Pharmacology & Pharmacy, 2015, 6, 75‐85
Published Online February 2015 in SciRes. http://www.scirp.org/journal/pp http://dx.doi.org/10.4236/pp.2015.62010

Published Online February 2015 in SciRes. http://www.scirp.org/journal/pp http://dx.doi.org/10.4236/pp.2015.62010

Ruth Gallily1, Zhannah Yekhtin1, Lumír Ondřej Hanuš2
1The Lautenberg Center for General and Tumor Immunology, The Hadassah Medical School, The Hebrew University of Jerusalem, Jerusalem, Israel
2Department of Medicinal and Natural Products, Institute for Drug Research, The Hadassah Medical School, The Hebrew University of Jerusalem, Jerusalem, Israel
Email: ruthg@ekmd.huji.ac.il
Received 12 November 2014; accepted 7 February 2015; published 10 February 2015
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/
1The Lautenberg Center for General and Tumor Immunology, The Hadassah Medical School, The Hebrew University of Jerusalem, Jerusalem, Israel
2Department of Medicinal and Natural Products, Institute for Drug Research, The Hadassah Medical School, The Hebrew University of Jerusalem, Jerusalem, Israel
Email: ruthg@ekmd.huji.ac.il
Received 12 November 2014; accepted 7 February 2015; published 10 February 2015
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/
Abstract
Cannabidiol (CBD), a major constituent of Cannabis, has been shown to be a powerful anti‐inflammatory and anti‐anxiety drug, without exerting a psychotropic effect. However, when given either intraperitoneally or orally as a purified product, a bell‐shaped dose‐response was observed, which limits its clinical use. In the present study, we have studied in mice the anti‐inflammatory and anti‐nociceptive activities of standardized plant extracts derived from the Cannabis sativa L., clone 202, which is highly enriched in CBD and hardly contains any psychoactive ingredients. In stark contrast to purified CBD, the clone 202 extract, when given either intraperitoneally or orally, provided a clear correlation between the anti‐inflammatory and anti‐nociceptive responses and the dose, with increasing responses upon increasing doses, which makes this plant medicine ideal for clinical uses. The clone 202 extract reduced zymosan‐induced paw swelling and pain in mice, and prevented TNFα production in vivo. It is likely that other components in the extract synergize with CBD to achieve the desired anti‐inflammatory action that may contribute to overcoming the bell‐shaped dose‐response of purified CBD. We therefore propose that Cannabis clone 202 (Avidekel) extract is superior over CBD for the treatment of inflammatory conditions.
Keywords
Cannabis sativa L. Clone 202, Cannabidiol, Anti‐Inflammation, Anti‐Nociceptive, TNFα
Cannabidiol (CBD), a major constituent of Cannabis, has been shown to be a powerful anti‐inflammatory and anti‐anxiety drug, without exerting a psychotropic effect. However, when given either intraperitoneally or orally as a purified product, a bell‐shaped dose‐response was observed, which limits its clinical use. In the present study, we have studied in mice the anti‐inflammatory and anti‐nociceptive activities of standardized plant extracts derived from the Cannabis sativa L., clone 202, which is highly enriched in CBD and hardly contains any psychoactive ingredients. In stark contrast to purified CBD, the clone 202 extract, when given either intraperitoneally or orally, provided a clear correlation between the anti‐inflammatory and anti‐nociceptive responses and the dose, with increasing responses upon increasing doses, which makes this plant medicine ideal for clinical uses. The clone 202 extract reduced zymosan‐induced paw swelling and pain in mice, and prevented TNFα production in vivo. It is likely that other components in the extract synergize with CBD to achieve the desired anti‐inflammatory action that may contribute to overcoming the bell‐shaped dose‐response of purified CBD. We therefore propose that Cannabis clone 202 (Avidekel) extract is superior over CBD for the treatment of inflammatory conditions.
Keywords
Cannabis sativa L. Clone 202, Cannabidiol, Anti‐Inflammation, Anti‐Nociceptive, TNFα
How to cite this paper: Gallily, R., Yekhtin, Z. and Hanuš, L.O. (2015) Overcoming the Bell‐Shaped Dose‐Response of Can‐ nabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacology & Pharmacy, 6, 75‐85. http://dx.doi.org/10.4236/pp.2015.62010
1. Introduction
 Inflammation and pain have accompanied human life for ages. Many anti-inflammation and anti-pain medications and various approaches have been employed through the centuries and in recent time. Many of used drugs, however, impose severe side effects. Cannabis from various origins and species has been employed in various forms as anti-pain agents for thousands of years [1]-[3]. One example is the legitimated drug Sativex® (Nabiximols) that is used in the treatment of severe spasticity in patients with multiple sclerosis [4]. Two other drugs, Marinol (Dronabinol) and Cesamet, have been approved for use in cancer related anorexia-cachexia syndrome as well as for nausea and vomiting [3]. But a major disadvantage of Cannabis phytomedicine is its psychoactive effects due to the presence of 9-Tetrahydrocannabinol (THC).
Inflammation and pain have accompanied human life for ages. Many anti-inflammation and anti-pain medications and various approaches have been employed through the centuries and in recent time. Many of used drugs, however, impose severe side effects. Cannabis from various origins and species has been employed in various forms as anti-pain agents for thousands of years [1]-[3]. One example is the legitimated drug Sativex® (Nabiximols) that is used in the treatment of severe spasticity in patients with multiple sclerosis [4]. Two other drugs, Marinol (Dronabinol) and Cesamet, have been approved for use in cancer related anorexia-cachexia syndrome as well as for nausea and vomiting [3]. But a major disadvantage of Cannabis phytomedicine is its psychoactive effects due to the presence of 9-Tetrahydrocannabinol (THC).
Recently, a science-based approach is being conducted to specify the benefits of Cannabis and its many constituents. A Cannabis plant contains hundreds of different chemicals with about 60 – 80 chemicals known as cannabinoids [5]. The major Cannabis psychoactive molecule is the -tetrahydrocannabinol, known as THC, which binds with high affinity (Ki = 3 – 5 nM) [6] to both the cannabinoid CB1 receptor expressed in the brain and the CB2 receptor expressed on cells of the immune system [7]. Another major constituent is Cannabidiol (CBD) which is devoid of psychotropic effects and binds only with very low affinity (Ki > 10 μM) [6] to the CB1/CB2 receptors. The other cannabinoids are present in minute amounts. Stimulation of CB1 receptor is responsible for the Cannabis psychoactivity, while activation of the CB2 receptor leads to attenuated inflammation, decreased injury and accelerated regeneration in many disease states [7]. CBD has been shown to activate central nervous system’s limbic and paralimbic regions, which can reduce autonomic arousal and feeling of an- xiety [3]. This is in contrast to THC which can be anxiogenic [3]. CBD has also been shown to have anti-emetic, anti-inflammatory and anti-psychotic effects [3]. Studies are looking for potential benefits of phytocannabinoids in management of neuropathic pain, hypertension, post-stroke neuroprotection, multiple sclerosis, epilepsy and cancer [3]. Doses up to 1500 mg per day as well as chronic use of CBD have been reported as being well tolerated by humans [3].
During the last 10 – 15 years, many studies have focused on the anti-inflammatory effects of purified CBD in various animal models, including rheumatoid arthritis, diabetes type 1, inflammatory bowel disease and multiple sclerosis [8]-[13]. These studies showed that purified CBD gives a bell-shaped dose-response curve. Healing was only observed when CBD was given within a very limited dose range, whereas no beneficial effect was achieved at either lower or higher doses. This trait of purified CBD imposes serious obstacles in planning human and animal studies. The aim of the present study was to find a CBD source that could eliminate the bell-shaped dose-response of purified CBD. We found that by using standardized plant extracts from the Cannabis clone 202 obtained from Tikun Olam, Israel, which is highly enriched in CBD and barely contains THC, a correlative anti- inflammatory and anti-pain dose-response could be achieved when applied either intraperitoneally or orally in an inflammatory mouse model.
2. Material and Methods
2.1. CBD and Cannabis Clone 202 (Avidekel) Extract
Purified CBD was purchased from THC Pharm. GmbH, Frankfurt, Germany. Cannabis sativa L. flowers from the clone 202 (Avidekel) rich in CBD while low in any psychotropic constituents was supplied by Tikun Olam Company (a government-approved farm growing medicinal Cannabis), Israel. CBD-enriched extract was pre- pared from the flowers of Cannabis clone 202 grown under controlled temperature and light conditions. 100% ethanol (20 ml) was added to the chopped Cannabis dry flowers (200 mg) for 24 – 48 hrs, with occasional shak- ing at room temperature. Following filtration, samples were taken for analysis. Ethanol solutions of Cannabis clone 202 extracts (10 mg/ml – 20 mg/ml) were kept at −20 ̊C in the dark. The extract was evaporated on Rota- vapor (BÜCHI Labortechnik AG, Switzerland). For intraperitoneal injection, the dried Cannabis clone 202 ex- tract was emulsified in a vehicle composed of ethanol:Cremophor:saline at a 1:1:18 ratio. Purified CBD was emulsified in the same vehicle. For oral administration, the dried Cannabis clone 202 extract and the purified CBD were dissolved in olive oil.
2.2. Analysis of the Cannabis Clone 202 Extract by Thin‐Layer Chromatography (TLC) Cannabis clone 202 extract (1 μl) was separated on TLC Silica Gel 60 F254 aluminium sheets (Merck, Darmstadt, Germany) using hexane:dioxane (4:1) as a solvent in a chamber of 13 × 9 × 12 cm. The separated com- ponents were detected by spraying the plates with a freshly prepared solution of 0.5 g Fast Blue B (D9805, Sigma) in acetone/water (9:1; v/v). Cannabinoids in the dried plant material predominately appeared as cannabinoid acids. The TLC analysis shows two major spots corresponding to the acid and neutral form of CBD, respectively, with only a minor spot corresponding to the acid form of THC (Figure 1(a)).
 Inflammation and pain have accompanied human life for ages. Many anti-inflammation and anti-pain medications and various approaches have been employed through the centuries and in recent time. Many of used drugs, however, impose severe side effects. Cannabis from various origins and species has been employed in various forms as anti-pain agents for thousands of years [1]-[3]. One example is the legitimated drug Sativex® (Nabiximols) that is used in the treatment of severe spasticity in patients with multiple sclerosis [4]. Two other drugs, Marinol (Dronabinol) and Cesamet, have been approved for use in cancer related anorexia-cachexia syndrome as well as for nausea and vomiting [3]. But a major disadvantage of Cannabis phytomedicine is its psychoactive effects due to the presence of 9-Tetrahydrocannabinol (THC).
Inflammation and pain have accompanied human life for ages. Many anti-inflammation and anti-pain medications and various approaches have been employed through the centuries and in recent time. Many of used drugs, however, impose severe side effects. Cannabis from various origins and species has been employed in various forms as anti-pain agents for thousands of years [1]-[3]. One example is the legitimated drug Sativex® (Nabiximols) that is used in the treatment of severe spasticity in patients with multiple sclerosis [4]. Two other drugs, Marinol (Dronabinol) and Cesamet, have been approved for use in cancer related anorexia-cachexia syndrome as well as for nausea and vomiting [3]. But a major disadvantage of Cannabis phytomedicine is its psychoactive effects due to the presence of 9-Tetrahydrocannabinol (THC).Recently, a science-based approach is being conducted to specify the benefits of Cannabis and its many constituents. A Cannabis plant contains hundreds of different chemicals with about 60 – 80 chemicals known as cannabinoids [5]. The major Cannabis psychoactive molecule is the -tetrahydrocannabinol, known as THC, which binds with high affinity (Ki = 3 – 5 nM) [6] to both the cannabinoid CB1 receptor expressed in the brain and the CB2 receptor expressed on cells of the immune system [7]. Another major constituent is Cannabidiol (CBD) which is devoid of psychotropic effects and binds only with very low affinity (Ki > 10 μM) [6] to the CB1/CB2 receptors. The other cannabinoids are present in minute amounts. Stimulation of CB1 receptor is responsible for the Cannabis psychoactivity, while activation of the CB2 receptor leads to attenuated inflammation, decreased injury and accelerated regeneration in many disease states [7]. CBD has been shown to activate central nervous system’s limbic and paralimbic regions, which can reduce autonomic arousal and feeling of an- xiety [3]. This is in contrast to THC which can be anxiogenic [3]. CBD has also been shown to have anti-emetic, anti-inflammatory and anti-psychotic effects [3]. Studies are looking for potential benefits of phytocannabinoids in management of neuropathic pain, hypertension, post-stroke neuroprotection, multiple sclerosis, epilepsy and cancer [3]. Doses up to 1500 mg per day as well as chronic use of CBD have been reported as being well tolerated by humans [3].
During the last 10 – 15 years, many studies have focused on the anti-inflammatory effects of purified CBD in various animal models, including rheumatoid arthritis, diabetes type 1, inflammatory bowel disease and multiple sclerosis [8]-[13]. These studies showed that purified CBD gives a bell-shaped dose-response curve. Healing was only observed when CBD was given within a very limited dose range, whereas no beneficial effect was achieved at either lower or higher doses. This trait of purified CBD imposes serious obstacles in planning human and animal studies. The aim of the present study was to find a CBD source that could eliminate the bell-shaped dose-response of purified CBD. We found that by using standardized plant extracts from the Cannabis clone 202 obtained from Tikun Olam, Israel, which is highly enriched in CBD and barely contains THC, a correlative anti- inflammatory and anti-pain dose-response could be achieved when applied either intraperitoneally or orally in an inflammatory mouse model.
2. Material and Methods
2.1. CBD and Cannabis Clone 202 (Avidekel) Extract
Purified CBD was purchased from THC Pharm. GmbH, Frankfurt, Germany. Cannabis sativa L. flowers from the clone 202 (Avidekel) rich in CBD while low in any psychotropic constituents was supplied by Tikun Olam Company (a government-approved farm growing medicinal Cannabis), Israel. CBD-enriched extract was pre- pared from the flowers of Cannabis clone 202 grown under controlled temperature and light conditions. 100% ethanol (20 ml) was added to the chopped Cannabis dry flowers (200 mg) for 24 – 48 hrs, with occasional shak- ing at room temperature. Following filtration, samples were taken for analysis. Ethanol solutions of Cannabis clone 202 extracts (10 mg/ml – 20 mg/ml) were kept at −20 ̊C in the dark. The extract was evaporated on Rota- vapor (BÜCHI Labortechnik AG, Switzerland). For intraperitoneal injection, the dried Cannabis clone 202 ex- tract was emulsified in a vehicle composed of ethanol:Cremophor:saline at a 1:1:18 ratio. Purified CBD was emulsified in the same vehicle. For oral administration, the dried Cannabis clone 202 extract and the purified CBD were dissolved in olive oil.
2.2. Analysis of the Cannabis Clone 202 Extract by Thin‐Layer Chromatography (TLC) Cannabis clone 202 extract (1 μl) was separated on TLC Silica Gel 60 F254 aluminium sheets (Merck, Darmstadt, Germany) using hexane:dioxane (4:1) as a solvent in a chamber of 13 × 9 × 12 cm. The separated com- ponents were detected by spraying the plates with a freshly prepared solution of 0.5 g Fast Blue B (D9805, Sigma) in acetone/water (9:1; v/v). Cannabinoids in the dried plant material predominately appeared as cannabinoid acids. The TLC analysis shows two major spots corresponding to the acid and neutral form of CBD, respectively, with only a minor spot corresponding to the acid form of THC (Figure 1(a)).
2.3. Analysis of the Cannabis Clone 202 Extract by Gas Chromatography and Mass Spectrophotometry (GC/MS)
For analysis of the composition of the ethanol extracts of medicinal Cannabis clone 202, the ethanol was evaporated and the resin dissolved in 20 ml of methanol and filtered through cotton in a capillary. The concentration of the extract was adjusted to 1 mg/ml to which 50 μg internal standard (Tetracosane, Acros Organics, USA) was added. One l of this sample was applied for the GC/MS analysis. The quantitative analysis of the samples by GC/MS was performed in a Hewlett Packard G 1800B GCD system with a HP-5971 gas chromatograph with electron ionization detector. The software used was GCD Plus ChemStation. The column used was SPB-5 (30 m × 0.25 mm × 0.25 μm film thickness). Experimental conditions were: inlet, 250 ̊C; detector, 280 ̊C; splitless in- jection/purge time, 1.0 min; initial temperature, 100 ̊C; initial time, 2.0 min; rate, 10 ̊C/min; final temperature, 280 ̊C. The helium flow rate was 1 ml/min. Calibration curve was made from 25.0 to 100 μg/ml Cannabidiol (CBD), 9-Tetetrahydrocannabinol (THC) or Cannabinol (CBN) together with 50.0 μg/ml tetracosane as internal standard. The cannabinoid composition of Cannabis clone 202 extract is presented in Figure 1(b), Figure 1(c) and Table 1.
2.4. Commercial Anti‐Nociceptive and Anti‐Inflammatory Drugs
The non-steroid anti-inflammatory drug (NSAID) aspirin (acetylsalicylic acid) was purchased from Sigma and dissolved in olive oil. Fifty mg of aspirin was given per os per kg in a volume of 40 μl. The opioid anti-noci- ceptive Tramadol hydrochloride was obtained from Grunenthal and dissolved in saline. Five mg of Tramadol was given per os per kg.
For analysis of the composition of the ethanol extracts of medicinal Cannabis clone 202, the ethanol was evaporated and the resin dissolved in 20 ml of methanol and filtered through cotton in a capillary. The concentration of the extract was adjusted to 1 mg/ml to which 50 μg internal standard (Tetracosane, Acros Organics, USA) was added. One l of this sample was applied for the GC/MS analysis. The quantitative analysis of the samples by GC/MS was performed in a Hewlett Packard G 1800B GCD system with a HP-5971 gas chromatograph with electron ionization detector. The software used was GCD Plus ChemStation. The column used was SPB-5 (30 m × 0.25 mm × 0.25 μm film thickness). Experimental conditions were: inlet, 250 ̊C; detector, 280 ̊C; splitless in- jection/purge time, 1.0 min; initial temperature, 100 ̊C; initial time, 2.0 min; rate, 10 ̊C/min; final temperature, 280 ̊C. The helium flow rate was 1 ml/min. Calibration curve was made from 25.0 to 100 μg/ml Cannabidiol (CBD), 9-Tetetrahydrocannabinol (THC) or Cannabinol (CBN) together with 50.0 μg/ml tetracosane as internal standard. The cannabinoid composition of Cannabis clone 202 extract is presented in Figure 1(b), Figure 1(c) and Table 1.
2.4. Commercial Anti‐Nociceptive and Anti‐Inflammatory Drugs
The non-steroid anti-inflammatory drug (NSAID) aspirin (acetylsalicylic acid) was purchased from Sigma and dissolved in olive oil. Fifty mg of aspirin was given per os per kg in a volume of 40 μl. The opioid anti-noci- ceptive Tramadol hydrochloride was obtained from Grunenthal and dissolved in saline. Five mg of Tramadol was given per os per kg.
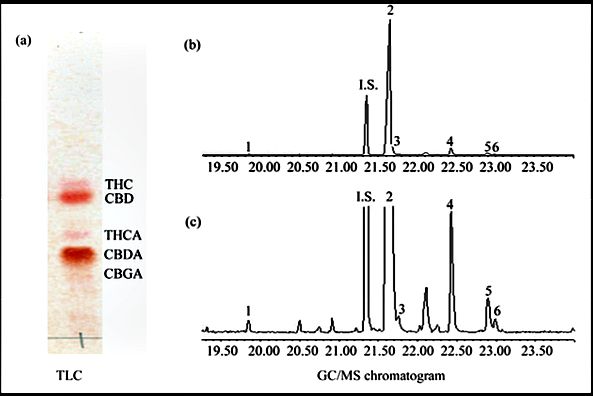
- Figure 1. (a) TLC analysis of clone 202 extract. 1 l of the extract was run on TLC as de- scribed in the Method section. CBD = Cannabidiol. CBDA = Cannabidiolic acid; (b) (c) GC/ MS chromatograms of an extract from Cannabis clone 202. (b) The full chromatogram. (c) Magnification of weaker signals. Number keys: 1: Cannabidivarol (CBDV); 2: Cannabidiol (CBD); 3: Cannabichromene (CBC); 4: 9-Tetrahydrocannabinol (9-THC); 5: Cannabigerol (CBG); 6: Cannabinol (CBN); I.S.-Internal Standard (Tetracosane).
- As cannabinoid acids during injection to the GC/MS decarboxylate, the results are a total sum of neutral cannabinoids and cannabinoid acids that have decarboxylated into neutral cannabinoids. The content is the mass fraction (% w/w) of the given constituent in the extract.
2.5. Animals
Six to eight week old female Sabra mice (Israel) were maintained in the SPF unit of the Hebrew University- Hadassah Medical School, Jerusalem, Israel. The experimental protocols were approved by the Animal Care Ethical Committee of the Hebrew University-Hadassah Medical School, Jerusalem, Israel. The animals were maintained on standard pellet diet and water ad libitum. The animals were maintained at a constant temperature (20 ̊C – 21 ̊C) and a 12 h light/dark cycle.
2.6. Induction of Paw Inflammation in Mice and Treatment with Purified CBD or Clone 202 Extract
To induce inflammation, 40 μl of 1.5% (w/v) zymosan A (Sigma) suspended in 0.9% saline was injected into the sub-planter surface of the right hind paw of the mice. Immediately after zymosan injection, CBD or Cannabis clone 202 extract was injected intraperitoneally (i.p.) or given orally. For intraperitoneal injection, these agents were dissolved in 0.1 ml vehicle containing ethanol:Cremophore:saline at a ratio of 1:1:18. Control mice were injected with the vehicle only. For per os administration, the agents were dissolved in olive oil, each mouse re- ceiving 40 μl. Control mice got 40 μl olive oil. After 2, 6 and 24 hrs, paw swelling and pain perception were measured. Serum TNFα titers were determined after 24 hrs. The effects of CBD and Cannabis clone 202 extract were compared to those of aspirin (50 mg/kg per os) and tramadol (5 mg/kg, i.p.).
2.7. Measurement of Oedema Formation
The paw swelling (thickness) was measured by calibrated calipers (0.01 mm), 2, 6 and 24 hrs following injec- tions of zymosan alone or with CBD or Cannabis clone 202 extracts.
2.8. Pain Assay
The hyperalgesia was evaluated by the paw withdrawal von Frey test at 2, 6, and 24 hrs following injections of zymosan and/or the test compounds. In the von Frey nociceptive filament assay, von Frey calibrated monofila- ment hairs of logarithmically incremental stiffness (0.008 – 300 g corresponding to 1.65 – 6.65 log of force). In our study, only 1.4 – 60 g corresponding to 4.17 to 5.88 log of force was used, to test the mouse sensitivity to a mechanical stimulus on the swollen paw. The measurements were performed in a quiet room. Before paw pain measurements, the animals were held for 10 sec. The trained investigator applied the filament to the central area of the hind paw with gradual increasing size. The test consisted of poking the middle of the hind paw to provoke a flexion reflex followed by a clear flinch response after paw withdrawal. Each one of the von Frey filaments was applied for approximately 3 – 4 s to induce the end-point reflex. The first testing was done by using the force filament of 1.4 g. If there was no withdrawal response, the next higher stimulus was tried. The mechanical threshold force (in grams (g)) was defined as the lowest force imposed by two von Frey monofilaments of vari- ous sizes, required to produce a paw retraction. The untreated left hind paw served as a control.
2.6. Induction of Paw Inflammation in Mice and Treatment with Purified CBD or Clone 202 Extract
To induce inflammation, 40 μl of 1.5% (w/v) zymosan A (Sigma) suspended in 0.9% saline was injected into the sub-planter surface of the right hind paw of the mice. Immediately after zymosan injection, CBD or Cannabis clone 202 extract was injected intraperitoneally (i.p.) or given orally. For intraperitoneal injection, these agents were dissolved in 0.1 ml vehicle containing ethanol:Cremophore:saline at a ratio of 1:1:18. Control mice were injected with the vehicle only. For per os administration, the agents were dissolved in olive oil, each mouse re- ceiving 40 μl. Control mice got 40 μl olive oil. After 2, 6 and 24 hrs, paw swelling and pain perception were measured. Serum TNFα titers were determined after 24 hrs. The effects of CBD and Cannabis clone 202 extract were compared to those of aspirin (50 mg/kg per os) and tramadol (5 mg/kg, i.p.).
2.7. Measurement of Oedema Formation
The paw swelling (thickness) was measured by calibrated calipers (0.01 mm), 2, 6 and 24 hrs following injec- tions of zymosan alone or with CBD or Cannabis clone 202 extracts.
2.8. Pain Assay
The hyperalgesia was evaluated by the paw withdrawal von Frey test at 2, 6, and 24 hrs following injections of zymosan and/or the test compounds. In the von Frey nociceptive filament assay, von Frey calibrated monofila- ment hairs of logarithmically incremental stiffness (0.008 – 300 g corresponding to 1.65 – 6.65 log of force). In our study, only 1.4 – 60 g corresponding to 4.17 to 5.88 log of force was used, to test the mouse sensitivity to a mechanical stimulus on the swollen paw. The measurements were performed in a quiet room. Before paw pain measurements, the animals were held for 10 sec. The trained investigator applied the filament to the central area of the hind paw with gradual increasing size. The test consisted of poking the middle of the hind paw to provoke a flexion reflex followed by a clear flinch response after paw withdrawal. Each one of the von Frey filaments was applied for approximately 3 – 4 s to induce the end-point reflex. The first testing was done by using the force filament of 1.4 g. If there was no withdrawal response, the next higher stimulus was tried. The mechanical threshold force (in grams (g)) was defined as the lowest force imposed by two von Frey monofilaments of vari- ous sizes, required to produce a paw retraction. The untreated left hind paw served as a control.
2.9. Tumor Necrosis Factor α (TNFα) Plasma Levels
Plasma levels of TNFα were measured using a mouse TNFα ELISA kit (R&D System), according to the manu-
facturer’s instructions.
2.10. Statistical Analysis
The results are presented as average ± standard error. Mice treated with CBD or Cannabis clone 202 extracts were compared with control mice receiving the vehicle only. Statistical significance was calculated using the ANOVA analysis of variance and Wilcoxon signed-rank test. Differences between the various doses of CBD and clone 202 extracts were analyzed for significance using the repeated measures ANOVA procedure with Post-Hoc test. All tests were 2-tailed and a p-value below 0.05 was considered statistically significant. A mini- mum of three to four animals was used in each treatment group for each experiment unless otherwise stated. Each experiment was performed at least three times. The graphs represent the average of all mice from the three different experiments. Thus, each bar corresponds to the average of 10 – 12 mice for each treatment group, for each time point, unless otherwise stated.
3. Results
3.1. Effect of CBD and CBD Enriched Clone 202 Extract on Inflammation and Hyperalgesia (Pain Sensation)
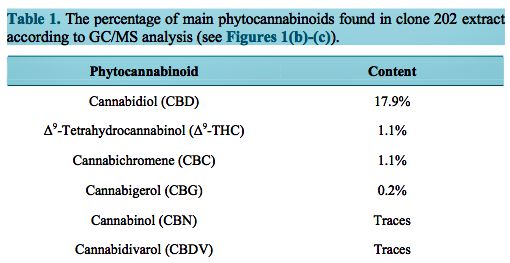
In this study we have used the well accepted mouse model of zymosan-induced inflammation [14] to investigate the anti-inflammatory and antinociceptive activities of Cannabis clone 202 extract versus purified CBD. The extent of hind paw swelling was determined 2, 6 and 24 hrs following paw injection of 60 μg zymosan together with either intraperitoneal injection or per os administration of various amounts of either purified CBD or Cannabis clone 202 extract, as indicated in the graphs (Figure 2, Figure 3). Following intraperitoneal injection of 1, 5, 25 and 50 mg/kg of purified CBD, a bell-shaped dose-response is observed (Figure 2(a)). The maximum inhibition of inflammation occurred after an injection of 5 mg/kg CBD with 50% and 57% inhibition after 6 and 24 hrs, respectively (p < 0.001), while a lower dose (1 mg/kg) being ineffective and higher doses (25 and 50 mg/kg) being less effective with 20% – 25% and 14% – 28% inhibition only, after 6 and 24 hrs, respectively (Figure 2(a)). In accordance with these findings, the anti-nociceptive effect, as determined by the von Frey monofilament assay, peaked at 5 mg/kg CBD (p < 0.001) (Figure 2(c)). The anti-nociceptive effect occurred prior (2 hrs) to inhibition of swelling (6 hrs), and peaked at 6 hrs. Higher concentrations of CBD had less an- ti-nociceptive effects (Figure 2(c)), again getting a bell-shaped dose-response. However, when clone 202 extract was used, a correlative dose-response was observed with increased inhibition of inflammation upon increased doses of the extract, reaching 43% and 64% inhibition at 25 mg and 50 mg, respectively, after 24 hrs (p < 0.001) (Figure 2(b)). These two dosages of clone 202 extract also showed strong anti-nociceptive effects after 6 and 24 hrs (p < 0.001) (Figure 2(d)). Although the anti-inflammatory effect of clone 202 extract was higher at 50 mg/kg than at 25 mg/kg with a p = 0.001, the antinociceptive effect was only slightly higher (p = 0.01), suggesting that a plateau has been reached. The clone 202 extract was more efficient for alleviating the pain than CBD (p = 0.01) (Figure 2(d) versus Figure 2(c)).
Plasma levels of TNFα were measured using a mouse TNFα ELISA kit (R&D System), according to the manu-
facturer’s instructions.
2.10. Statistical Analysis
The results are presented as average ± standard error. Mice treated with CBD or Cannabis clone 202 extracts were compared with control mice receiving the vehicle only. Statistical significance was calculated using the ANOVA analysis of variance and Wilcoxon signed-rank test. Differences between the various doses of CBD and clone 202 extracts were analyzed for significance using the repeated measures ANOVA procedure with Post-Hoc test. All tests were 2-tailed and a p-value below 0.05 was considered statistically significant. A mini- mum of three to four animals was used in each treatment group for each experiment unless otherwise stated. Each experiment was performed at least three times. The graphs represent the average of all mice from the three different experiments. Thus, each bar corresponds to the average of 10 – 12 mice for each treatment group, for each time point, unless otherwise stated.
3. Results
3.1. Effect of CBD and CBD Enriched Clone 202 Extract on Inflammation and Hyperalgesia (Pain Sensation)
In this study we have used the well accepted mouse model of zymosan-induced inflammation [14] to investigate the anti-inflammatory and antinociceptive activities of Cannabis clone 202 extract versus purified CBD. The extent of hind paw swelling was determined 2, 6 and 24 hrs following paw injection of 60 μg zymosan together with either intraperitoneal injection or per os administration of various amounts of either purified CBD or Cannabis clone 202 extract, as indicated in the graphs (Figure 2, Figure 3). Following intraperitoneal injection of 1, 5, 25 and 50 mg/kg of purified CBD, a bell-shaped dose-response is observed (Figure 2(a)). The maximum inhibition of inflammation occurred after an injection of 5 mg/kg CBD with 50% and 57% inhibition after 6 and 24 hrs, respectively (p < 0.001), while a lower dose (1 mg/kg) being ineffective and higher doses (25 and 50 mg/kg) being less effective with 20% – 25% and 14% – 28% inhibition only, after 6 and 24 hrs, respectively (Figure 2(a)). In accordance with these findings, the anti-nociceptive effect, as determined by the von Frey monofilament assay, peaked at 5 mg/kg CBD (p < 0.001) (Figure 2(c)). The anti-nociceptive effect occurred prior (2 hrs) to inhibition of swelling (6 hrs), and peaked at 6 hrs. Higher concentrations of CBD had less an- ti-nociceptive effects (Figure 2(c)), again getting a bell-shaped dose-response. However, when clone 202 extract was used, a correlative dose-response was observed with increased inhibition of inflammation upon increased doses of the extract, reaching 43% and 64% inhibition at 25 mg and 50 mg, respectively, after 24 hrs (p < 0.001) (Figure 2(b)). These two dosages of clone 202 extract also showed strong anti-nociceptive effects after 6 and 24 hrs (p < 0.001) (Figure 2(d)). Although the anti-inflammatory effect of clone 202 extract was higher at 50 mg/kg than at 25 mg/kg with a p = 0.001, the antinociceptive effect was only slightly higher (p = 0.01), suggesting that a plateau has been reached. The clone 202 extract was more efficient for alleviating the pain than CBD (p = 0.01) (Figure 2(d) versus Figure 2(c)).
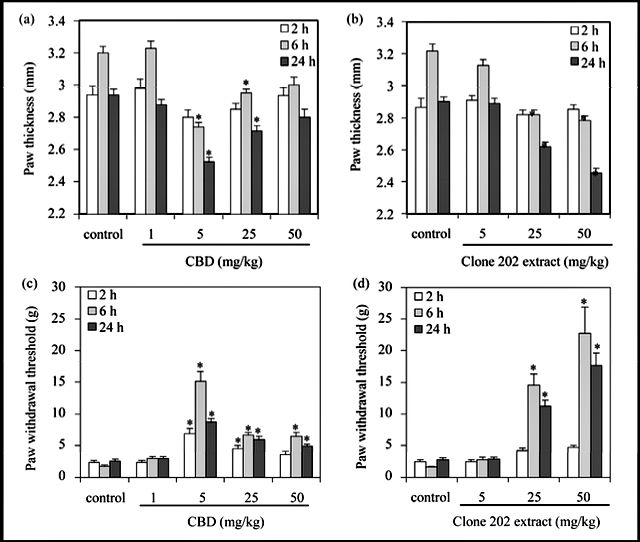
When CBD or Cannabis clone 202 extract was given orally, a similar response was observed. Namely, CBD gives a bell-shaped dose-response with an optimal inhibitory effect at 25 mg/kg (p < 0.001) (Figure 3(a) and Figure 3(c)), whereas Cannabis clone 202 extract provides a correlative dose-response curve with a maximum effect on swelling and pain relief at 50 and 150 mg/kg, respectively (p < 0.001) (Figure 3(b) and Figure 3(d)). Significant pain relief was already obtained with an oral clone 202 extract dose of 50 mg/kg (Figure 3(d)) that corresponds to about 10 mg/kg CBD (Table 1), while 25 mg/kg of purified CBD was needed to achieve the same effect (Figure 3(c)). This suggests for a better usage of clone 202 extract.
It should be noted that agents taken per os need to go through the enterohepatic route prior to exerting their effects, where the absorption rate and first-pass liver metabolism affect the blood drug level [15]. This may explain the higher doses required and the delayed response in comparison with the parenteral route, where the agents are immediately available for the blood circulation. The anti-inflammatory and anti-nociceptive effects peak at 6 hrs, which accords with the pharmacokinetics and pharmacodynamics of cannabinoids described by Grotenhermen [15].
It should be noted that agents taken per os need to go through the enterohepatic route prior to exerting their effects, where the absorption rate and first-pass liver metabolism affect the blood drug level [15]. This may explain the higher doses required and the delayed response in comparison with the parenteral route, where the agents are immediately available for the blood circulation. The anti-inflammatory and anti-nociceptive effects peak at 6 hrs, which accords with the pharmacokinetics and pharmacodynamics of cannabinoids described by Grotenhermen [15].
- Figure 2. Anti-inflammatory and antinociceptive effects of intraperitoneally injected CBD and CBD- enriched clone 202 extract. (a) (b) Prevention of zymosan-induced swelling of hind paw. 1.5% zymo- san in 40 μl was injected into the sub-planter surface of the right hind paw. Immediately thereafter, CBD (a) or Cannabis clone 202 extract (b) was injected intraperitoneally. The paw thickness indica- tive for paw swelling was measured 2, 6 and 24 hrs thereafter. The paw thickness of untreated mice was 2.0 – 2.2 mm, which made the baseline of the graph. N = 12 for each time point. *p < 0.001 com- pared to control mice. p < 0.001 for 50 mg/kg vs 25 mg/kg of clone 202 extract at 24 hrs; (c) (d) Anti- pain effect of CBD (c) and Cannabis clone 202 extract (d). The hyperalgesia was measured by using the von Frey nociceptive filament assay. The higher the paw withdrawal threshold, the higher is the anti-nociceptive effect of the drug. The experiments were repeated three times, each experiment with 4 mice in each treatment group. The graphs presents the average of all mice in the three experiments, meaning that the N = 12 for each time point. The bars represent standard error. *p < 0.001 compared to control mice. p < 0.01 for 50 mg/kg vs 25 mg/kg of clone 202 extract at 24 hrs. p < 0.01 for clone 202 extract vs CBD.
3.2. Suppression of TNFα Production by CBD and Clone 202 Extract
TNFα is a well-known pro-inflammatory cytokine secreted by activated macrophages upon inflammation that has been shown to be involved in initiation and amplification of inflammatory processes that ultimately leads to oedema [16]. Therefore, it was important to analyze the effect of CBD and clone 202 extracts on TNFα produc- tion. To this end, mice sera were analyzed for TNFα concentration by ELISA 24 hrs after treatment with zymosan in the absence or presence of CBD or clone 202 extract. When comparing the TNFα sera level in mice 24 hrs after injection of increasing doses of purified CBD, a bell-shaped dose-response curve of TNFα production was observed, with a maximum inhibitory effect (43%) achieved at 5 mg/kg (p < 0.001), while no inhibition was observed at either lower (1 mg/kg) or higher (25 and 50 mg/kg) doses (Figure 4(a)). In contrast, following injection of CBD-enriched clone 202 extract to mice, a clear dose dependent response was apparent. Increased in- hibition of TNFα production (39%; 46% and 57%, respectively) was observed following injections with increas- ing amounts of extract (5 mg/kg, 25 mg/kg and 50 mg/ml, respectively) with a p value less than 0.001 (Figure 4(b)). Already at 5 mg/kg did clone 202 extract lead to a strong reduction in TNFα production (Figure 4(b)),
TNFα is a well-known pro-inflammatory cytokine secreted by activated macrophages upon inflammation that has been shown to be involved in initiation and amplification of inflammatory processes that ultimately leads to oedema [16]. Therefore, it was important to analyze the effect of CBD and clone 202 extracts on TNFα produc- tion. To this end, mice sera were analyzed for TNFα concentration by ELISA 24 hrs after treatment with zymosan in the absence or presence of CBD or clone 202 extract. When comparing the TNFα sera level in mice 24 hrs after injection of increasing doses of purified CBD, a bell-shaped dose-response curve of TNFα production was observed, with a maximum inhibitory effect (43%) achieved at 5 mg/kg (p < 0.001), while no inhibition was observed at either lower (1 mg/kg) or higher (25 and 50 mg/kg) doses (Figure 4(a)). In contrast, following injection of CBD-enriched clone 202 extract to mice, a clear dose dependent response was apparent. Increased in- hibition of TNFα production (39%; 46% and 57%, respectively) was observed following injections with increas- ing amounts of extract (5 mg/kg, 25 mg/kg and 50 mg/ml, respectively) with a p value less than 0.001 (Figure 4(b)). Already at 5 mg/kg did clone 202 extract lead to a strong reduction in TNFα production (Figure 4(b)),
- Figure 3. Anti-inflammatory and anti-nociceptive effects of CBD and CBD-enriched clone 202 ex- tract administrated per os. (a) (b) Prevention of zymosan-induced swelling of hind paw. 1.5% zymo- san in 40 μl was injected into the sub-planter surface of the right hind paw. Immediately thereafter, CBD (a) or Cannabis clone 202 extract (b) was given per os dissolved in olive oil (40 μl). The paw thickness indicative for paw swelling was measured 2, 6 and 24 hrs thereafter. The paw thickness of untreated mice was 2.0 – 2.2 mm, which made the baseline of the graph. N = 12 for each time point. *p < 0.001 in comparison to control mice. The anti-inflammatory effects of 25, 50, 100 and 150 mg/kg of clone 202 extract were similar; (c) (d) Anti-pain effect of CBD (c) and Cannabis clone 202 extract (d) when given orally. The hyperalgesia was measured by using the von Frey nociceptive filament assay. The higher the paw withdrawal threshold, the higher is the anti-nociceptive effect of the drug. The experiments were repeated three times, each experiment with 4 mice in each treatment group. The graphs presents the average of all mice in the three experiments, meaning that the N = 12 for each time point. The bars represent standard error. *p < 0.001 in comparison to control mice. p < 0.001 for 50 mg/kg clone 202 extract (containing 8.9 mg/kg CBD) vs 10 mg/kg purified CBD. p < 0.05 of 100 mg/kg and 150 mg/kg vs 50 mg/kg of clone 202 extract at 6 hrs, indicating a dose- dependent effect.
even though this dose was insufficient in reducing paw swelling (Figure 2(b)) or relieve pain (Figure 2(d)). At least 25 mg/kg extract, which corresponds to about 5 mg CBD, was required to achieve the anti-inflammatory effect. These data show that TNFα secretion is more sensitive to inhibition by clone 202 extract, than paw swel- ling and pain.
Similar to the results obtained with intraperitoneal injection, orally administrated CBD gave a bell-shape response, with an optimal response using 25 mg/kg (p < 0.001), while higher or lower doses had less effect (Figure 4(c)). In contrast, orally delivered clone 202 extract showed an increased inhibitory effect on TNFα production with increased doses (Figure 4(d)). Already at 25 mg/kg an inhibition of 48% was achieved that in- creased further to 66% when given 150 mg/kg clone 202 extract (Figure 4(d)). The inhibition of TNF produc- tion was much stronger than the inhibitory effect on paw swelling of 27% – 35%.
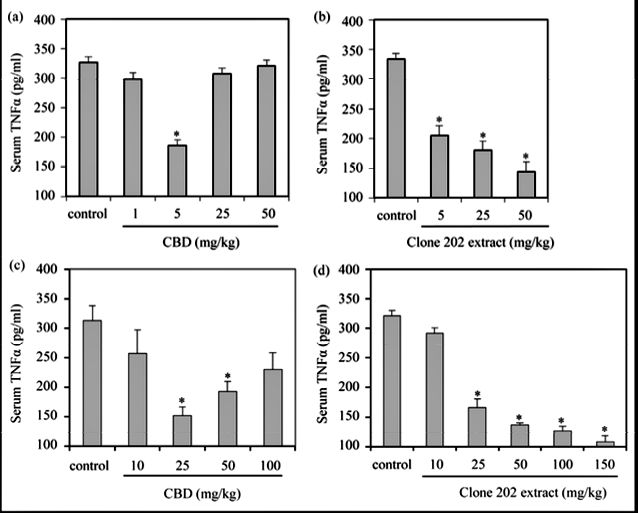
- Figure 4. Prevention of zymosan-induced TNFα production by purified CBD and clone 202 extract. (a) (b) Twenty four hours after injecting zymosan and an intraperitoneal dose of CBD (a) or clone 202 extract (b), or a per os dose of CBD (c) or clone 202 extract (d), the TNFα concentration in the serum was determined by ELISA. The experiments were repeated three times, each experiment with 4 mice in each treatment group. The graphs presents the average of all mice in the three experiments, meaning an N = 12 for each treatment. TNFα serum level of untreated mice was 15 pg/ml. The bars represent standard error. *p < 0.001 in comparison to control mice. p < 0.01 when comparing clone 202 extract with purified CBD. p < 0.01 when comparing an increasing doses of clone 202 extract, emphasizing a dose-dependent effect.
3.3. Comparison of CBD and Cannabis Clone 202 Extract with Commercial Anti‐Nociceptive and Anti‐Inflammatory Drugs
Since Cannabis clone 202 extract has profound anti-inflammatory and anti-nociceptive effects as described above, it was important to compare its potency with commercial anti-nociceptive and anti-inflammatory drugs. We chose to use tramadol, a strong atypical opioid analgesic drug, and aspirin, a well-known non-steroid anti-inflammatory drug (NSAID) that is also a pain reliever. Immediately after zymosan injection, mice were treated with aspirin (50 mg/kg per os), tramadol (5 mg/kg i.p.), CBD (5 mg/kg i.p.) or clone 202 extract (50 mg/kg i.p.). While aspirin had a moderate effect on paw swelling (p < 0.001 at 6 h), tramadol barely had any effect (Figure 5(a)). Both CBD and clone 202 extract markedly prevented paw swelling to a much larger extent than aspirin (p < 0.005) (Figure 5(a)). As expected, aspirin and tramadol had a strong anti-nociceptive effect that exceeded that of CBD and clone 202 extract (p < 0.01) (Figure 5(b)). Aspirin, but not tramadol, showed a slight inhibitory ef- fect on TNFα production, that was negligible in comparison to the strong inhibitory effect of CBD and clone 202 extract (p < 0.01) (Figure 5(c)). Thus, CBD and clone 202 extract are endowed with different traits than aspirin and tramadol, making them superior with respect to anti-inflammatory properties.
4. Discussion
In this manuscript we have observed different dose-response patterns when using purified CBD or plant extract
Since Cannabis clone 202 extract has profound anti-inflammatory and anti-nociceptive effects as described above, it was important to compare its potency with commercial anti-nociceptive and anti-inflammatory drugs. We chose to use tramadol, a strong atypical opioid analgesic drug, and aspirin, a well-known non-steroid anti-inflammatory drug (NSAID) that is also a pain reliever. Immediately after zymosan injection, mice were treated with aspirin (50 mg/kg per os), tramadol (5 mg/kg i.p.), CBD (5 mg/kg i.p.) or clone 202 extract (50 mg/kg i.p.). While aspirin had a moderate effect on paw swelling (p < 0.001 at 6 h), tramadol barely had any effect (Figure 5(a)). Both CBD and clone 202 extract markedly prevented paw swelling to a much larger extent than aspirin (p < 0.005) (Figure 5(a)). As expected, aspirin and tramadol had a strong anti-nociceptive effect that exceeded that of CBD and clone 202 extract (p < 0.01) (Figure 5(b)). Aspirin, but not tramadol, showed a slight inhibitory ef- fect on TNFα production, that was negligible in comparison to the strong inhibitory effect of CBD and clone 202 extract (p < 0.01) (Figure 5(c)). Thus, CBD and clone 202 extract are endowed with different traits than aspirin and tramadol, making them superior with respect to anti-inflammatory properties.
4. Discussion
In this manuscript we have observed different dose-response patterns when using purified CBD or plant extract
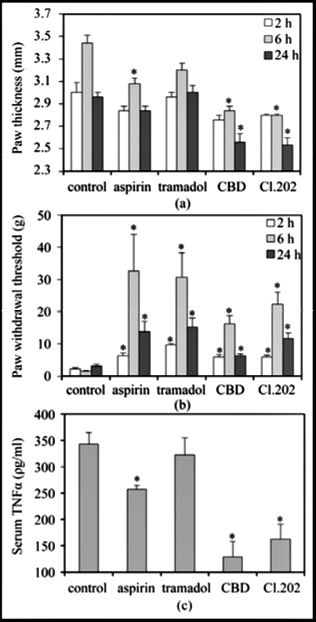
- Figure 5. Comparison of anti-inflammatory and anti-nociceptive effects of CBD and Cannabis clone 202 extract with the commercial drugs aspirin and tramadol. (a) Prevention of zymo- san-induced swelling of hind paw. 1.5% zymosan in 40 μl was injected into the sub-planter surface of the right hind paw. Immediately thereafter, aspirin (50 mg/kg per os), tramadol (5 mg/kg i.p.), CBD (5 mg/kg i.p.) or Cannabis clone 202 extract (50 mg/kg i.p.) was given. The paw thickness indicative for paw swelling was measured 2, 6 and 24 hrs later. The paw thickness of untreated mice was 2.0 – 2.2 mm, which made the baseline of the graph. N = 5 for each time point of each treatment group. *p < 0.001 in comparison to control mice. p < 0.005 when com- paring CBD and clone 202 extract with aspirin and tramadol; (b) Anti-pain effect of aspirin, tramadol, CBD and Cannabis clone 202 extract in mice treated as described in paragraph A. The hyperalgesia was measured by using the von Frey nociceptive filament assay. The higher the paw withdrawal threshold, the higher is the anti-nociceptive effect of the drug. N = 5 for each time point of each treatment group. The bars represent standard error. *p < 0.001 in comparison to control mice. p < 0.05 when comparing CBD and clone 202 extract with aspirin and tramadol; (c) The TNFα serum concentration at 24 hrs in mice that were treated as described in paragraph A. N = 5 for each treatment. The bars represent standard error. *p < 0.001 in comparison to control mice. p < 0.01 when comparing CBD and clone 202 extract with aspirin and tramadol.
of the Cannabis sativa L. clone 202, which is highly enriched in CBD. Purified CBD showed a bell-shaped dose-response, where a therapeutic response could only be achieved at a certain concentration. This narrow therapeutic window makes it difficult to use CBD in the clinics as a single agent. Therefore, we sought for a better preparation that can utilize the favorable therapeutic effects of CBD. We observed that plant extracts of the non-psychotropic clone 202 could fit this aim. A dose-dependent response was observed on all three parameters tested: namely, the extract prevented zymosan-induced paw oedema, zymosan-induced pain and zymosan- induced TNFα production in mice, with an improved therapeutic effect upon increased dosages. Thus, the limitation with purified CBD could be overcome when presented together with other natural components of the plant. Of note, TNFα secretion was more sensitive to clone 202 extract inhibition than paw swelling and pain.
Our finding that it is possible to get a correlative dose-response using Cannabis clone 202 extracts, makes it possible to use it in many pathological conditions. We suggest that clone 202 extracts may be a suitable substi- tute for the current used Cannabis strain in the clinics, especially taking into account that it does not have any psychotropic adverse effects. Following the clinical improvement by the clone 202 extracts, more tedious experiments with CBD might be planned.
Our findings that CBD in the presence of other plant constituents improve the dose-response are supported by some recent reports showing that CBD in a standardized Cannabis sativa extract is more potent or efficacious than pure CBD [17]-[19]. These research groups studied the anti-proliferative effect of CBD on tumor cells [17] [19] and the inhibitory effect of CBD on bladder contractility [18]. The higher efficiency of plant extract might be explained by additive or synergistic interactions between CBD and minor phytocannabinoids or non-canna- binoids presented in the extracts. Other phytocannabinoids, including Tetrahydrocannabivarin, Cannabigerol and Cannabichromene, exert additional effects of therapeutic interest [20]. A lot of research has been made to isolate and characterize isolated single constituents of traditional herbal medicine to find their rationale for therapeutic uses. However, our data together with those of others [21] provide legitimation to introduce a new generation of phytopharmaceuticals to treat diseases that have hitherto been treated using synthetic drugs alone. The therapeutic synergy observed with plant extracts results in the requirement for a lower amount of active components, with consequent reduced adverse effects.
5. Conclusion
In conclusion, we recommend standardized plant extract of the Cannabis clone 202 for treatment of various inflammatory conditions.
Acknowledgements
The authors would like to thank Dr. Ronit Sionov for her valuable editorial assistance.
Conflict of Interest
Prof. Ruth Gallily has been a consultant for Tikun Olam since 2013, and has received a research grant during the years 2012-2014 from Tikun Olam, Israel. There is no conflict of interest.
References
Our findings that CBD in the presence of other plant constituents improve the dose-response are supported by some recent reports showing that CBD in a standardized Cannabis sativa extract is more potent or efficacious than pure CBD [17]-[19]. These research groups studied the anti-proliferative effect of CBD on tumor cells [17] [19] and the inhibitory effect of CBD on bladder contractility [18]. The higher efficiency of plant extract might be explained by additive or synergistic interactions between CBD and minor phytocannabinoids or non-canna- binoids presented in the extracts. Other phytocannabinoids, including Tetrahydrocannabivarin, Cannabigerol and Cannabichromene, exert additional effects of therapeutic interest [20]. A lot of research has been made to isolate and characterize isolated single constituents of traditional herbal medicine to find their rationale for therapeutic uses. However, our data together with those of others [21] provide legitimation to introduce a new generation of phytopharmaceuticals to treat diseases that have hitherto been treated using synthetic drugs alone. The therapeutic synergy observed with plant extracts results in the requirement for a lower amount of active components, with consequent reduced adverse effects.
5. Conclusion
In conclusion, we recommend standardized plant extract of the Cannabis clone 202 for treatment of various inflammatory conditions.
Acknowledgements
The authors would like to thank Dr. Ronit Sionov for her valuable editorial assistance.
Conflict of Interest
Prof. Ruth Gallily has been a consultant for Tikun Olam since 2013, and has received a research grant during the years 2012-2014 from Tikun Olam, Israel. There is no conflict of interest.
References
- [1] Hazekamp, A., Ware, M.A., Muller-Vahl, K.R., Abrams, D. and Grotenhermen, F. (2013) The Medicinal Use of Cannabis and Cannabinoids—An International Cross-Sectional Survey on Administration Forms. Journal of Psychoactive Drugs, 45, 199-210. http://dx.doi.org/10.1080/02791072.2013.805976
- [2] Mechoulam, R. (2012) Cannabis—A Valuable Drug That Deserves Better Treatment. Mayo Clinic Proceedings, 87, 107-109. http://dx.doi.org/10.1016/j.mayocp.2011.12.002
- [3] Greydanus, D.E., Hawver, E.K., Greydanus, M.M. and Merrick, J. (2013) Marijuana: Current Concepts. Frontiers in Public Health, 1, 42. http://dx.doi.org/10.3389/fpubh.2013.00042
- [4] Syed, Y.Y., McKeage, K. and Scott, L.J. (2014) Delta-9-Tetrahydrocannabinol/Cannabidiol (Sativex®): A Review of Its Use in Patients with Moderate to Severe Spasticity Due to Multiple Sclerosis. Drugs, 74, 563-578. http://dx.doi.org/10.1007/s40265-014-0197-5
- [5] Brenneisen, R. (2007) Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents. Marijuana and the Cannabinoids, Chapter 2, 17-49. http://dx.doi.org/10.1007/978-1-59259-947-9_2
- [6] Pertwee, R.G. (2008) The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: Delta9-Tetra- hydrocannabinol, Cannabidiol and Delta9-Tetrahydrocannabivarin. British Journal of Pharmacology, 153, 199-215. http://dx.doi.org/10.1038/sj.bjp.0707442
- [7] Pacher, P. and Mechoulam, R. (2011) Is Lipid Signaling through Cannabinoid 2 Receptors Part of a Protective System? Progress in Lipid Research, 50, 193-211. http://dx.doi.org/10.1016/j.plipres.2011.01.001
- [8] Malfait, A.M., Gallily, R., Sumariwalla, P.F., Malik, A.S., Andreakos, E., Mechoulam, R. and Feldmann, M. (2000) The Nonpsychoactive Cannabis Constituent Cannabidiol Is an Oral Anti-Arthritic Therapeutic in Murine Collagen-In- duced Arthritis. Proceedings of the National Academy of Sciences USA, 97, 9561-9566.
[9] Mechoulam, R., Peters, M., Murillo-Rodriguez, E. and Hanus, L.O. (2007) Cannabidiol—Recent Advances. Chemistryhttp://dx.doi.org/10.1073/pnas.160105897
& Biodiversity, 4, 1678-1692. http://dx.doi.org/10.1002/cbdv.200790147- [10] Weiss, L., Zeira, M., Reich, S., Slavin, S., Raz, I., Mechoulam, R. and Gallily, R. (2008) Cannabidiol Arrests Onset of Autoimmune Diabetes in NOD Mice. Neuropharmacology, 54, 244-249. http://dx.doi.org/10.1016/j.neuropharm.2007.06.029
- [11] Kozela, E., Lev, N., Kaushansky, N., Eilam, R., Rimmerman, N., Levy, R., Ben-Nun, A., Juknat, A. and Vogel, Z. (2011) Cannabidiol Inhibits Pathogenic T Cells, Decreases Spinal Microglial Activation and Ameliorates Multiple Sclerosis-Like Disease in C57BL/6 Mice. British Journal of Pharmacology, 163, 1507-1519. http://dx.doi.org/10.1111/j.1476-5381.2011.01379.x
- [12] Esposito, G., Filippis, D.D., Cirillo, C., Iuvone, T., Capoccia, E., Scuderi, C., Steardo, A., Cuomo, R. and Steardo, L. (2013) Cannabidiol in Inflammatory Bowel Diseases: A Brief Overview. Phytotherapy Research, 27, 633-636. http://dx.doi.org/10.1002/ptr.4781
- [13] Jamontt, J.M., Molleman, A., Pertwee, R.G. and Parsons, M.E. (2010) The Effects of Delta-Tetrahydrocannabinol and Cannabidiol Alone and in Combination on Damage, Inflammation and in Vitro Motility Disturbances in Rat Colitis. British Journal of Pharmacology, 160, 712-723. http://dx.doi.org/10.1111/j.1476-5381.2010.00791.x
- [14] Gadó, K. and Gigler, G. (1991) Zymosan Inflammation: A New Method Suitable for Evaluating New Anti-Inflamma- tory Drugs. Agents and Actions, 32, 119-121. http://dx.doi.org/10.1007/BF01983335
- [15] Grotenhermen, F. (2003) Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clinical Pharmacokinetics, 42, 327-360. http://dx.doi.org/10.2165/00003088-200342040-00003
- [16] Rocha, A.C., Fernandes, E.S., Quintao, N.L., Campos, M.M. and Calixto, J.B. (2006) Relevance of Tumour Necrosis Factor-Alpha for the Inflammatory and Nociceptive Responses Evoked by Carrageenan in the Mouse Paw. British Journal of Pharmacology, 148, 688-695. http://dx.doi.org/10.1038/sj.bjp.0706775
- [17] Romano, B., Borrelli, F., Pagano, E., Cascio, M.G., Pertwee, R.G. and Izzo, A.A. (2014) Inhibition of Colon Carcino- genesis by a Standardized Cannabis Sativa Extract with High Content of Cannabidiol. Phytomedicine, 21, 631-639. http://dx.doi.org/10.1016/j.phymed.2013.11.006
- [18] Capasso, R., Aviello, G., Borrelli, F., Romano, B., Ferro, M., Castaldo, L., Montanaro, V., Altieri, V. and Izzo, A.A. (2011) Inhibitory Effect of Standardized Cannabis Sativa Extract and Its Ingredient Cannabidiol on Rat and Human Bladder Contractility. Urology, 77, 1006.e9-1006e15. http://dx.doi.org/10.1016/j.urology.2010.12.006
- [19] De Petrocellis, L., Ligresti, A., Schiano Moriello, A., Iappelli, M., Verde, R., Stott, C.G., Cristino, L., Orlando, P. and Di Marzo, V. (2013) Non-THC Cannabinoids Inhibit Prostate Carcinoma Growth in Vitro and in Vivo: Pro-Apoptotic Effects and Underlying Mechanisms. British Journal of Pharmacology, 168, 79-102. http://dx.doi.org/10.1111/j.1476-5381.2012.02027.x
- [20] Russo, E.B. (2011) Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. British Journal of Pharmacology, 163, 1344-1364. http://dx.doi.org/10.1111/j.1476-5381.2011.01238.x
- [21] Wagner, H. and Ulrich-Merzenich, G. (2009) Synergy Research: Approaching a New Generation of Phytopharmaceu- ticals. Phytomedicine, 16, 97-110. http://dx.doi.org/10.1016/j.phymed.2008.12.018
-
Download as PDF – Scientific Research Publishing

