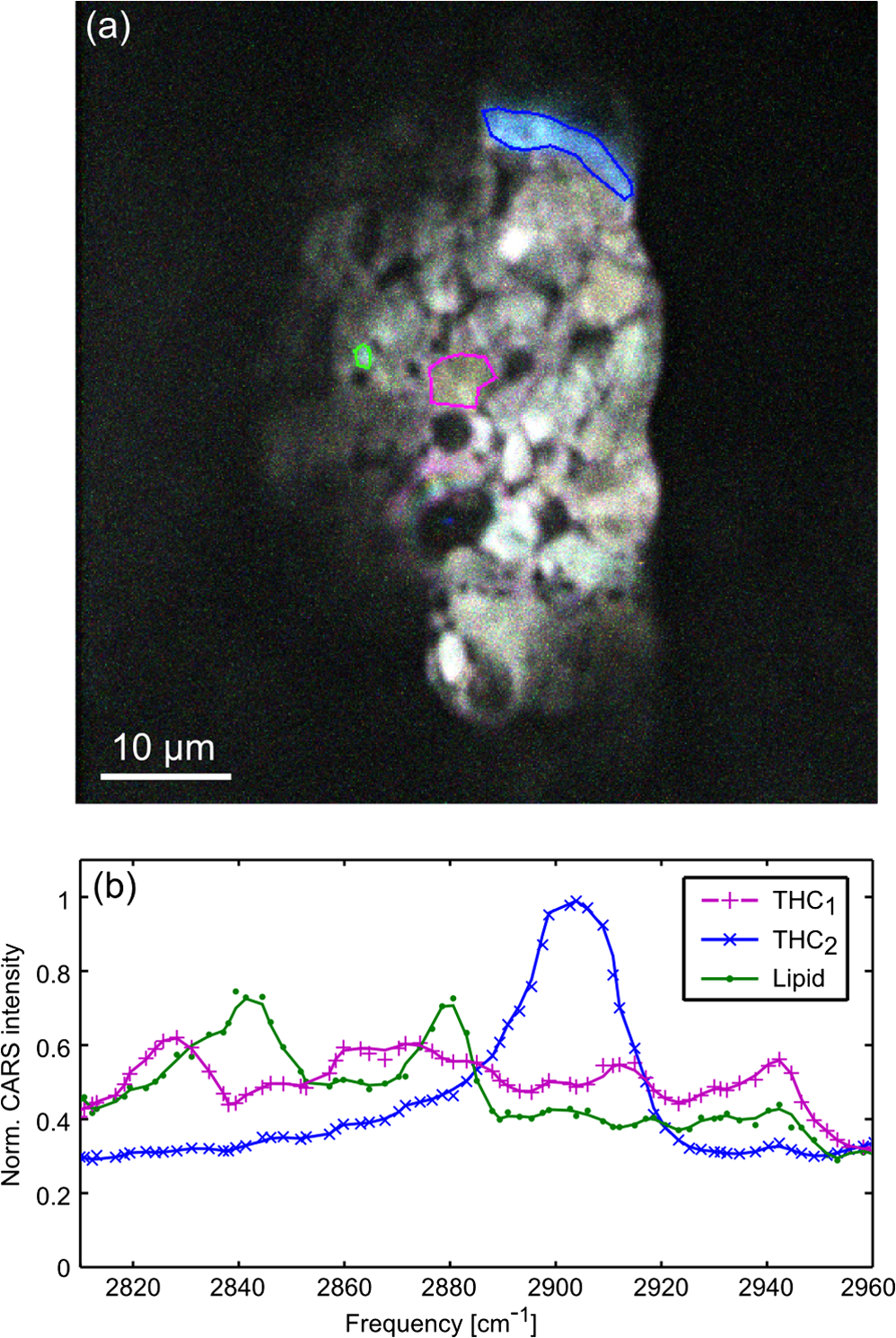
Fig. 4 Hyperspectral CARS image (a) and CARS spectra (b) of the interior of a stigma showing both representative THCa spectra (magenta and blue regions and curves) as well as native lipids (green region and curve). The presence of many compounds other than THCa in this section of the plant yields a complex mixture, from which it is impossible to find a pure THCa spectrum in any region of interest.
Conclusions
Acknowledgments
References
Erik T. Garbacik; Roza P. Korai; Eric H. Frater; Jeroen P. Korterik; Cees Otto, et al.
“In planta imaging of Δ9-tetrahydrocannabinolic acid in Cannabis sativa L. with hyperspectral coherent anti-Stokes Raman scattering microscopy”, J. Biomed. Opt. 18(4), 046009 (Apr 15, 2013). ;http://dx.doi.org/10.1117/1.JBO.18.4.046009