
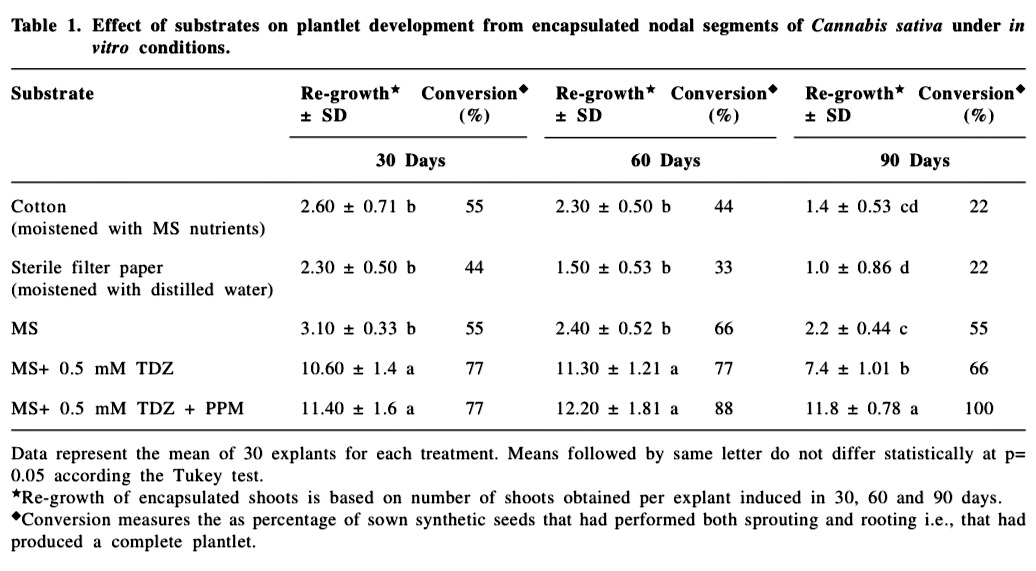
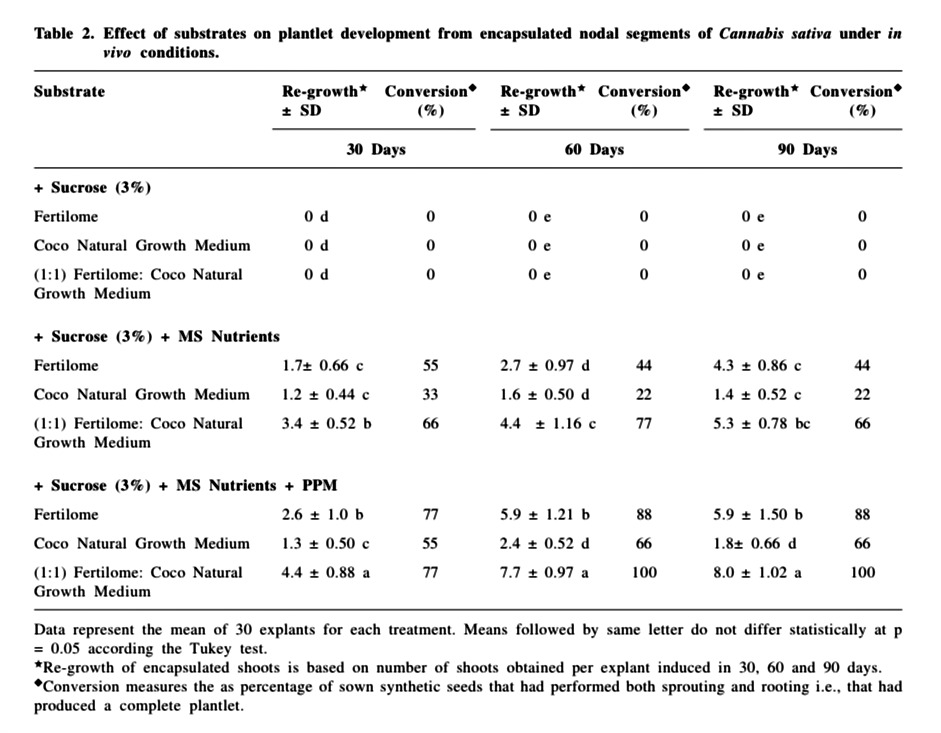
Abstract
Cannabis sativa L. (Cannabaceae) is an important medicinal plant well known for its pharmacologic and therapeutic potency. Because of allogamous nature of this species, it is difficult to maintain its potency and efficacy if grown from the seeds. Therefore, chemical profile-based screening, selection of high yielding elite clones and their propagation using biotechnological tools is the most suitable way to maintain their genetic lines. In this regard, we report a simple and efficient method for the in vitro propagation of a screened and selected high yielding drug type variety of Cannabis sativa, MX-1 using synthetic seed technology. Axillary buds of Cannabis sativa isolated from aseptic multiple shoot cultures were successfully encapsulated in calcium alginate beads. The best gel complexation was achieved using 5 % sodium alginate with 50 mM CaCl2.2H2O. Regrowth and conversion after encapsulation was evaluated both under in vitro and in vivo conditions on different planting substrates. The addition of antimicrobial substance — Plant Preservative Mixture (PPM) had a positive effect on overall plantlet development. Encapsulated explants exhibited the best regrowth and conversion frequency on Murashige and Skoog medium supplemented with thidiazuron (TDZ 0.5 μM) and PPM (0.075 %) under in vitro conditions. Under in vivo conditions, 100 % conversion of encapsulated explants was obtained on 1:1 potting mix- fertilome with coco natural growth medium, moistened with full strength MS medium without TDZ, supplemented with 3 % sucrose and 0.5 % PPM. Plantlets regenerated from the encapsulated explants were hardened off and successfully transferred to the soil. These plants are selected to be used in mass cultivation for the production of biomass as a starting material for the isolation of THC as a bulk active pharmaceutical.
Cannabis has acquired considerable importance as a medicinal crop all over the world. Indeed its medical use predates recorded history. The earliest written reference is to be found in the fifteenth century B.C., Chinese Pharmacopeia. Between 1840 and 1900, more than 100 articles on the therapeutic use of Cannabis were published in medical journals (Grinspoon and Bakalar, 1995). As a plant it is valued for its hallucinogenic and medicinal properties and has also been used to treat a variety of ailments including pain, glaucoma, nausea, asthma, depression, insomnia and neuralgia (Mechoulam et al., 1976; Duke and Wain, 1981). The therapeutic values of Cannabis derivatives have also been highlighted against HIV/AIDS (Abrams et al., 2007) and multiple sclerosis (Pryce and Baker, 2005). The pharmacologic and therapeutic potency of preparations of Cannabis sativa L. and it’s main active constituent Δ9-tetrahydrocannabinol (THC) has been extensively reviewed (Sirikantaramas et al., 2007; Long et al., 2005; Brenneisen et al., 1996; Mattes et al., 1994).
On other hand, Cannabis is one of the oldest sources of textile fiber. Hemp grown for fiber was introduced in western Asia and Egypt, and subsequently to Europe between 1000 and 2000 BCE. Cultivation of hemp in Europe became widespread after 500 CE. The crop was first brought to South America in 1545, in Chile, and to North America in Port Royal, Acadia in 1606 (Small and Marcus, 2002).
This extraordinary versatile important plant material naturally calls for development of suitable protocols for production of sufficient number of uniform planting materials from ‘elite’ Cannabis varieties through biotechnological intervention. Among the biotechnological approaches, micropropagation is one of the most viable techniques, allows efficient and rapid clonal propagation of many economically important crops. Since C. sativa is a cross pollinated crop, the allogamous (cross fertilization) nature of species makes it impossible to maintain elite cultivar/clones by seed. Synthetic seed technology can be considered an important application of micropropagation, that could be a valuable aid to economical large-scale clonal propagation and germplasm conservation of the screened and selected elite germplasm of C. sativa.
According to Bornman (1993), synthetic seeds may provide the only technology realistically amenable to the extensive scale up required for the commercial production of some clones. In addition, Mathur et al. (1989) reported that the use of this technology economized upon the medium, space and time requirements. Successful cases of synthetic seeds production and plantlet regeneration have been reported for cereals, vegetables, fruits, ornamentals, aromatic grass and conifers (Fowke et al., 1994; Piccioni and Standardi, 1995; Castillo et al., 1998; Ganapathi et al., 2001; Brischia et al., 2002; Hao and Deng, 2003). However, in most cases somatic embryos were used in the encapsulation process. Some authors (Mathur et al., 1989; Ganapathi et al., 1992; Sharma et al., 1994; Piccioni and Standardi, 1995; Pattnaik and Chand, 2000) described the encapsulation of vegetative propagules such as axillary buds or shoot tips, which could be used for mass clonal propagation as well as in long- term conservation of germplasm.
Although there have been few reports on tissue culture of C. sativa (Richez- Dumanois et al., 1986; Mandolino and Ranalli, 1999; Slusarkiewicz-Jarzina et al., 2005; Bing et al., 2007), no reports are available on in vitro propagation using synthetic seed technology. In our study, the possibility of using the non- embryogenic, in vitro-derived vegetative propagules for encapsulation has been explored. The strategy used is of particular significance for a plant like Cannabis, in which the process of somatic embryogenesis has not been documented, and therefore somatic embryos are not available for the production of synthetic seeds. This study reports a high frequency propagation of encapsulated axillary buds of a high yielding elite Mexican variety (MX-1) of C. sativa in calcium alginate gel, and their morphogenic response to various planting media.
MATERIALS AND METHODS
Plant material
Explants of nodal segements containing axillary buds, were excised from multiple shoot cultures of high yielding C. sativa variety (MX-1) maintained on Murashige and Skoog’s medium (Murashige and Skoog, 1962) containing 3 % (w/v) sucrose, 0.8 % (w/v) Type E Agar suppplemented with TDZ (0.5 μM) adjusted to pH 5.7 (Lata et al., 2008, accepted) The subculturing was done every four weeks on sterile medium dispensed (25 ml) in glass culture vessels (4 cm diameter x 9.5 cm high, baby food jars with magenta B caps).
Encapsulation Procedure
Encapsulation Matrix : Sodium alginate was added in the range of 2-6 % (w/v) to full strength Murashige and Skoog’s medium (MS) with or without 3 % sucrose. The solutions were supplemented with 0.5 μM Thidiazuron (TDZ) and 2.5 μM indole-3-butyric acid (IBA). A broad spectrum fungicide, Plant preservative mixture (PPM) in a range of 0.3-0.5 % was added to the gel matrix for in vivo experiments. For complexation, different concentrations (25-100 mM) of complexing agent (CaCl2.2H2O) were prepared in liquid MS medium containing the same adjuvents as the sodium alginate matrix but excluding the PPM. Both the solutions were autoclaved separately for 15 min at a pressure of 1.1 kg cm-2 and temperature of 121 oC after adjusting the pH to 5.8.
Formation of beads : The beads were formed by dropping explants mixed with sodium alginate solution into CaCl2.2H2O in a flask, placed on an orbital shaker at 80 rpm. The resulting beads (0.5-0.8 cm in diameter) containing the entrapped nodal segments were left in the calcium chloride solution for 30 min for complexation. These were retrieved using a nylon mesh and the traces of calcium chloride was removed by washing with sterilized distilled water. The encapsulated nodal explants now called as synthetic seed / beads were inoculated onto the different planting substrata for growth and development studies as detailed in results (Fig. 1A).
Planting substrata and culture conditions for in vitro germination
For in vitro experiments, in order to assess the germination potential, the synthetic seeds prepared were separately placed on these media viz. (a) cotton moistened with Murashige and Skoog’s medium, (b) sterile filter paper moistened with distilled water at room temperature, (c) Murashige and Skoog’s medium (MS) (Murashige and Skoog, 1962), (d) MS supplemented with cytokinin, Thidiazuron (TDZ) (0.5 μM), (e) MS supplemented with TDZ (0.5 μM) and Plant preservative mixture (PPM) (Caisson laboratories, USA) (0.075 %). Each treatment consisted of thirty beads and was repeated two times. Beads (10±1) were inoculated in glass culture vessels (4 cm diameter x 9.5 cm high, baby food jars with magenta B caps) jars and cultures were maintained at 25 ± 2 °C and 60-70 % humidity incubated with 16 h photoperiod under fluorescent light with a photon flux of 52 μmol m-2 s-1. After sprouting the encapsulated shoot buds were transferred to baby food jars with magenta B caps (two sprouted buds per flask) containing the same medium as that on which the encapsulated buds were sprouted. Three week-old shoots with fully expanded leaves emerging through the ruptured capsule were placed in half-strength MS medium fortified with 2.5 μM IBA concentrations to trigger root development. A set of non-encapsulated axillary buds were plated on the media described above and maintained under similar in order to serve as a control.
Planting substrata and culture conditions for in vivo germination
For in vivo experiments, the synthetic seeds were directly placed in a pot containing substrates (marketed by Canna Continental, Los angeles, CA, www. canna-hydroponics. com) – non sterile potting mix-fertilome, coco natural growth medium and 1:1 potting mix- fertilome with coco natural growth medium. The substrates were divided into three groups,
1) Each moistened with full strength MS medium without TDZ, supplemented with 3 % sucrose, 2) Each moistened with full strength MS medium + TDZ without 3 % sucrose, 3) Each moistened with full strength MS medium without TDZ supplemented with 3 % sucrose and 0.5 % PPM (Table 2). Each treatment had 30 beads and was repeated two times. The substrates were autoclaved prior to use. Encapsulated buds entrapped in calcium alginate capsules were plated onto the media, which were contained in pots that were placed in an indoor cultivation facility housed at Coy-Waller laboratory, University of Mississippi, at a temperature ranging from 25-30 °C. In order to maintain high humidity around the beads, they were covered with polyethylene sheets until the emergence of shoots and roots (7-14 days). A set of non-encapsulated axillary buds were plated onto the same substrates and maintained under similar conditions in order to serve as a control.
Data analysis
A total of ninety explants were inoculated for each replicate per treatment. Each replicate consisted of thirty explants removed at 30 day interval during the growing period of 90 days. After 30, 60 and 90 days, regrowth and conversion of plantlets were evaluated. Regrowth of encapsulated shoots is based on number of shoots obtained per explant induced in 30, 60 and 90 days. Conversion was considered as percentage of sown synthetic seeds that had performed both sprouting and rooting i.e., that had produced a complete plantlet. The data were submitted to statistical analyses by analysis of variance followed by the Tukey test with the level of significance set at 5 % using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Transfer of plants to soil
Rooted shoots were carefully taken out of the medium and washed thoroughly in running tap water to remove all traces of medium attached to the roots without damaging the roots. Plantlets were preincubated in fertilome in thermocol cups (marketed by Wal-mart Stores, Inc.) for 10 days and irrigated with tap water supplemented with 0.5 % PPM. The cups were covered with polythene bags to maintain humidity and kept in growroom and later acclimatized in non sterile 1:1 potting mix- fertilome with coco natural growth medium irrigated with regular tap water. All these plantlets were kept under similar environmental conditions grown in an indoor cultivation facility housed at Coy-Waller laboratory, University of Mississippi. Light was provided with full spectrum 1000 watts HID (high intensity discharge) lamps (Sun Systems, CA) hung on the top of plants. A hot air suction fan was attached and about 3 to 4 feet distance between plants and bulb was maintained to avoid heating due to HID bulbs. Using an automatic electric timer artificial day/night cycle was regulated with a 16 h photoperiod. Growroom temperature and relative humidity was kept at nearly 25- 30 °C and 60 % respectively.
RESULTS AND DISCUSSION
Characteristics of encapsulated buds
About 220-250 characteristic beads (hydrogels) were formed using 100 ml of sodium alginate and 200 ml CaC12.2H20 solution. The beads differed morphologically with respect to texture, shape and transparency, with different combinations of sodium alginate and calcium chloride, and with diameters ranging from 4.5 to 6.2 mm. An encapsulation matrix of 5 % sodium alginate with 50 mm CaC12.2H20 with an ion exchange duration of 30 min, was most suitable for the formation of ideal beads (Fig. 1). Lower concentration of Na alginate (2-4 %) resulted in the formation of too soft beads that were difficult to handle, while at higher concentrations the beads were iso-diametric but were hard enough to cause considerable delay in sprouting. The reduction in the gelling ability of lower concentrations of Na alginate after exposure to high temperatures during autoclaving has been reported by Larkin et al. (1988). The use of agar as a gel matrix was deliberately avoided because it has been described as inferior to alginate with respect to long-term storage (Bapat et al., 1987). Lower levels of CaC12.2H20 prolonged the complexation time whereas higher concentrations adversely affected the bead quality. Therefore, synthetic seeds formed with 5 % sodium alginate with 50 mm CaC12.2H20 for 30 min were used in all the subsequent experiments.
The encapsulated nodal segments containing axillary buds were cultured on different substrates and the effect of these substrates was evaluated both under in vitro and in vivo conditions (Table 1 & 2). The most desirable feature of the encapsulated explants is their capability to retain viability in terms of regrowth and conversion abilities after encapsulation (Micheli et al., 2007; Adriani et al., 2000). In the present investigation, regrowth (defined as the number of shoots obtained per explant induced in 30, 60 and 90 days) and conversion (defined as the percentage of sown synthetic seeds that had performed both sprouting and rooting i.e., that had produced a complete plantlet) were the parameters used to measure the plantlet development. Differences in regrowth and conversion were observed after different treatments of the synthetic seeds.

Fig. 1. Propagation of Cannabis sativa through encapsulated nodal segments containing axillary buds. A. Calcium alginate encapsulated axillary buds, B&C. Rupturing of synthetic seeds showing proliferation of encapsulated axillary buds, D. Conversion- showing sprouting and rooting. E&F. Regenerated plant and a fully grown hardened micropropagated plant on 1:1 potting mix- fertilome with coco natural growth medium.
Under in vitro conditions, encapsulated explants exhibited shoot development on each of the five planting substrates. Shoot development was induced within 6-8 days on substrates, MS medium supplemented with 0.5 μM TDZ alone and MS medium supplemented with 0.5 μM TDZ and antimicrobial Plant Preservative Mixture (PPM) (Fig. 1B & C). This period was longer (15-20 days) in cotton, filter paper and MS medium alone. The effect of the addition to the bead of PPM did not compromise with the conversion of the encapsulated explants sown in in vitro conditions. The addition of PPM had a positive effect on overall plantlet development. The regrowth and conversion were observed maximum in the substrate containing PPM (Table 1, Fig. 1D). Roots developed from the sprouted buds within 21 days of culture. The encapsulated explants cultured on cotton and filter paper showed the emergence of weak shoots and few with no roots, which failed to continue and died immediately. Similar results have been obtained by Ganapathi et al., (2001) who encapsulated somatic embryos of banana and cultured them on different media (MS supplemented with different growth regulators) and substrates (cotton, soilrite and blotting paper) moistened with 1⁄4 strength liquid MS medium. They reported that the frequency of conversion on these substrates (20 % on cotton and soil rite) was much lower than on nutrient MS medium (66 %). On the contrary, non-encapsulated explants of the same size (used as control) lengthened the sprouting time to 30- 35 days with retarded (less than 2.0-2.5 cm height) growth of plantlets as compared to those developed from encapsulated explants, which grew to a height of 5.0-6.0 cm with 3-4 nodes and a well developed root system within 3 weeks of culture.
In vivo germination
Under in vivo conditions, the synthetic beads moistened with full strength MS medium without plant growth regulators (TDZ) supplemented with 3 % sucrose, when placed directly in pots containing non sterile fertilome, coco natural growth medium or 1:1 potting mix- fertilome with coco natural growth medium, did not form plantlets and showed severe fungal and bacterial contamination. When the substrates were devoid of 3 % sucrose but was moistened with only MS nutrients, the conversion up to 90 days was to the extent of 66 % on 1:1 potting mix-fertilome with coco natural growth medium, 44 % on fertilome and 22 % on coco natural growth medium.
Consequently the addition of PPM (0.5 %) to the three substrates resulted in remarkable improvement in regrowth and conversion frequency. PPM is a heat stable biocide compound, effective against a wide spectrum of common contaminants (Guri and Patel, 1998). Similar non phytotoxic effects of PPM have been reported by Micheli et al. (2002) with encapsulated in vitro derived apical buds of apple rootstock. Mathur et al. (1989) and Pattnaik et al., 1995 have also reported beneficial role of antimicrobial agents, for in vivo germination. Since the most important requirement for synthetic seeds to be used for mass clonal propagation is high and uniform germination under non-sterile conditions; e.g., nursery bed in a greenhouse or in field (Fujii et al., 1993; Onishi et al., 1994), our results show that over a period of 90 days, 100 % conversion was obtained on 1:1 potting mix- fertilome with coco natural growth medium, 88 % on fertilome and 66 % on coco natural growth medium (Table 2). Visual observation after sowing the synthetic seeds showed the necessity of irrigating the substrate once every 2 days, because fertilome and coco natural growth medium tended to dry very quickly. The non encapsulated buds (control) inoculated on the above planting substrates turned necrotic within 6-7 days and failed to germinate. Bapat and Rao, (1990), under non-aseptic conditions, encapsulated axillary buds of mulberry in autoclaved alginate matrix MS medium without sucrose and reported that 60 % of the beads showed shoot emergence.
Plants regenerated from the encapsulated explants of C. sativa were hardened off and were successfully transferred to thermocol cups containing potting mix and new growths were observed after 2 weeks (Fig. 1E). The plants attained 14-16 cm height within 6 weeks of transfer (Fig. 1F). These plants exhibited 100 % survival rate 8 weeks after transfer. The acclimatized plants exhibited normal development and no gross morphological variation was observed.
Furthermore our results in the present investigation of encapsulating the nodal segments containing axillary buds has resulted in scaling up the micropropagation technique for Cannabis sativa previously reported by us (Lata et al., 2008, accepted). At the same time, it has efficiently economized upon the medium, space and time requirements as follows:
Encapsulated explants utilize merely 300 ml of MS medium (100 ml for sodium alginate solution and 200 ml for CaCl2, 2H2O solution) for the preparation of nearly 250 beads. Each bead contains an explant, thereby producing 250 plantlets under in vitro conditions. On the other hand nearly 6.51 of MS medium is required for producing the same number of plants (25-ml medium/ glass culture vessel, 4 cm diameter x 9.5 cm high, baby food jars with magenta B caps) when non-encapsulated explants were used. Further, the culture vessels can accommodate only 3 non-encapsulated explants whereas the same space is sufficient for placing 20 beads, reducing thereby the culture space required otherwise. Moreover, each shoot bud explant has to be slightly embedded into the medium to allow efficient nutrient uptake. The roots under such conditions generally grow into the medium and thus at the time of transplantation to soil the plant has to be handled very carefully. Plants from encapsulated explants are very easily retrieved by picking with a forceps or just shaking the beads out. In addition to the above advantages, the technique offers easy transportation of large numbers of plants in low bulk.
In conclusion, synthetic seed technology would allow production of mass propagation material of C. sativa possessing the ability of regrowth, rooting and developing into plantlet for in vitro and ex vitro usage. This technique has a tremendous potential for scaling up the micropropagation procedure while at the same time economizing upon time, space and cost. The inclusion of antimicrobial substances in the planting substrate would allow possible future sowing of synthetic seeds in greenhouse (ex vitro), without sterility requirements. Conversion of encapsulated nodal segments into plantlets in extremely simplified medium such as 1:1 potting mix- fertilome with coco natural growth medium indicates that this method could be used in developing a cost effective propagation protocol for the mass propagating elite varieties of C. sativa. Besides, the use of vegetative propagules assures a high degree of genetic uniformity and stability, minimizing the occurrence of somaclonal variations. The procedure reported here for the production of synthetic seed by encapsulating nodal segments in calcium alginate and retrieving plants under both in vitro and in vivo conditions remains a preliminary investigation. A more thorough study of nutritive conditions in the planting substrate and encapsulation matrix is needed, especially in view of using this synthetic seed technology for germplasm storage of high yielding elite varieties of C. sativa. These studies are currently in progress.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams D.I., Jay C.A., Shade S.B., Vizoso H., Reda H., Press S., Kelly M.E., Rowbotham M.C., Petersen K.L. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurol. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [PubMed][Cross Ref]
- Adriani M., Piccioni E., Standardi A. Effect of different treatments on the conversion of ‘Hayward’ kiwifruit synthetic seeds to whole plants following encapsulation of in vitro-derived buds. N Z J Crop Horti Sci. 2000;28:59–67.
- Bapat V.A., Mhatre M., Rao P.S. Propagation of Morus indica L. (mulberry) by encapsulated shoot buds. Plant Cell Rep. 1987;6:393–395. doi: 10.1007/BF00269570. [PubMed] [Cross Ref]
- Bapat V.A., Rao P.S. In vitro growth of encapsulated axillary buds of mulberry (Morus indica L.) Plant Cell Tiss Org Cult. 1990;20:69–70. doi: 10.1007/BF00034760. [Cross Ref]
- Bing X, Ning L, Jinfeng T and Nan G (2007). Rapid tissue culture method of Cannabis sativa for industrial uses. CN 1887043 A 20070103 Patent, p 9.
- Bornman C.H. Maturation of Somatic Embryos. In: Redenbaugh K., editor. Synseeds: Application of Synthetic Seeds to Crop Improvement. Boca Raton, Florida: CRC Press; 1993. pp. 105–114.
- Brenneisen R., Egli A., Elsohly M.A., Henn V., Spiess Y. The effect of orally and rectally administered -tetrahydrocannabinol on spasticity. A pilot study with two patients. Int J Clin Pharm Ther. 1996;34(1):446. [PubMed]
- Brischia R., Piccioni E., Standardi A. Micropropagation and synthetic seed in M.26 apple rootstock (II): A new protocol for production of encapsulated differentiating propagules. Plant Cell Tiss Org Cult. 2002;68:137–141. doi: 10.1023/A:1013802723018. [Cross Ref]
- Castillo B., Smith M.A.L., Yadava U.L. Plant regeneration from encapsulated somatic embryos of Carica papaya L. Plant Cell Rep. 1998;17:172–176. doi: 10.1007/s002990050373. [Cross Ref]
- Duke J.A., Wain K.K. Medicinal Plants of the world, Computer index with more than 85.000 entries. In: Duke J.A., editor. Handbook of Medicinal Herbs. Boca Raton, Florida: CRC press; 1981. p. 96.
- Fowke L.C., Attree S.M., Pometry M.K. Production of vigorous desiccation-tolerant white spruce (Picea glauca {Moench} Voss.) synthetic seeds in a bioreactor. Plant Cell Rep. 1994;13:601–606. doi: 10.1007/BF00232931. [PubMed] [Cross Ref]
- Fujii J.A., Slade D., Redenbaugh K. Planting of artificial seeds and somatic embryos. In: Redenbaugh K., editor. Synseeds: Application of Synthetic Seeds to Crop Improvement. Boca Raton, Florida: CRC Press; 1993. pp. 183–202.
- Ganapathi T.R., Suprasanna P., Bapat V.A., Rao P.S. Propagation of banana through encapsulated shoot tips. Plant Cell Rep. 1992;11:571–575. doi: 10.1007/BF00233095. [PubMed] [Cross Ref]
- Ganapathi T.R., Srinivas I., Suprasanna P., Bapat V.A. Regeneration of plants from alginated-encapsulated somatic embryos of banana cv. Rasthali (Musa spp. AAB group) Biol Plant. 2001;37:178–181.
- Grinspoon L., Bakalar J.B. Marihuana as medicine. JAMA. 1995;273:1875–1876. doi: 10.1001/jama.273.23.1875. [PubMed] [Cross Ref]
- Guri A.Z., Patel K.N. Compositions and methods to prevent microbial contamination of plant tissue culture media. United States Patent. 1998;5:750.
- Hao Y.J., Deng X.X. Genetically stable regeneration of apple plants from slow growth. Plant Cell Tiss Org Cult. 2003;72:253–260. doi: 10.1023/A:1022388728497. [Cross Ref]
- Larkin P.J., Davies P.A., Tanner G.J. Nurse culture of low number of Medicago and Nicotianaprotoplasts using calcium alginate beads. Plant. Sci. 1988;58:203–210. doi: 10.1016/0168-9452(88)90010-6. [Cross Ref]
- Lata H, Chandra S, Khan I and ElSohly MA (2008). Thidiazuron induced high frequency direct shoot organogenesis of Cannabis sativa L. In vitro Cell Dev Biol Plant (accepted).
- Long L.E., Malone D.T., Taylor D.A. The pharmacological actions of cannabidiol. Drugs of the Future. 2005;30(7):747. doi: 10.1358/dof.2005.030.07.915908. [Cross Ref]
- Mandolino G., Ranalli P. Advances in biotechnological approaches for hemp breeding and industry. In: Ranalli P., editor. Advances in hemp research. New York: Haworth Press; 1999. pp. 185–208.
- Mathur J., Ahuja P.S., Lal N., Mathur A.K. Propagation of Valeriana wallichii DC using encapsulated apical and axial shoot buds. Plant Sci. 1989;60:111–6. doi: 10.1016/0168-9452(89)90050-2. [Cross Ref]
- Mattes R.D., Egelman K., Shaw L.M., Elsohly M.A. Cannabinoids appetite stimulation. Pharmacol Biochem Behav. 1994;44(3):745–747. doi: 10.1016/0091-3057(93)90194-X. [PubMed] [Cross Ref]
- Mechoulam S., Lander N., Dikstein S., Carlini E.A., Blumenthal M. On the Therapeutic Possibilities of Some Cannabinoids. In: Cohen S., Stillman R., editors. The Therapeutic Potential of Marihuana. New York: Plenum Press; 1976. p. 36.
- Micheli M., Pellegrino S., Piccioni E., Standardi A. Effects of double encapsulation and coating on synthetic seed conversion in M.26 apple rootstock. J. Microencap. 2002;19(3):347–356. doi: 10.1080/02652040110105337. [PubMed] [Cross Ref]
- Micheli M., Hafiz I.A., Standardi A. Encapsulation of in vitro-derived explants of olive (Olea europaea L. cv. Moraiolo) II Effects of storage on capsule and derived shoots performance. Sci Horti. 2007;113:286–292. doi: 10.1016/j.scienta.2007.04.001. [Cross Ref]
- Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [Cross Ref]
- Onishi N., Sakamoto Y., Hirosawa T. Synthetic seeds as an application of mass production of somatic embryos. Plant Cell Tiss Org Cult. 1994;39:137–145. doi: 10.1007/BF00033921.[Cross Ref]
- Pattnaik S., Chand P.K. Morphogenic response of the alginate encapsulated axillary buds from in vitro shoot cultures of six mulberries. Plant Cell Tiss Org Cult. 2000;64:177–185. doi: 10.1023/A:1006424626433. [Cross Ref]
- Piccioni E., Standardi A. Encapsulation of micropropagated buds of six woody species. Plant Cell Tiss Org Cult. 1995;42:221–226. doi: 10.1007/BF00029990. [Cross Ref]
- Pryce G., Baker D. Emerging properties of cannabinoid medicines in management of multiple sclerosis. Trends in Neurosci. 2005;28(5):272–276. doi: 10.1016/j.tins.2005.03.006. [PubMed][Cross Ref]
- Richez-Dumanois C., Braut-Boucher F., Cosson L., Paris M. Multiplication vegetative in vitro du chanvre (Cannabis sativa L.) Application a la conservation des clones selectiones. Agronomie. 1986;6:487–495. doi: 10.1051/agro:19860510. [Cross Ref]
- Statistical Analysis Systems User’s guide: Statistics version 9.1. Cary, NC: SAS Institute; 2003.
- Sharma T.R., Singh B.M., Chauhan R.S. Production of disease free encapsulated buds of Zingiber officinale Rose. Plant Cell Rep. 1994;13:300–302. doi: 10.1007/BF00233325. [PubMed][Cross Ref]
- Sirikantaramas S., Taura F., Morimoto S., Shoyama Y. Recent Advances in Cannabis sativaResearch: Biosynthetic Studies and Its Potential in Biotechnology. Curr Pharma Biotechnol. 2007;8(4):237–243. doi: 10.2174/138920107781387456. [PubMed] [Cross Ref]
- Slusarkiewicz-Jarzina A., Ponitka A., Kaczmarek Z. Influence of cultivar, explant source and plant growth regulator on callus induction and plant regeneration of Cannabis sativa L. Acta Biol Craco Series Bot. 2005;47(2):145–151.
- Small E., Marcus D. Hemp: A new crop with new uses for North America. In: Janick J., Whipkey A., editors. Trends in new crops and new uses. Alexandria, VA: ASHS Press; 2002. pp. 284–326.


Women’s Short


