Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Nov 5;10(4):20552173241282379. doi: 10.1177/20552173241282379
Abstract
Background
One of the most disabling symptoms of patients with multiple sclerosis (MS) is spasticity which affects their quality of life. Nowadays, cannabinoids are used for spasticity control in patients with MS, while the efficacy and safety are not clearly understood. So, we designed this systematic review and meta-analysis to assess the efficacy of cannabinoids for controlling MS-related spasticity.
Methods
PubMed, Scopus, EMBASE, Web of Science, and Google Scholar were systematically searched by two independent researchers on 1 May 2023. They also searched gray literature (references of included studies, as well as conference abstracts).
Results
A literature search revealed 6552 records, 95 full-texts were evaluated, and finally, 31 studies remained for systematic review. Among included studies, six randomized trials were included. Nabiximols was the most commonly used medication for controlling MS-related spasticity. Mean Expanded Disability Status Scale ranged between 4.6 and 7. Most studies (17 studies) were done in Italy, followed by Germany (4 studies). The pooled standardized mean difference (SMD) of NRS (Numeric Rating Scale) (after–before) is estimated as −1.41 (95% confidence interval (CI): −1.65, −1.17) (I2 = 97%, p < 0.001). The pooled standardized mean difference (SMD) of Ashworth (after-before) is estimated as −0.39 (95% CI: −0.72, −0.06) (I2 = 69.9%, p = 0.005).
Conclusion
The results of this systematic review and meta-analysis showed that nabiximols was the most common cannabinoid which was used to control MS-related spasticity, and it was effective in controlling MS-related spasticity (significantly decreased SMD of NRS, and Ashworth after treatment).
Introduction
Multiple sclerosis (MS) is a chronic disabling disease of the central nervous system (CNS), characterized by demyelinating plaques, and significant physical complications such as walking difficulties, gait imbalance, and spasticity.1,2
One of the most disabling symptoms of patients with MS is spasticity, affecting more than half of the patients, while literature shows that near three-fourths of affected individuals suffer from spasticity 15 years after disease progression.3Muscle hypertonia, stiffness, weakness, and following insomnia will result in interfering with daily activities, and quality of life impairment.4
The common treatment includes antispastic medications such as baclofen, tizanidine, or dantrolene in combination with physiotherapy, with not always fully satisfactory effects.3,5 Withdrawal is common as the side effects include falling, sedation, dizziness, and withdrawal syndrome.6
Currently, Onabotulinumtoxin (BOTOX®, Allergan, Inc., Irvine, CA) injection has become more popular for controlling spasticity, but the duration of action is short, and administration of botox needs a high rate of specialization.7,8
These days, people with MS admit to consuming cannabinoids to control different symptoms such as pain, anxiety, spasticity, and sleep disturbances.9
Cannabis is cultivated all over the world, containing over 483 identifiable chemicals, while only 80 cannabinoids are isolated from the plant, but the most famous ones are tetrahydrocannabinol (THC) and cannabidiol (CBD).10
Novotna et al.11for the first time introduced the application of oral spray of cannabinoids for MS-related spasticity, and nabiximols has been approved for MS-related spasticity treatment.
Various studies show that cannabinoids are used for spasticity control in patients with MS, while the efficacy and safety are not clearly understood. So, we designed this systematic review and meta-analysis to assess the efficacy of cannabinoids for controlling MS-related spasticity.
Methods
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 for reporting our systematic review, and meta-analysis.12
Eligibility criteria
Inclusion criteria: We included trials and observational studies that evaluated the effects of cannabinoids on spasticity in patients with MS.
Exclusion criteria: Case reports, case series, letters to editors.
We excluded studies that had no clear data for meta-analysis.
Information sources
PubMed, Scopus, EMBASE, Web of Science, and Google Scholar were systematically searched by two independent researchers on 1 May 2023. They also searched gray literature (references of included studies, as well as conference abstracts).
Search strategy
| (((((((((((((((((((((((((((((((((((((((((((((((((((((((Cannabinoids[MeSH Terms]) OR (Cannabis[MeSH Terms])) OR (Dronabinol[MeSH Terms])) OR (Nabilone[MeSH Terms])) OR (Cannabidiol[MeSH Terms])) OR (Phytocannabinoids[MeSH Terms])) OR (Nabiximols[MeSH Terms])) OR (Cannabinol[MeSH Terms])) OR (Cannabigerol[MeSH Terms])) OR (Cannabichromene[MeSH Terms])) OR (Cannabinoid*[Text Word])) OR (Cannabis[Text Word])) OR (Cannabi[Text Word])) OR (Hemp Plant*[Text Word])) OR (Plant, Hemp[Text Word])) OR (Plants, Hemp[Text Word])) OR (Marihuana[Text Word])) OR (Marijuana[Text Word])) OR (Cannabis indica[Text Word])) OR (Cannabis sativa[Text Word])) OR (Hemp*[Text Word])) OR (Hashish*[Text Word])) OR (Bhang*[Text Word])) OR (Ganja*[Text Word])) OR (Dronabinol[Text Word])) OR (9-ene-Tetrahydrocannabinol[Text Word])) OR (9 ene Tetrahydrocannabinol[Text Word])) OR (THC[Text Word])) OR (Tetrahydrocannabinol[Text Word])) OR (Tetrahydrocannabinol, (6a-trans)-Isomer[Text Word])) OR (Tetrahydrocannabinol, Trans-Isomer[Text Word])) OR (Tetrahydrocannabinol, Trans Isomer[Text Word])) OR (Marinol[Text Word])) OR (Tetrahydrocannabinol, (6aR-cis)-Isomer[Text Word])) OR (Nabilone[Text Word])) OR (nabilone, (6aR-trans)-isomer[Text Word])) OR (Cesamet[Text Word])) OR (Lilly 109514[Text Word])) OR (LY 109514[Text Word])) OR (nabilone, (6aS-trans)-isomer[Text Word])) OR (Cannabidiol[Text Word])) OR (Epidiolex[Text Word])) OR (Phytocannabinoid*[Text Word])) OR (Nabiximol*[Text Word])) OR (tetrahydrocannabinol-cannabidiol combination[Text Word])) OR (GW 1000[Text Word])) OR (GW1000[Text Word])) OR (GW-1000[Text Word])) OR (SAB 378[Text Word])) OR (SAB378[Text Word])) OR (SAB-378[Text Word])) OR (Sativex[Text Word])) OR (Cannabinol[Text Word])) OR (Cannabigerol[Text Word])) OR (Cannabichromene[Text Word])) AND (((((((((((((Multiple sclerosis[MeSH Terms]) OR (Muscle Spasticity[MeSH Terms])) OR (Multiple sclerosis[Text Word])) OR (Disseminated sclerosis[Text Word])) OR (Sclerosis, disseminated[Text Word])) OR (Sclerosis, Multiple[Text Word])) OR (Multiple Sclerosis, Acute Fulminating[Text Word])) OR (Muscle Spasticity[Text Word])) OR (Spasticity, Muscle[Text Word])) OR (Spastic[Text Word])) OR (Clasp-Knife Spasticity[Text Word])) OR (Clasp Knife Spasticity[Text Word])) OR (Spasticity, Clasp-Knife[Text Word])) |
Selection process and collection
After the primary search, the obtained results were imported to ENDNOTE software. Duplicates were deleted, then titles and abstracts of eligible studies were assessed. Potential full texts were obtained, and were evaluated by two independent researchers.
Researchers extracted data and entered it in separate Excel files. If discrepancies were present, the third one solved the issue.
Data items
The first author of the publication, country of the study, publication year, duration of the study, number of study participants, total female, and male cases, type of MS, cannabinoid type, mean age at disease onset, mean Expanded Disability Status Scale (EDSS), type of the disease, numeric rating scale for spasticity, and Modified Ashworth Scale were extracted from included studies.
Study risk of bias assessment
ROBINS-I tool was used for Quality assessment of nonrandomized studies, while ROB2 was applied for quality assessment of randomized trials.13,14
Effect measures
We calculated standardized mean difference (SMD) for NRS, and Ashworth scale.
Synthesis methods
All statistical analysis was done using STATA (Version 14.0; Stata Corp LP, College Station, TX, USA). The p-values <0.05 were considered significant.
Certainty assessment
For all estimated effect sizes, we reported 95% CI. For studies that reported more than one endpoint outcome, we considered the final one.
Results
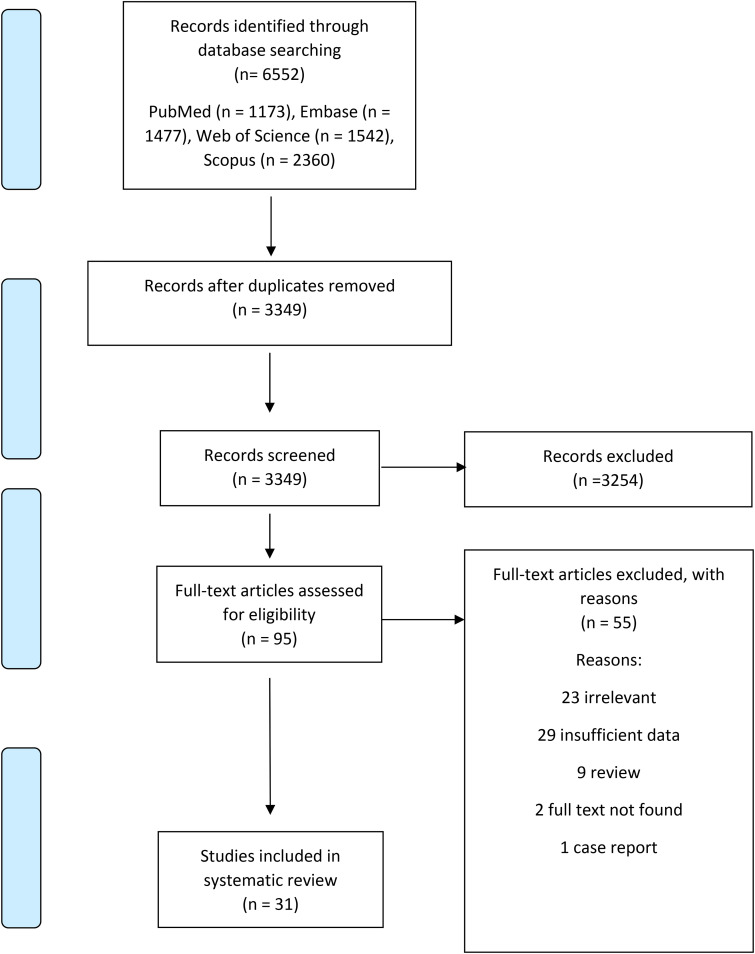
A literature search revealed 6552 records, 95 full-texts were evaluated, and finally, 31 studies remained for systematic review (Figure 1).
Figure 1.
Included studies published between 2002 and 2023. Most studies were done in Italy, followed by Germany.
The number of patients in studies ranged between 8 and 1845, and the duration of studies ranged between 4 weeks and 1 year. Among included studies, six randomized trials were included. Except for four studies, all others used nabiximols. Mean EDSS ranged between 4.6 and 7. Most studies (17 studies) were done in Italy, followed by Germany (4 studies) (Table 1).
Table 1.
Included study (design and results).
| Author | Year | Country | Study design | Study population | Age | Gender | Cannabinoid type | Study duration | Disease duration | EDSS | Disease type | Age at diagnosis | NRS: numeric rating scale for spasticity | Modified Ashworth Scale | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||||||||
| Vecchio et al. 15 | 2020 | Italy | Nonrandomized trial | 15 | 55.5 ± 5.2 | 11 F, 4 M | Nabiximols | 6 weeks | 17.4 ± 6.2 | Median (range) 6 (2–8) |
All progressive | Median (range) 5 (1–10) |
Median (range) 2 (0–8) |
Median (range) 14 (4–32) |
Median (range) 4 (1–16) |
|
| Koehler et al. 16 | 2013 | Germany | Observational study | 166 | 69 F, 97 M | Nabiximols | 9 months | 133 SPMS, 20 PPMS, 13 RRMS | Mean (range) 7 (4–10) (N = 120) |
Mean (range) 3 (0–6) (N = 120) |
||||||
| Collin et al. 17 | 2010 | UK | Double-blind, randomized, placebo-controlled, parallel-group study | 337 (experiment: 167/control: 170) | Experiment: 48 ± 10.06/Control: 47.1 ± 10.15 | Experiment: 106 F, 61 M/control: 101 F, 69 M | Nabiximols | 15 weeks | Experiment: 14.4 ± 8.29/ control: 16 ± 8.48 | Experiment: 6 ± 1.56/control: 6 ± 1.50 | Mean (SE) experiment: 6.77 (0.14) control: 6.49 (0.09) |
Mean (SE) experiment: week 2: 6.16 (0.10) week 4: 5.81 (0.14) week 6: 5.59 (0.18) week 8: 5.49 (0.14) week 10: 5.35 (0.15) week 12: 5.36 (0.18) week 14: 5.34 (0.18) control: week 2: 5.96 (0.09) week 4: 5.73 (0.14) week 6: 5.64 (0.14) week 8: 5.70 (0.14) week 10: 5.75 (0.16) week 12: 5.59 (0.15) week 14: 5.58 (0.13) | ||||
| Squintani et al. 18 | 2016 | Italy | Nonrandomized trial | 19 | 56.1 ± 8.9 | 14 F, 5 M | Nabiximols | 4 weeks | 17.6 ± 11.3 | 6.1 ± 1.4 | 1.1 ± 0.6 | 0.7 ± 0.5 | ||||
| Freidel et al. 19 | 2014 | Germany | Observational study | 33 | 48.1 | 21 F, 12 M | Nabiximols | 6 weeks | 4.6 | Mean, median (Q1, Q2,Q3, Q4) 6.03, 6 (3,5,7,10) |
Mean, median (Q1,Q2, Q3,Q4) 3.61, 3 (1,2,5,7) |
|||||
| Marinelli et al. 20 | 2016 | Italy | Nonrandomized trial | 36 | 53.77 ± 10.32 | 21 F, 15 M | Nabiximols | 4 weeks | 181.28 ± 87.62 months | 6.83 ± 0.55 | 29 SPMS, 6 PPMS, 1 RRMS | 6.8 ± 1.7 | 5.7 ± 2.1 | |||
| Patti et al. 21 | 2015 | Italy | Cohort study | 1615 | 51 ± 9.5 | 849 F, 766 M | Nabiximols | 24 weeks | 17.5 ± 8.6 | Median (range) 6.5 (1.5–9.5) |
7.5 ± 1.4 (N = 1597) | Week 4: 5.9 ± 1.6 (N = 1432) week 12: 5.1 ± 1.6 (N = 889) week 24: 4.8 ± 1.7 (N = 593) |
||||
| Vaney et al. 22 | 2004 | Switzerland | Randomized, double-blind, placebo-controlled cross-over parallel group study | 57 | 54.9 ± 10 | 29 F, 28 M | Cannabis extract | 31 days (14 treatment, 3 washout, 14 placebo) | 17 ± 8.4 | Median 7 | Treatment period: 12.2 ± 6.4 Placebo period: 13.1 ± 6.3 (N = 50) |
Treatment period: 11.6 ± 6.5 Placebo period: 11.5 ± 6.1 (N = 50) |
||||
| Centonze et al. 23 | 2009 | Italy | Non randomized trial | 20 | 13 F, 7 M | Nabiximols | 6 weeks | 5.36 ± 1.69 | Week 1: 5.86 ± 1.98 Week 2: 5.85 ± 2.5 Week 3: 5.73 ± 2.48 Week 4: 5.56 ± 2.18 Week 5: 5.76 ± 2.1 Week 6: 5.71 ± 2.52 |
5.14 ± 2.12 | Week 1: 5.11 ± 2.12 Week 2: 4.91 ± 2.01 Week 3: 4.97 ± 1.94 Week 4: 4.94 ± 2.02 Week 5: 5.14 ± 1.97 Week 6: 4.91 ± 1.98 |
|||||
| Paolicelli et al. 24 | 2016 | Italy | Cohort | 102 | 48.8 ± 10.4 | 52 F, 50 M | Nabiximols | 40 weeks | 19.2 ± 8 | 6.7 ± 1.1 | 59 SPMS, 25 RRMS, 10 PPMS, 8 PRMS | 8.7 ± 1.3 | Month 1: 6.2 ± 1.8 Month 3: 5.9 ± 1.6 Month 6: 6.1 ± 1.4 Year 1: 6.2 ± 1.4 |
|||
| Flachenecker et al. 25 | 2014 | Germany | Observational, prospective, multicenter, noninterventional study | 52 | 49.4 ± 8.6 | 29 F, 23 M | Nabiximols | 1 year | 14.1 ± 8.0 | Median (range) 6 (3–8) |
34 SPMS, 10 RRMS, 8 PPMS | 6.2 ± 1.8 | 4.6 ± 2.1 (N = 51) | |||
| Flachenecker et al. 26 | 2014 | Germany | Observational, prospective, multicenter, noninterventional study | 276 | 50.0 ± 9.4 | 168 F, 108 M | Nabiximols | 3 months | 15.4 ± 9.0 | Median (range) 6 (1–9) |
168 SPMS, 72 RRMS, 34 PPMS, 2 PRMS | 6.1 ± 1.7 | Week 4: 5.2 ± 1.9 (N = 210) | 3.0 ± 0.8 | Week 4: 2.7 ± 0.9 (N = 260) Week 12: 2.6 ± 1.0 (N = 95) |
|
| Sartori et al. 27 | 2021 | Italy | Retrospective single-center study | 36 | 53.9 ± 8.7 | 18 F, 18 M | Nabiximols | Median (range) 178 (8−447) months |
Median (range) 6.75 (2.5−9) |
19 SPMS, 9 RRMS, 8 PPMS | Median (range) 2 (0–4) |
Median (range) 2 (0–4) |
||||
| Chisari et al. 28 | 2020 | Italy | Prospective observational multicenter | 1845 | 50.9 ± 12.3 | 1226 F, 619 M | Nabixomols | 18 months | 16.3 ± 8.8 | Median (Range) 6.5 (4–8.5) |
1283 SPMS, 333 PPMS, 229 RRMS | 35.9 ± 11.7 | 7.8 ± 1.7 | Week 4: 5.7 ± 1.6 (N = 1502) Month 3: 5.2 ± 1.4 (N = 1241) Month 6: 5.0 ± 1.7 (N = 1017) Month 12: 4.8 ± 1.8 (N = 853) Month 18: 4.7 ± 2.0 (N = 777) |
||
| Lus et al. 29 | 2018 | Italy | Open-label, prospective, multicenter, nonpharmacological, randomized, minor interventional, postmarketing authorization pilot project | 52 | 51.9 ± 9.1 | 32 F, 20 M | Nabiximols (chewing gum, cold bottle) | 4 weeks | 13.2 ± 7.5 | 6.2 ± 1.4 | 27 SPMS, 12 RRMS, 11 PPMS, 2 PRMS | 6.1 ± 2.2 | 5.4 ± 2.2 (N = 46) | |||
| Novotna et al. 30 | 2011 | UK | Nonrandomized trial | 331 | 49.1 ± 9.85 | 202 F, 129 M | Nabiximols | 4 weeks | 12.3 ± 7.49 | 6 ± 1.4 | 6.91 ± 1.25 | 3.9 ± 1.51 | ||||
| Gustavsen et al. 31 | 2020 | Denmark | Observational study | 28 (24 THC, 4 CBD) | 50 | 21 F, 7 M | Cannabis oil (THC rich, CBD rich) | 4 weeks | Median (range) 11 (1–28) | Median (range) 4.5 (2–9) |
15 RRMS, 8 SPMS, 5 PPMS | Median (range) THC: 6 (1–10) CBD: 6 (4–8) |
Median (range) THC: 2.5 (0–7) (N = 18) CBD: 2 (2–2) (N = 3) |
|||
| D’hooghe et al. 32 | 2021 | Belgium | Retrospective study | 238 | Nabiximols | 12 weeks | 8.1 ± 1.08 | Week 4: 5.2 ± 1.85 (N = 229) Week 8: 4.6 ± 1.69 (N = 188) Week 12: 4.1 ± 1.78 (N = 96) |
||||||||
| Messina et al. 33 | 2017 | Italy | Observational study | 1597 | 51 | 841 F, 756 M | Nabiximols | 6 months | 17.5 ± 8.6 | Median (range) 6.5 (1.5–9.5) |
1029 SPMS, 311 RRMS, 255 PPMS | 7.5 ± 1.4 | Month 1: 5.9 ± 1.6 Month 3: 5.1 ± 1.6 Month 6: 4.8 ± 1.7 |
|||
| Serpell et al. 34 | 2012 | UK | Open-label trial | 146 | 50 ± 9 | 94 F, 52 M | Nabiximols | 52 weeks | Mean (SEM) 5.68 (0.22) |
Mean (SEM) 3.85 (0.25) |
||||||
| Corey-Bloom et al. 35 | 2012 | USA | Randomized, double-blind, placebo-controlled crossover design | 30 | 51 ± 8 | 19 F, 11 M | Smoked cannabis | 2 weeks | 8.5 ± 7.4 | 5.3 ± 1.5 | 20 SPMS, 10 RRMS | Mean (95%CI) treatment: 9.13 (8.21–10.07) placebo: 8.92 (8.03–9.79) |
Mean (95%CI) treatment: 6.18 (5.13–7.21) placebo: 8.71 (7.57–9.71) |
|||
| Vermersch et al. 36 | 2016 | Italy | Observational study | 433 | 50.4 ± 10.4 | 239 F, 194 M | Nabiximols | 3 months | 13.7 ± 7.9 | 5.94 ± 1.38 | 223 RRMS, 137 RRMS, 72 PPMS | 6.9 ± 1.9 (N = 394) | 5.3 ± 1.8 (N = 253) | |||
| Maniscalco et al.37 | 2017 | Italy | Observational study | 15 | 56.1 ± 8.6 | 7 F, 8 M | Nabiximols | 4 weeks | Median (range) 91 (4–276) months | Median (range) 8 (4–10) |
Median (range) 6 (2–8) |
|||||
| Carotenuto et al.38 | 2016 | US | Nonrandomized trial | 10 | 51.10 ± 9.96 | 10 F, 10 M | Nabiximols | 1 year | Median (range) 9.21 (2–39) | 4.70 ± 1.08 | 12 progressive, 8 relapsing | 37.28 ± 13.61 | 8 ± 1.93 (N = 10) | 5.50 ± 2.45 (N = 10) | ||
| Coghe et al.39 | 2015 | Italy | Nonrandomized trial | 20 | 49.6 ± 9.11 | 11 F, 9 M | Nabiximol | 1 month | 5.3 ± 0.81 | 4 RRMS, 1 PRMS, 1 SPMS | 7.1 ± 1.22 | 5.24 ± 1.39 | ||||
| Ferrante et al.40 | 2019 | Italy | Retrospective cohort study | 37 | 56 ± 9 | 26 F, 11 M | Nabiximols | 7.86 ± 1.00 | 5.66 ± 1.04 | |||||||
| Patti et al.41 | 2022 | Italy | Observational study | 1138 | 51.5 ± 9.8 | 621 F, 517 M | Nabiximols | 18 month | 19.8 ± 10.5 | 6.5 ± 1.16 | 761 SPMS, 193 RRMS, 183 PPMS | 7.8 ± 1.25 | Week 4: 5.9 ± 1.5 Month 3: 5.3 ± 1.3 (N = 760)Month 6: 5.1 ± 1.2 (N = 653) Month 12: 5.1 ± 1.2 (N = 473) Month 18: 5.1 ± 1.2 (N = 397) | |||
| Pau et al.42 | 2023 | Italy | Nonrandomized trial | 13 | 51.2 ± 11.8 | 9 F, 4 M | Nabixiomols | 4 weeks | 5.4 ± 1.6 | 11 SPMS, 1 RRMS, 1 PPMS | 6.3 ± 1.3 | 4.2 ± 1.3 | ||||
| Guger et al. 43 | 2023 | Austria | Prospective observational study | 55 | 52.5 ± 9.6 | 33 F, 22 M | Nabiximols | 3 month | 15.1 ± 9.1 | 5.3 ± 1.3 | 28 SPMS, 18 PPMS, 9 RRMS | 6.4 ± 1.8 | Month 1: 4.8 ± 1.7 (N = 43) Month 3: 3.9 ± 2.0 (N = 40) |
|||
| Gajofatto et al.44 | 2023 | Italy | Nonrandomized trial | 12 | Median (range) 51 (36–73) |
5 F, 7 M | Nabiximols | 8 weeks | Median (range) 21.5 (10–37) |
Median (range) 6 (4.5–8) |
10 SPMS, 2 RRMS | Median (range) 8 (5–9) |
Median (range) 6.5(4–8) |
|||
| Killestein et al.45 | 2002 | The Netherland | Randomized, double-blind, placebo-controlled, twofold crossover study | 16 (each group 8) | 46 ± 7.9 | Dronabinol | 4 weeks | 15 ± 10.7 | 6.2 ± 1.2 | 10 SPMS, 6 PPMS | Placebo: 1.15 (0.94–1.37) plant extract: 1.20 (0.97–1.39) drobinidol: 1.13 (0.92–1.36) | Placebo: 1.03 (0.81–1.23) plant extract: 0.94 (0.74–1.14) drobinidol: 0.97 (0.77–1.18) | ||||
CBD: cannabidiol; EDSS: Expanded Disability Status Scale; F: female; M, male; NRS: Numeric Rating Scale; PPMS: primary progressive multiple sclerosis; PRMS: progressive-relapsing multiple sclerosis; RRMS: relapsing-remitting mustiple sclerosis; SE: standard error; SEM: standard error of the mean; SPMS: slowly progressive multiple sclerosis; THC: tetrahydrocannabinol.
The quality assessment of trials and observational studies are summarized in Tables 2 and 3.
Table 2.
Quality assessment of nan-randomized studies (ROBINS-I).
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Vecchio et al.15 | Low | Low | Low | Low | Low | Low | Low | Low |
| Koehler et al.16 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Squintani et al.18 | Low | Low | Low | Low | Low | Low | Low | Low |
| Freidel et al. 19 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Marinelli et al. 20 | Low | Low | Low | Low | Low | Low | Low | Low |
| Patti et al.21 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Centonze et al.23 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Paolicelli et al.24 | Low | Low | Low | Low | Low | Low | Low | Low |
| Flachenecker et al.25 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Flachenecker et al.26 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Sartori et al.27 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Chisari et al.28 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Novotna et al.30 | Low | Low | Low | Low | Low | Low | Low | Low |
| Gustavsen et al.31 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| D’hooghe et al.32 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Messina et al.33 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Serpell et al.34 | Low | Low | Low | Low | Low | Low | Low | Low |
| Vermersch et al.36 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Maniscalco et al.37 | Low | Low | Low | Low | Low | Low | Low | Low |
| Carotenuto et al.38 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Coghe et al.39 | Low | Low | Low | Low | Low | Low | Low | Low |
| Ferrante et al.40 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Patti et al.41 | Low | Moderate | Low | Low | Moderate | Low | Low | Moderate |
| Pau et al.42 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Guger et al.43 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Gajofatto et al.44 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
Table 3.
Quality assessment of randomized trials (ROB2).
| Study | Randomization process | Deviations from the intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall bias |
|---|---|---|---|---|---|---|
| Collin et al.17 | Some concerns | Low | Low | Low | Low | Some concerns |
| Vaney et al.22 | Low | Low | Low | Low | Low | Low |
| Lus et al.29 | Some concerns | Low | Some concerns | Low | Low | Some concerns |
| Corey-Bloom et al.35 | Some concerns | Low | Low | Low | Low | Some concerns |
| Killestein et al.45 | Low | Low | Some concerns | Low | Low | Some concerns |
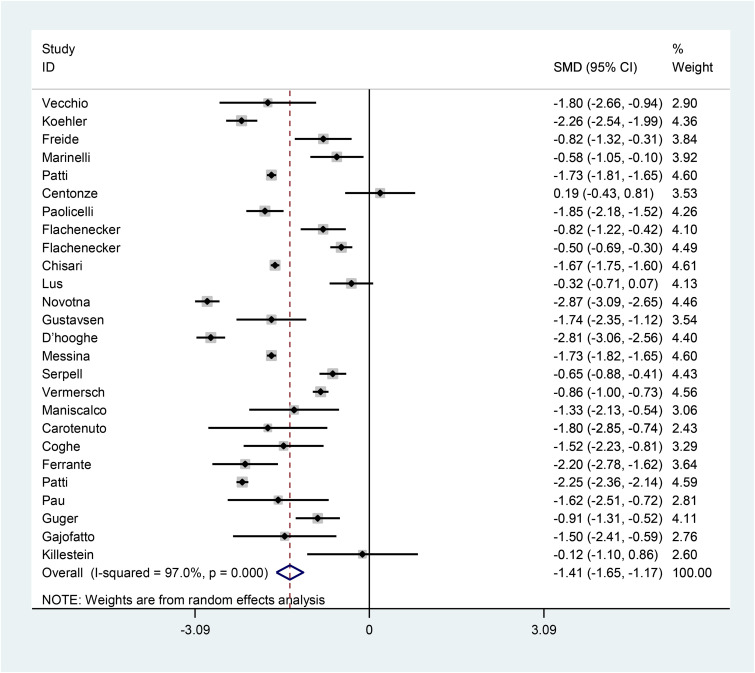
The pooled SMD of NRS (after–before) is estimated as −1.41 (95% CI: −1.65, −1.17) (I2 = 97%, p < 0.001) (Figure 2), indicating that cannabis use is effective in decreasing numeric rating scale of spasticity in patients with MS.
Figure 2.
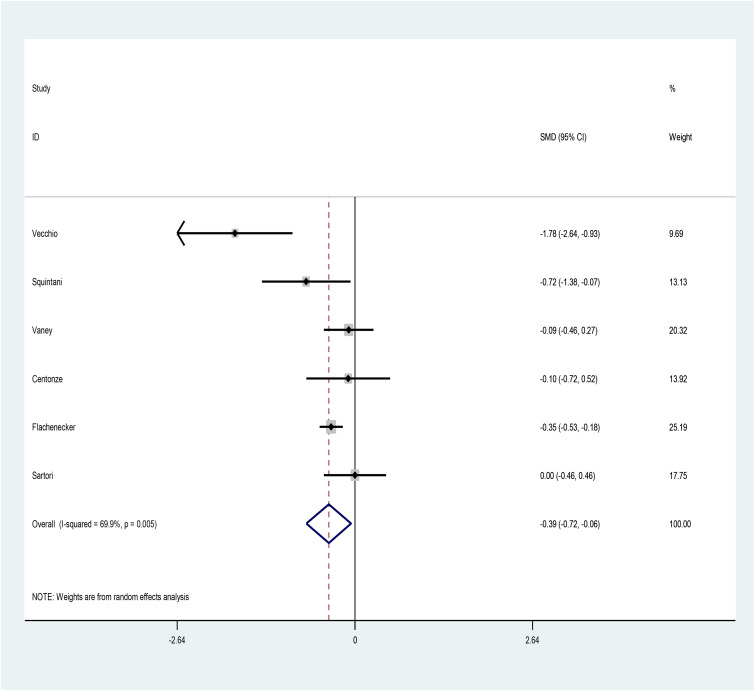
The pooled SMD of Ashworth (after–before) is estimated as −0.39 (95% CI: −0.72, −0.06) (I2 = 69.9%, p = 0.005) (Figure 3), indicating that cannabis use helps reducing Ashworth spasticity scale in patients with MS.
Figure 3.
Discussion
To our knowledge, this is the first systematic review and meta-analysis in this field. According to our results, the administration of cannabinoids for controlling spasticity in patients with MS is helpful as the pooled SMD of NRS and Ashworth were significantly improved after administration of these medications. As our results show, the SMD of Ashworth scale was −1.78, which showed a great impact of cannabinoids on MS-related spasticity.
In a multicentric observational study, Guger et al. enrolled patients with MS who suffered from spasticity, and evaluated spasticity-treatment using nabiximols oromucosal spray. Their results showed near 40% reduction in NRS for spasticity after administration of the medication.43
In a single-center study, Sartori et al., evaluated the effects of botulinum toxin injections (BTI), and nabiximols on MS-related spasticity in patients with MS. Their results showed that BTI was more effective than nabiximols in treating MS-related spasticity.27
Vecchio et al., evaluated the effects of cannabinoids (cannabinoid spray) on spasticity in patients with MS. Participants were allowed to use the maximum dose of 12 puffs per day. They found that after 6 weeks, pain, and spasticity were improved significantly.15
Among included studies, some found that cannabinoids are not effective in controlling spasticity, while others did find. The difference among the findings is due to different inclusion and exclusion criteria, diverse definitions of spasticity, and follow-up duration variation.
The first large-scale clinical trial to evaluate the effects of cannabinoids on MS-related spasticity was developed by Zajicek et al. in 2005, and 630 patients were recruited.
They were assigned to oral cannabis extract, Δ9-tetrahydrocannabinol, or placebo.
They reported improvement in spasticity as 61% in the first group, 60% in the second group, and 46% in the placebo group. We did not include this study as they reported a mean change of Modified Ashworth Scale for spasticity not crude numbers.46
Patients with MS suffer from spasticity based on demyelinating plaques of CNS, and damage to descending spinal pathways (corticospinal, reticulospinal, and vestibulospinal).47Factors such as male sex, duration of MS disease, higher level of disability, and relapses play roles in developing MS-related spasticity.
Urinary tract infections, distension of the urinary bladder and rectum, pain, and pressure sores could lead to development, and aggravation of spasticity in MS.48
The most common medication that is used for MS-related spasticity is baclofen followed by benzodiazepines, while their efficacy is not very satisfactory. Botulinum toxin type A is another medication that could reduce muscle tone, but it is partially effective.48
In animal models of MS, both endogenous and exogenous cannabinoids improve spasticity and tremors.49
Cannabinoids activate G protein-coupled receptors (GPCRs), leading to increased synthesis of cyclic adenosine monophosphate (cAMP), and activation of cAMP-dependent protein kinase (PKA) that helps phosphorylation of channel protein. All these effects result in ionic permeability modification.50
The administration of cannabinoids to control MS-related spasticity may help patients, but larger multicentric studies are needed.
This study had some limitations. First, the inclusion and exclusion criteria differed between included studies. Second, some studies applied NRS while others used Ashworth scale for spasticity evaluation. Third, the duration of disease differed between studies.
Conclusion
The results of this systematic review and meta-analysis showed that nabiximols was the most common cannabinoid which was used to control MS-related spasticity, and it was effective in controlling MS-related spasticity (significantly decreased SMD of NRS, and Ashworth after treatment).
Footnotes
Authors’ contribution: MA was involved in conceptualization, investigation, writing—original draft, and writing—review & editing; MP in conceptualization, formal analysis, andwriting—review & editing; FG in investigation, data curation, and writing—review & editing; SZE-R in project administration, supervision, visualization, and writing—review & editing; ETB in visualization, data curation, and writing—review & editing; MG in methodology, software, formal analysis, and writing—original draft; and MR in methodology, software, formal analysis, and writing—original draft.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ensieh Taftian-Banadkouki https://orcid.org/0000-0002-4305-6966
Contributor Information
Mohaddeseh Azadvari, Physical Medicine and Rehabilitation Department, Sina Hospital, School of medicine, Tehran University of Medical Sciences, Tehran, Iran; Urology Research Center, Tehran University of Medical Sciences, Tehran, Iran.
Maryam Pourshams, Department of Psychiatry, Golestan Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Fatemeh Guitynavard, Urology Research Center, Thran University of Medical Sciences, Tehran, Iran; Urology Department, Sina Hospital, School of medicine, Tehran University of Medical Sciences, Tehran, Iran.
Seyede Zahra Emami-Razavi, Physical Medicine and Rehabilitation, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran; Joint Reconstruction Research Center (JRRC), Imam Khomeini Hospital complex, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Ensieh Taftian-Banadkouki, Physical Medicine and Rehabilitation, Shariati Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
References
- 1.Ghajarzadeh M, Jalilian R, Sahraian MA, et al. Pain in patients with multiple sclerosis. Maedica (Buchar) 2018; 13: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghajarzadeh M, Mahsa O, Sauraian MA, et al. Emotional intelligence (EI) of patients with multiple sclerosis (MS). Iran J Public Health 2014; 43: 1550. [PMC free article] [PubMed] [Google Scholar]
- 3.Izquierdo G. Multiple sclerosis symptoms and spasticity management: new data. Neurodegener Dis Manag 2017; 7: 7–11. [DOI] [PubMed] [Google Scholar]
- 4.Patti F, Chisari CG, Solaro C, et al. Effects of THC/CBD oromucosal spray on spasticity-related symptoms in people with multiple sclerosis: results from a retrospective multicenter study. Neurol Sci 2020; 41: 2905–2913. [DOI] [PubMed] [Google Scholar]
- 5.Otero-Romero S, Sastre-Garriga J, Comi G, et al. Pharmacological management of spasticity in multiple sclerosis: systematic review and consensus paper. Mult Scler J 2016; 22: 1386–1396. [DOI] [PubMed] [Google Scholar]
- 6.Zettl UK, Rommer P, Hipp Pet al. et al. Evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management of patients with spasticity due to multiple sclerosis. Ther Adv Neurol Disord 2016; 9: 9–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnitzler A, Ruet A, Baron S, et al. Botulinum toxin A for treating spasticity in adults: costly for French hospitals? Ann Phys Rehabil Med 2015; 58: 265–268. [DOI] [PubMed] [Google Scholar]
- 8.Cameron MH, Bethoux F, Davis Net al. et al. Botulinum toxin for symptomatic therapy in multiple sclerosis. Curr Neurol Neurosci Rep 2014; 14: 1–7. [DOI] [PubMed] [Google Scholar]
- 9.Ingram G, Pearson OR. Cannabis and multiple sclerosis. Pract Neurol 2019; 19: 310–315. [DOI] [PubMed] [Google Scholar]
- 10.Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep 2018; 18: 1–10. [DOI] [PubMed] [Google Scholar]
- 11.Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 2011; 18: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021; 88: 105906. [DOI] [PubMed] [Google Scholar]
- 13.Thomson H, Craig P, Hilton-Boon M, et al. Applying the ROBINS-I tool to natural experiments: an example from public health. Syst Rev 2018; 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minozzi S, Cinquini M, Gianola S, et al. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol 2020; 126: 37–44. [DOI] [PubMed] [Google Scholar]
- 15.Vecchio D, Varrasi C, Virgilio E, et al. Cannabinoids in multiple sclerosis: a neurophysiological analysis. Acta Neurol Scand 2020; 142: 333–338. [DOI] [PubMed] [Google Scholar]
- 16.Koehler J, Feneberg W, Meier Met al. et al. Clinical experience with THC: CBD oromucosal spray in patients with multiple sclerosis-related spasticity. Int J Neurosci 2014; 124: 652–656. [DOI] [PubMed] [Google Scholar]
- 17.Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res 2010; 32: 451–459. [DOI] [PubMed] [Google Scholar]
- 18.Squintani G, Donato F, Turri M, et al. Cortical and spinal excitability in patients with multiple sclerosis and spasticity after oromucosal cannabinoid spray. J Neurol Sci 2016; 370: 263–268. [DOI] [PubMed] [Google Scholar]
- 19.Freidel M, Tiel-Wilck K, Schreiber H, et al. Drug-resistant MS spasticity treatment with sativex® add-on and driving ability. Acta Neurol Scand 2015; 131: 9–16. [DOI] [PubMed] [Google Scholar]
- 20.Marinelli L, Mori L, Canneva S, et al. The effect of cannabinoids on the stretch reflex in multiple sclerosis spasticity. Int Clin Psychopharmacol 2016; 31: 232–239. [DOI] [PubMed] [Google Scholar]
- 21.Patti F, Messina S, Solaro C, et al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J Neurol Neurosurg Psychiatry 2016; 87: 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaney C, Heinzel-Gutenbrunner M, Jobin P, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler J 2004; 10: 417–424. [DOI] [PubMed] [Google Scholar]
- 23.Centonze D, Mori F, Koch G, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci 2009; 30: 531–534. [DOI] [PubMed] [Google Scholar]
- 24.Paolicelli D, Direnzo V, Manni A, et al. Long-term data of efficacy, safety, and tolerability in a real-life setting of THC/CBD oromucosal spray-treated multiple sclerosis patients. J Clin Pharmacol 2016; 56: 845–851. [DOI] [PubMed] [Google Scholar]
- 25.Flachenecker P, Henze T, Zettl UK. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur Neurol 2014; 72: 95–102. [DOI] [PubMed] [Google Scholar]
- 26.Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice-results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol 2014; 71: 271–279. [DOI] [PubMed] [Google Scholar]
- 27.Sartori A, Dinoto A, Stragapede L, et al. Nabiximols and botulinum toxin injections for patients with multiple sclerosis: efficacy on spasticity and spasms in a single-centre experience. Neurol Sci 2021; 42: 1–7. [DOI] [PubMed] [Google Scholar]
- 28.Chisari CG, Solaro C, Annunziata P, et al. Nabiximols discontinuation rate in a large population of patients with multiple sclerosis: a 18-month multicentre study. J Neurol Neurosurg Psychiatry 2020; 91: 914–920. [DOI] [PubMed] [Google Scholar]
- 29.Lus G, Cantello R, Danni MC, et al. Palatability and oral cavity tolerability of THC: CBD oromucosal spray and possible improvement measures in multiple sclerosis patients with resistant spasticity: a pilot study. Neurodegener Dis Manag 2018; 8: 105–113. [DOI] [PubMed] [Google Scholar]
- 30.Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols*(Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 2011; 18: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 31.Gustavsen S, Søndergaard H, Linnet K, et al. Safety and efficacy of low-dose medical cannabis oils in multiple sclerosis. Mult Scler Relat Disord 2021; 48: 102708. [DOI] [PubMed] [Google Scholar]
- 32.D’hooghe M, Willekens B, Delvaux V, et al. Sativex®(nabiximols) cannabinoid oromucosal spray in patients with resistant multiple sclerosis spasticity: the Belgian experience. BMC Neurol 2021; 21: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messina S, Solaro C, Righini I, et al. Sativex in resistant multiple sclerosis spasticity: discontinuation study in a large population of Italian patients (SA. FE. study). PLoS ONE 2017; 12: e0180651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol 2013; 260: 285–295. [DOI] [PubMed] [Google Scholar]
- 35.Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ 2012; 184: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vermersch P, Trojano M. Tetrahydrocannabinol: cannabidiol oromucosal spray for multiple sclerosis-related resistant spasticity in daily practice. Eur Neurol 2016; 76: 216–226. [DOI] [PubMed] [Google Scholar]
- 37.Maniscalco GT, Aponte R, Bruzzese D, et al. THC/CBD oromucosal spray in patients with multiple sclerosis overactive bladder: a pilot prospective study. Neurol Sci 2018; 39: 97–102. [DOI] [PubMed] [Google Scholar]
- 38.Carotenuto A, Iodice R, Petracca M, et al. Upper motor neuron evaluation in multiple sclerosis patients treated with Sativex®. Acta Neurol Scand 2017; 135: 442–448. [DOI] [PubMed] [Google Scholar]
- 39.Coghe G, Pau M, Corona F, et al. Walking improvements with nabiximols in patients with multiple sclerosis. J Neurol 2015; 262: 2472–2477. [DOI] [PubMed] [Google Scholar]
- 40.Ferrante F, Polito G, Ferraro M, et al. 5PSQ-081 Δ-9-tetrahydrocannabinol (sativex) for the treatment of multiple sclerosis spasticity: evaluation of effectiveness and safety. Eur J Hosp Pharm 2019; 26. [Google Scholar]
- 41.Patti F, Chisari CG, Fernández O, et al. A real-world evidence study of nabiximols in multiple sclerosis patients with resistant spasticity: analysis in relation to the newly described ‘spasticity-plus syndrome. Eur J Neurol 2022; 29: 2744–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pau M, Porta M, Spinicci G, et al. Change in upper limb function in people with multiple sclerosis treated with nabiximols: a quantitative kinematic pilot study. Neurol Sci 2023; 44: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guger M, Hatschenberger R, Leutmezer F. Non-interventional, prospective, observational study on spasticity-associated symptom control with nabiximols as add-on therapy in patients with multiple sclerosis spasticity in Austria. Brain Behav 2023; 13: e2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gajofatto A, Cardobi N, Gobbin F, et al. Resting-state functional connectivity in multiple sclerosis patients receiving nabiximols for spasticity. BMC Neurol 2023; 23: 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Killestein J, Hoogervorst ELJ, Reif M, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002; 58: 1404–1407. [DOI] [PubMed] [Google Scholar]
- 46.Zajicek JP, Sanders H, Wright D, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 2005; 76: 1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Safarpour Y, Mousavi T, Jabbari B. Botulinum toxin treatment in multiple sclerosis—a review. Curr Treat Options Neurol 2017; 19: 1–14. [DOI] [PubMed] [Google Scholar]
- 48.Malfitano AM, Proto MC, Bifulco M. Cannabinoids in the management of spasticity associated with multiple sclerosis. Neuropsychiatr Dis Treat 2008; 4: 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol 2007; 150: 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Śmiarowska M, Białecka M, Machoy-Mokrzyńska A. Cannabis and cannabinoids: pharmacology and therapeutic potential. Neurol Neurochir Pol 2022; 56: 4–13. [DOI] [PubMed] [Google Scholar]