Abstract
Cannabidiol (CBD) has become a highly attractive entity in therapeutics. However, its low aqueous solubility, instability and handling problems limit the development of effective CBD formulations. Subcutaneously administered CBD-loaded polycaprolactone microparticles (MP) represent an interesting strategy to overcome these challenges. This work focuses on evaluating the pharmacokinetics of CBD formulated in polymer microparticles for subcutaneous administration and characterising its release. The mean release time (MRLT) parameter is used to compare the release of CBD from two microparticle formulations in vitro and in a mouse model. After the administration of CBD in solution, a bicompartmental distribution is observed due to the extensive diffusion to the brain, being the brain/blood AUC ratio 1.29. The blood and brain mean residence time (MRT) are 0.507 ± 0.04 and 0.257 ± 0.0004 days, respectively. MP prepared with two drug/polymer ratios (15/150-MP and 30/150-MP) are designed, showing similar in vitro dissolution profiles (similarity factor (f2) is 63.21), without statistically significant differences between MRLTin vitro values (4.68 ± 0.63 and 4.32 ± 0.05 days). However, considerable differences in blood and brain profiles between both formulations are detected. The blood and brain MRT values of 15/150-MP are 6.44 ± 0.3 days and 6.15 ± 0.25 days, respectively, whereas significantly lower values 3.91 ± 0.29 days and 2.24 ± 0.64 days are obtained with 30/150-MP. The extended release of CBD during 10 days after a single subcutaneous administration is achieved.
Keywords: Cannabidiol, Cannabinoids, Pharmacokinetics, Polymeric microparticles, Subcutaneous administration, Sustained release
© 2023. Controlled Release Society.
References
-
- Bains S, Mukhdomi T. Medicinal cannabis for treatment of chronic pain, in StatPearls. 2023, StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL).
-
- Borrelli F, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl). 2009;87(11):1111–21. – PubMed
Grants and funding
Correction to: Polycaprolactone microparticles for the subcutaneous administration of cannabidiol: in vitro and in vivo release
Correction: to: Drug Delivery and Translational Research https://doi.org/10.1007/s13346-023-01444-2
The authors make the following corrections and clarifications of omissions:
Authorship and Roles
The name of Dolores Hernán is incorrect in the original article. It is correct as reflected here. This paper is based primarily on the dissertation of Dr. Hernán Pérez de la Ossa. Her roles on the manuscript included: conceptualization, experimental design, formal analysis and investigation, and preparation of the original draft of this publication. The roles of JLP were methodology and formal analysis as well as investigation. The roles of AHL were funding acquisition, resources, and supervision.
Material and methods
Drug content and drug release studies
The validation of the HPLC method used to quantify CBD in both drug content and drug release studies is included in the dissertation of Hernán Pérez de la Ossa [1].
Animal experimental procedure
Experiments conducted at Virginia Commonwealth University used male ICR mice from Harlan Laboratories (Dublin, VA) and the experiments conducted at Complutense University of Madrid used ICR mice from Charles River Laboratories (Massachusetts, United States).
Analytical procedure to determine CBD blood and brain levels
The analytical procedure included in the original publication is the one used at Virginia Commonwealth University. The procedure used at Complutense University is as follows:
- The quantification of CBD in blood and brain tissues from 15/150-MP and 30/150-MP treated mice was carried by LC-MS following the protocol described in the methods of the original manuscript with few modifications. In brief, blood and brain samples were homogenized with 1 mL of ice-cold 50 mMTris-HCl, pH 7.4. Subsequently, two consecutive extractions were carried out. For each extraction, 2 mL of cold acetonitrile (stored for 5 min at − 20 ºC) was added and samples were vortexed for 2 min. The samples were then centrifuged (Universal 32R centrifuge; Hettich, Germany) at 4 ºC and 152 RFC for 15 min. The supernatants were carefully recovered and stored overnight at -20 ºC. The acetonitrile layer was carefully recovered and evaporated to dryness using a Savant SpeedVac concentrator SPD121P (Holbrook, N.Y., USA). Finally, the dried samples were resolubilised in 0.1 mL of acetonitrile, filtered using a PTFE 0.22 µM syringe filter 4 mm and placed into vials with 100 µL inserts for LC-MS analysis.
- The LC-MS quantification of CBD was conducted using a previously described method with slight modifications [2]. A liquid chromatography coupled to a triple quadruple mass spectrometer equipped with a turbo ion spray source operating in positive ion mode (LCMS 8030, Shimadzu) was used. Chromatographic separation was performed using a Phenomenex Gemini C18 column (110 Å, 150 × 2 mm), with an injection volume 10 µL, a flow rate of 0.5 mL/min, and run time of 10 min. The mobile phase consisted of phase A (0.1% formic acid in water) and phase B (0.1% formic acid in acetonitrile). The analysis was carried out in gradient mode: from 5 to 50% phase B – 5 min; 95% phase B – 7 min; 5% phase B – 8 min; until 10 min initial conditions. Ions were analysed in a MRM mode. Mass transitions of m/z 314.9 > 192.9 (CE = − 32 V) were used for quantification and m/z 314.9 > 123.0 (CE = − 33 V) for identification.
The LC-MS quantification of CBD was conducted using a previously described method with slight modifications [2]. A liquid chromatography coupled to a triple quadruple mass spectrometer equipped with a turbo ion spray source operating in positive ion mode (LCMS 8030, Shimadzu) was used. Chromatographic separation was performed using a Phenomenex Gemini C18 column (110 Å, 150 × 2 mm), with an injection volume 10 µL, a flow rate of 0.5 mL/min, and run time of 10 min. The mobile phase consisted of phase A (0.1% formic acid in water) and phase B (0.1% formic acid in acetonitrile). The analysis was carried out in gradient mode: from 5 to 50% phase B – 5 min; 95% phase B – 7 min; 5% phase B – 8 min; until 10 min initial conditions. Ions were analysed in a MRM mode. Mass transitions of m/z 314.9 > 192.9 (CE = − 32 V) were used for quantification and m/z 314.9 > 123.0 (CE = − 33 V) for identification.
Calibration curves were prepared for each assay with mouse whole blood and brains obtained from untreated animals, and adding 0.5–250 ng of CBD; and CBD solutions in ACN from 2 to 2500 ng/ml.
Results
In vivo studies
Figure 4A, left panel: The x-axis of the graph has been modified to show the 16-day study duration. This update does not affect the results or conclusions of this article.
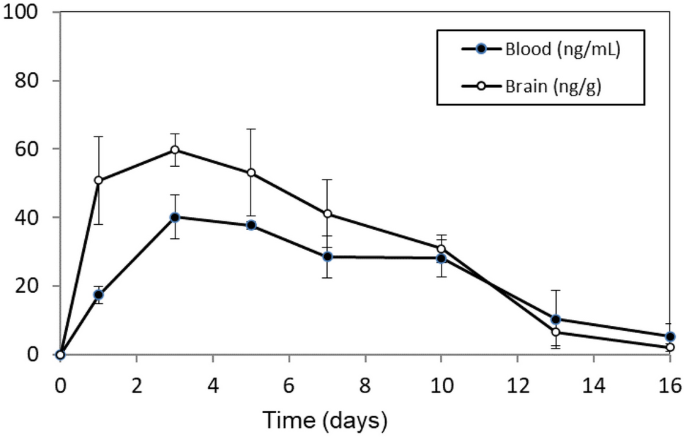
There are typographical errors in the text regarding the MRT values in blood and brain after administration of 15/150 MP, reported to be 6.27 and 5.54 days respectively. The correct values are those reflected in Table 2 and 3: 6.44 and 6.15 days, respectively.
Acknowledgements
The investigators are grateful for the support and guidance from the late Dr Billy R. Martin (VCU) who contributed to the design of the study and the mentorship of Dolores Hernán Pérez de la Ossa.
Funding
This work was partially funded by the National Institutes of Health (P50 DA005274, R01 DA-03672, and P30 DA033934) and the Santander-UCM Research Group 910939: Parenteral Administration of Drugs. D. Hernán Pérez de la Ossa received a FPU fellowship (AP2005-0184) from the Spanish Ministry of Education and Science.
Ethical statement
Vertebrate animal experiments conducted at VCU were approved by the VCU Institutional Animal Care and Use Committee.
The original article has been corrected.
References
-
Hernán Pérez de la Ossa D. Development and in vitro in vivo evaluation of biodegradable microspheres for cannabinoid vehiculization (2010). Universidad Complutense de Madrid, Servicio de Publicaciones. SN 978-84-693-7813-7. https://dialnet.unirioja.es/servlet/tesis?codigo=92690&orden=1&info=link.
-
Molnar A, Fu S, Lewis J, Allsop DJ, Copeland J. The detection of THC, CBD and CBN in the oral fluid of Sativex(R) patients using two on-site screening tests and LC-MS/MS. Forensic Sci Int. 2014;238:113–9. https://doi.org/10.1016/j.forsciint.2014.03.004.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fraguas-Sánchez, A.I., Hernán Pérez de la Ossa, D., Montejo, C. et al. Correction to: Polycaprolactone microparticles for the subcutaneous administration of cannabidiol: in vitro and in vivo release. Drug Deliv. and Transl. Res. (2023). https://doi.org/10.1007/s13346-023-01507-4
- Published
- DOIhttps://doi.org/10.1007/s13346-023-01507-4


