Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Nov 1;25(21):11764. doi: 10.3390/ijms252111764
Abstract
Alzheimer’s disease (AD) is a challenging medical issue that requires efficacious treatment options to improve long-term quality of life. Cannabidiol (CBD) is a cannabis-derived phytocannabinoid with potential health benefits, including reports from our laboratory and others showing a therapeutic role in the pre-clinical treatment of AD; however, the mechanisms whereby CBD affects AD progression remain undefined. Innate lymphoid cells (ILCs) are recently discovered immune cells that initiate and orchestrate inflammatory responses. ILC2, a sub-class of ILCs, is proposed to have a role in cognitive function via unknown mechanisms. In this present study, we explored whether CBD ameliorates AD symptoms via the enhancement of acetylcholine (ACh), a cholinergic neurotransmitter involved in cognition that may regulate ILC2. 5xFAD mice were chronically treated by inhalation of a formulation of broad-spectrum CBD for seven months. ACh production, ILC2s profile, brain histopathology, and long-term behavior were assessed. Together, our studies showed that long-term inhalation of CBD improved cognitive function and reduced senile plaques in a murine AD model, effects that were associated with enhanced ACh production and altered ILC2s distribution within the CNS. These findings indicate that inhaled CBD could offer a cost-effective, non-invasive, and effective treatment for managing AD. The beneficial effects of CBD inhalation may be linked to increased ACh production and an altered distribution of ILC2s, highlighting the need for further research in this area.
1. Introduction
Alzheimer’s disease (AD) is a multifaceted disorder characterized by progressive and irreversible cognitive deterioration, which imposes a significant emotional and financial strain on society [1]. Often referred to as the “Silver Tsunami” of the 21st century, if the burden continues to grow at the same rate as in the past decade, AD is projected to impact over 150 million people worldwide with a financial encumbrance of $1.1 trillion by 2050, becoming the most challenging and expensive medical disorder globally. Although there have been gradual improvements in the treatment and understanding of Alzheimer’s disease (AD) pathophysiology, no definitive therapy exists to prevent or slow its progression [2,3]. Current treatments address only the symptoms rather than the complex underlying causes, leading to incomplete and ineffective management of the disease. Recent medications for AD, such as aducanumab and lecanemab, have received approval from the Food and Drug Administration (FDA) and show promising therapeutic potential. However, their long-term side effects and overall efficacy are still being investigated [4].
Cannabidiol (CBD) is a relatively safe, non-psychoactive phytocannabinoid produced by cannabis plants. Recent work carried out at our laboratory and others suggests a beneficial effect of CBD, either alone or in combination with other cannabinoids, in pre-clinical models of AD and neurodegeneration [1,5]. In particular, we reported that short-term administration of CBD alleviated the symptoms of AD, including reduced β-amyloid peptide (Aβ) deposition, in 5xFAD mice via modulation of TREM2 and IL-33 [1]. However, the molecular and cellular mechanisms underlying the ability of CBD to slow neurodegenerative processes are largely unknown. Moreover, the efficacy of chronic inhalant CBD remains unexplored as a therapy for AD.
The interplay between the nervous and immune systems is critical for the maintenance of physiological homeostasis [6,7]. In particular, the cholinergic system orchestrates bi-directional crosstalk between the nervous system and immunologic components. The cholinergic system consists of neurotransmitters, receptors, and enzymes that dynamically work in concert to transmit signals from the central and peripheral nervous systems to the immune network [8,9]. Acetylcholine (ACh), the most abundant neurotransmitter, is composed of an ester linking acetic acid and choline [10,11]. ACh regulates a diverse set of physiologic processes, including muscle contraction, sensory gating, arousal, learning, attention, and memory [10,11,12]. In fact, the introduction of the “cholinergic hypothesis” of Alzheimer’s disease (AD) was based on ACh deficiency as a way to explain the excessive neuroinflammation, extracellular deposition of β-amyloid (Aβ), and irregular phosphorylation of tau protein, all of which affect cognitive function [13,14]. Given the excitatory nature of ACh, any disturbance in cholinergic signaling may profoundly and irreversibly affect neuro-immune homeostasis [15], accelerating cognitive demise and earlier onset dementia [16,17]. Thus, targeted modulation of ACh may provide an attractive therapeutic approach to ameliorate the negative consequences of AD [18,19,20].
Although mainly produced by cholinergic neurons, immune cells of lymphocytic lineage, including B and T cells, and innate lymphoid cells (ILCs), may also produce ACh [21,22,23,24]. ILCs are functionally diverse immunomodulatory cells with crucial roles in the initiation and development of inflammatory responses, as well as tissue remodeling and homeostasis [25,26]. ILCs are tissue-resident cells, usually functionally quiescent and in a state of homeostasis, characterized by several unique cellular features that distinguish them from other lymphocytes and myeloid cells [27,28]. ILCs, which are divided into three subgroups (ILC1, ILC2, and ILC3) based on phenotype and function, lack known lineage markers, do not express antigen-specific B and T cell receptors, and exhibit a lower frequency than classical adaptive lymphocytes [29,30]. ILCs strategically reside at tissue gateways and barriers, rapidly responding to dynamic stimuli to orchestrate and coordinate immune responses to distinct challenges [25,31].
Several studies have indicated that ILC2s play a significant role in improving cognitive function and alleviating pathologic age-related symptoms [32,33]. Further, large accumulations of IL-5/IL-13-expressing ILC2s in the choroid plexus of aged mice have already been reported [32,34]. The induction of IL-5 and IL-13 by ILC2s has been shown to regulate neuroinflammatory responses, improving symptoms of age-related cognitive decline [33,35]. Such a protective role suggests that ILC2s could be a potential target as an immunotherapeutic modality in the treatment of several age-related disorders and deserves further investigation.
Herein, we showed that long-term cognitive benefits of inhalant CBD were associated with enhanced ACh production and changes in the profile of ILCs in a mouse model of AD, suggesting an immune-cholinergic approach in the treatment of AD.
2. Results
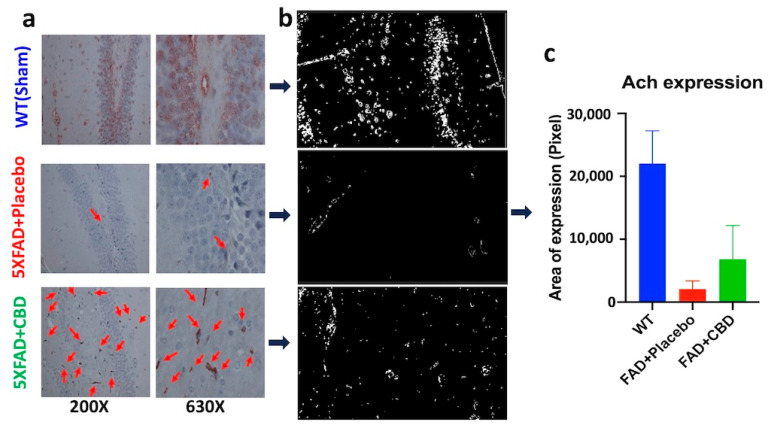
2.1. Long-Term Treatment with Inhaled CBD Increased Expression of Hippocampal ACh in AD Mice
Long-term treatment with inhaled CBD for seven months induced brain ACh expression in 5xFAD mice compared to those given the placebo treatment (Figure 1a). Hippocampal ACh expression in CBD-treated mice was significantly higher than ACh expression in AD mice treated with placebo (p > 0.05). ACh expression in all experimental groups was quantified using the ImageJ software version 1.53e (Figure 1b,c).
Figure 1.
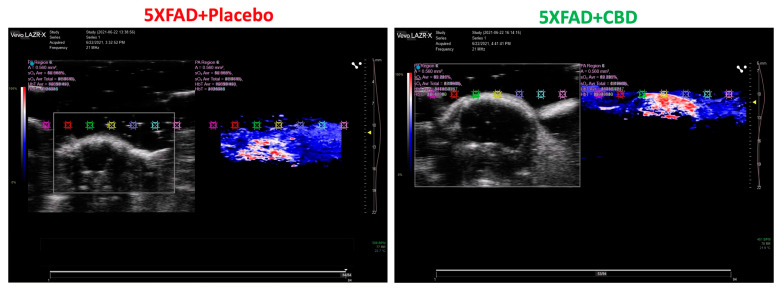
2.2. Inhalant CBD Improved Cerebral Blood Flow in AD
Photoacoustic imaging of the brain demonstrated that inhalant CBD improved the cerebral blood flow significantly in 5xFAD mice compared to the placebo-treated group (Figure 2). The visual overview of blood flow was obtained by color doppler and was based on the color intensity.
Figure 2.
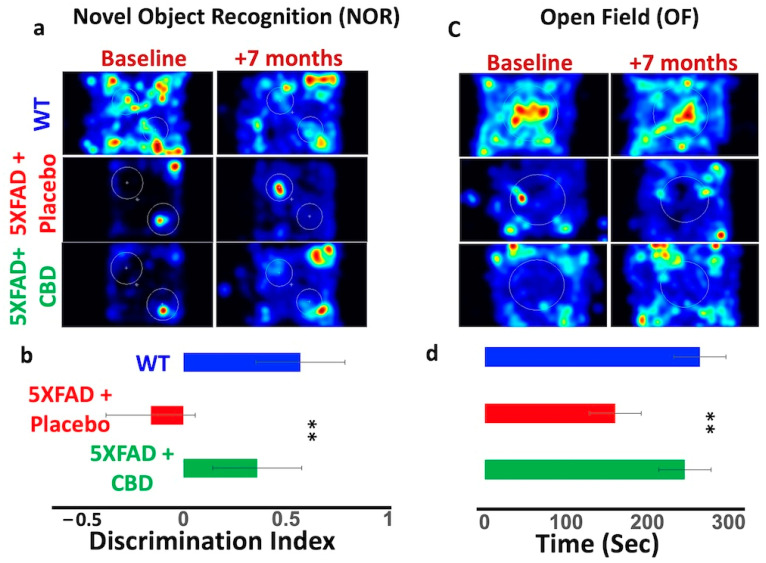
2.3. CBD-Induced ACh Was Associated with Improvement in Cognitive Function in AD
A seven-month treatment with inhaled CBD improved cognitive function in 5xFAD mice compared to placebo-treated mice (Figure 3). The NOR test suggested an improvement in cognitive function in CBD-treated mice, evidenced by more time spent on exploration compared to the placebo group (DI increased to 0.4 ± 0.9 from −0.25 ± 0.7, p ≤ 0.03) (Figure 3a,b). Similarly, the OF test indicated differences in spatial distribution of activity, with the CBD-treated 5xFAD group showing increased time in the central zone (260 ± 70 s) compared to placebo-treated mice (180 ± 80 s, p ≤ 0.05) (Figure 3c,d).
Figure 3.
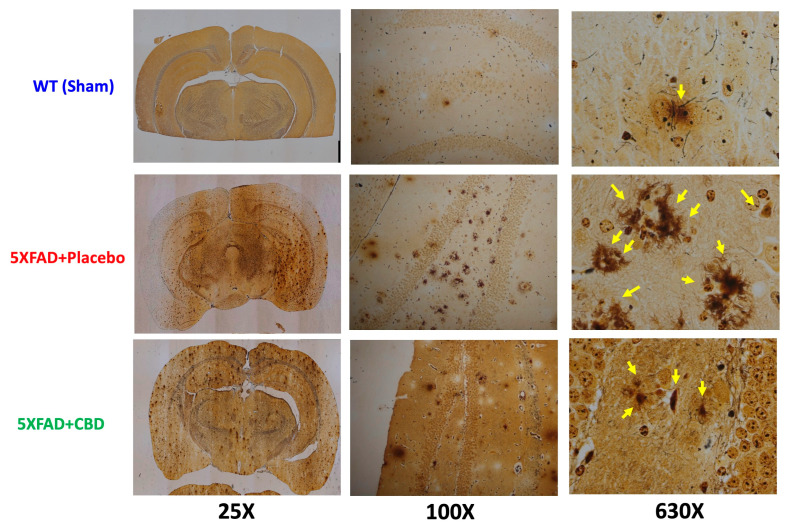
2.4. Long-Term Inhaled CBD Reduced Senile Plaques in AD
The Bielschowsky silver stain demonstrated that treatment with inhaled CBD reduced the occurrence of neurofibrillary tangles and senile plaques (amyloid plaques) compared to placebo-treated 5xFAD mice (Figure 4). Senile plaques are extracellular deposits of the amyloid beta (Aβ) protein, and their reduction in CBD-treated subjects was consistent with improvements in cognitive function.
Figure 4.
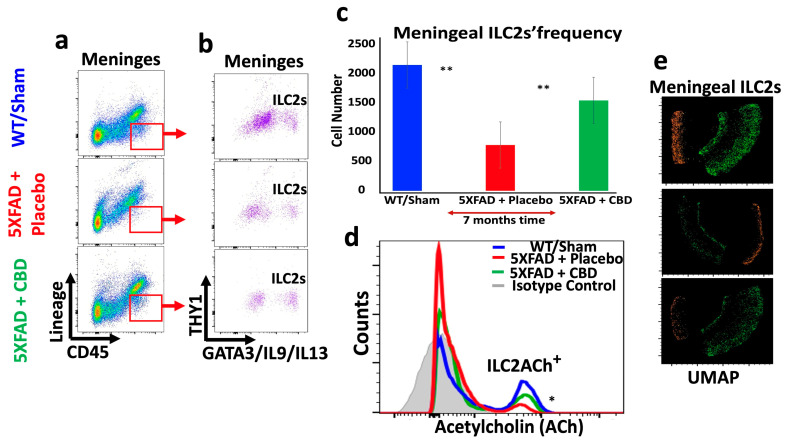
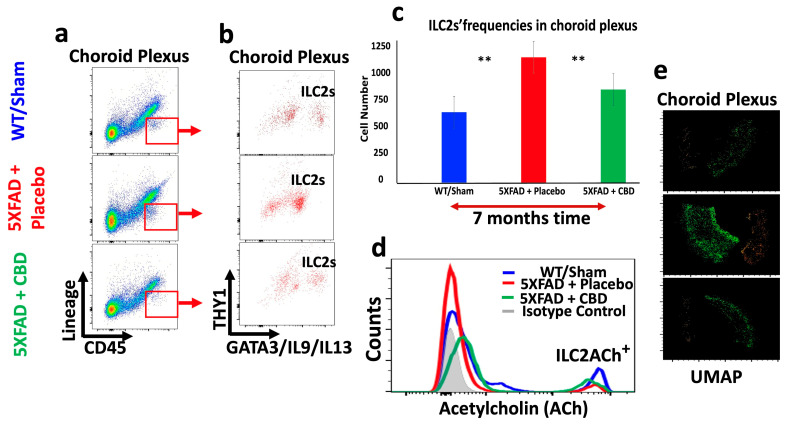
2.5. Inhaled CBD Changed the Profile of ILC2s in the Meninges and Choroid Plexus of 5xFAD Mice and Their Level of ACh Expression
Long-term treatment with inhaled CBD had profound impacts on the immunologic profile of brain of 5xFAD mice. CBD treatment reversed the frequency of meningeal ILC2s towards a level closer to WT mice, significantly higher than in the meninges of 5xFAD mice treated with placebo (p < 0.04) (Figure 5a–c). Concurrently, CBD treatment reduced the frequency of ILC2s in the CP of 5xFAD mice, significantly lower than that in placebo-treated mice (** p ≤ 0.02), and closer to the level in WT mice (Figure 6a–c). These novel findings are important since ILC2s are playing crucial roles in shaping brain function, orchestrating neuroinflammation, and optimizing cognitive function. More importantly, long-term treatment of 5xFAD mice with inhaled CBD increased ACh production in their meningeal ILC2s significantly compared to their counterparts treated with placebo (Figure 5d and Figure 6d). Given the nature of ACh as a signaling molecule, its expression by ILC2s could suggest a major regulatory mechanism in containing the excessive neuroinflammatory responses in AD. Additionally, the UMAP analysis showed a profound alteration in the distribution pattern of ILC2s in both meninges and the CP after CBD treatment compared to the placebo-treated 5xFAD mice (Figure 5e and Figure 6e).
Figure 5.
Figure 6.
3. Discussion
The findings presented here in this current study are significant and novel at several levels. This is the first report to suggest that the interplay between CBD and the cholinergic system could be crucial for the beneficial effects of CBD in the treatment of AD in a pre-clinical setting. We had previously shown that CBD ameliorated the adversarial symptoms and improved cognitive function in a murine model of AD [1]. The exact mechanisms by which CBD affects the pathophysiology of AD are still not completely understood [36]. However, various studies indicate that CBD may offer symptomatic relief or potentially slow the progression of AD through its anti-inflammatory, antioxidative, and neurogenic properties [36]. At the molecular level, CBD is recognized as an inverse agonist of cannabinoid receptors, and this modulation has been shown to be beneficial in preventing AD-related pathology [36]. Importantly, it is proposed that long-term treatment with CBD may ameliorate the AD symptoms through the upregulation of mRNA levels and its role in the amendment and optimization of autophagy in AD [36]. Interestingly, it is shown that activation of the alpha7 nicotinic acetylcholine receptor (a7 nAChR) can enhance autophagy and contributes to neuronal survival [37]. Therefore, it is plausible to hypothesize that long-term CBD inhalation increases ACh levels and its receptors, leading to the modulation of impaired autophagy in AD. Further, it is well established that a deficiency of ACh in patients with AD results in a gradual and considerable decline in cognitive and behavioral functions [19]. Our results demonstrated that long-term CBD treatment was able to increase ACh levels in a murine model of AD with no additional complications. Thus, it is rational to propose that CBD could provide a safe, natural, and affordable booster of ACh to improve the cognitive function and to achieve better outcomes in the treatment of AD, and warrants further investigations.
Increasing evidence accentuate that ACh is a key molecule in the regulation of immunoinflammatory responses and plays a crucial role in maintaining homeostasis [38]. In this study, we showed for the first time that long-term inhalation of broad-spectrum CBD induced ACh expression within the hippocampus and meningeal ILC2, paralleling reduced amyloid accumulation and cognitive improvements in 5xFAD mice. Given the role of ACh in regulating the inflammation [38], our results support the notion that targeting the cholinergic system provides an effective therapeutic modality in the treatment of AD and other neurodegenerative diseases. Enhancement of ACh expression by CBD maybe an important factor by which CBD could modulate the neuroinflammation within CNS and to mitigate the adversarial symptoms of AD.
Regulation of the cholinergic network may provide a mechanism to explain how CBD exerts potent anti-inflammatory effects in AD. ILCs maintain homeostasis and regulate the initiation and development of inflammatory responses during injury and infection. Of the three sub-classes of ILCs, ILC2s are specifically associated with cognitive function [32,39]. Notably, ILC2s accumulate within the choroid plexus during neuropathology and in the late stages of natural aging [32,33,40], while the cerebral meninges are a major residential site for ILC2s in younger ages and under healthy status [29,34]. The shift in location and accumulation of ILC2s from meninges to the CP during the aging process is associated with cognitive deficiency and impairment [32,33,41]. Our findings indicate that inhalation of CBD reverses the depletion of ILC2s from meninges and normalizes ILC2 numbers within the choroid plexus, which may calibrate the function of the choroid plexus and meninges, re-establish immune balance, and improve cognitive function. Given the beneficial effects of ACh in the treatment of AD, our observation that inhaled CBD increased ACh-expressing ILCs may provide a framework for development of a mechanistically innovative immunotherapeutic for the treatment of AD.
Moreover, inhalation as a mode of drug delivery has a number of advantages. Inhaled CBD is non-invasive, easy to apply, highly accurate with respect to dosing and target engagement, affordable, and readily available. Our studies suggest long-term CBD inhalation is both safe and efficacious in a murine AD model; however, further studies are needed in other species and models to establish whether CBD may exhibit high translational value.
Study Limitations
This study has potential limitations. The expression of ACh in different brain areas, timing/stages of disease, CBD dosing/formulations, gender effect, and profile of different sub-classes of ILCs are examples of such limitations, and warrant further research. Thus, a more thorough investigation is essential to assess the safety, pharmacokinetics, pharmacodynamics, and, most critically, the efficacy of cannabinoid-based drugs in the treatment of AD.
4. Materials and Methods
4.1. Declaration Regarding Humane Use of Animals
All mouse experiments were conducted in accordance with the principles of the ‘Three Rs’ (Replacement, Reduction, and Refinement) and were approved by the Institutional Animal Care and Use Committee (IACUC) at Augusta University (Augustsa, GA, USA). Procedures were designed to minimize discomfort and distress to the animals, and all animal care practices adhered to the highest standards of humane treatment as outlined by the NIH (National Institute of Helath, Bethesda, Maryland, USA) guidelines.
4.2. Experimental Design and Treatment Protocol
Adult male 5xFAD mice were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). 5xFAD mice express human amyloid precursor protein (APP) and preseniln-1 (PSEN1) transgenes with five AD-linked mutations, used as a pre-clinical model of AD [1]. At five months, mice were randomized into two groups (n = 10/group), receiving either placebo or inhaled CBD (10 mg/mouse, Thriftmaster Global Bioscience, Dallas, TX, USA) every other day for seven consecutive months. Each CBD inhaler contained 985 mg of broad-spectrum CBD (winterized crude hemp extract) plus 15 mg of co-solvent, surfactant, and propellant, with a total volume of 1000 mg (5 mg dose per actuation, with 200 mL/min flow rate). For the placebo, the 985 mg of broad-spectrum CBD was replaced with 985 mg of hemp seed oil. As described previously [42], inhalers were modified by adding an extra nozzle piece to adjust to the mouse model and to further control the intake of CBD.
4.3. Photoacoustic Imaging of Brain
Cerebral blood flow was measured using a FujiFilm VEVO 3100 imaging system. Ultrasound imaging (FUJIFILM VisualSonics Corporation, Bothell, WA, USA) was used to determine the morphologic regularity and blood flow in brain tissues, measured using the laser photoacoustic method. The key imaging parameters were frequency, dynamic range, depth of field, gain setting, and frame rate. After scanning, multiple frames of ultrasound data were captured to create a comprehensive view of the anatomical structures. Advanced algorithms were employed to enhance image quality, improve contrast, and reduce noise, providing clearer visuals for analysis.
4.4. Behavioral Tests
Open Field (OF) and Novel Object Recognition (NOR) tests were used to assess cognitive outcomes as described previously [1]. For NOR testing, mice were placed in an enclosed box with two identical objects that were placed within a 10 cm circle, at a set distance apart. Mice were then removed from the environment for a predetermined amount of time, and one of the two previously used (familiar) objects was replaced with a novel object that was different from the familiar object in shape, texture, and appearance. The ability of the mouse to discriminate between the familiar and novel object was quantified as a discrimination index, DI = (Tn − Tf)/(Tn + Tf), where Tn is the time spent by the mouse with the novel object, and Tf indicates the time spent with the familiar object. In OF evaluation, mice were tested in a square box (40 cm by 40 cm by 40 cm) for 10 min, and activity was digitally recorded. Distance traveled, mean velocity, and time spent in the center zone were analyzed with Ethovision XT video-tracking 17.5 software (Noldus Information Technology, Leesburg, VA, USA).
4.5. Immunohistochemistry
ACh immunostaining was performed, as previously described [1,42]. Briefly, 5 μm formalin-fixed and paraffin-embedded brain tissue sections were mounted onto glass slides and incubated with a specific antibody against ACh (LS-C295819, LSBio, Seattle, WA, USA), and incubated at 4 °C overnight. Preparations were counterstained with hematoxylin and mounted in Faramount. Slides were imaged by a blinded investigator using bright-field microscopy and quantified using the ImageJ software (version 1.53e).
4.6. Bielschowsky Silver Staining
Freshly harvested brain tissue was fixed in formalin and embedded in paraffin. Serial 8 μm coronal brain sections were cut using a sliding microtome and then sections were deparaffinized prior to staining with a Bielschowsky Stain Kit (Polysciences, Warrington, PA, USA, cat# 25994-250). Slides were rinsed in tap water, fixed in 5% sodium thiosulfate, dehydrated through alcohols and xylene, treated with ammonical silver, and then reduced to visible metallic silver to label senile plaques, axons, and neurofibrillary tangles. Slides were imaged by a blinded investigator with the use of bright-field microscopy, and quantified using the ImageJ software (version 1.53e).
4.7. Analytical Flow Cytometry
Meninges and choroid plexuses were carefully harvested, processed, and analyzed by flow cytometry as previously described [29,42]. ILC2s were identified as Lin−CD45+CD127+GATA3+; with expression of Interleukin-5 (IL-5) and IL-13. All antibodies from BioLegend (San Diego, CA, USA) unless otherwise noted. Cells were then run through a NovoCyte Quanteun flow cytometer (Agilent Technologies, Santa Clara, CA, USA). Cells were gated based on forward and side scatter properties and on marker combinations to select viable cells of interest. Single stains were used to set compensation, and isotype controls were used to determine the level of nonspecific binding. Quantified measurement was performed using FlowJo (version 11.0) analytical software (FlowJo LLC, Ashland, OR, USA). Further analysis was performed by measuring ACh expression by ILC2s (LS-C691651, LSBio, Seattle, WA, USA). As previously described [43], the FlowJo plugin for the algorithm “uniform manifold approximation and projection” (UMAP) was used to perform and display the pattern distribution of meningeal ILC2s.
4.8. Statistical Analysis
Brown-Forsythe and Welch ANOVA were employed to determine significance (p < 0.05) among the groups and for statistical analysis. These methods assessed not only the equality of group means but also adjusted the denominator of the F ratio to ensure it had the same expected value as the numerator.
5. Conclusions
The new findings from this study suggest a possible mechanism connecting the immuno-protective effects of CBD in AD to the expression of ACh in the cholinergic system. These compelling results indicate that, by enhancing ACh expression as a crucial regulatory molecule, CBD helps restore homeostasis by modulating inflammatory responses driven by ILC2s. The interaction between CBD and ACh influences the frequency, distribution, and function of ILC2s in the meninges and the choroid plexus, proposing a novel neuro-immunotherapeutic role for CBD as a safe and cost-effective treatment for Alzheimer’s disease.
Clues for Future Directions
Although our new findings indicate promising CBD-based therapeutic approaches for AD, they also highlight the intricate interactions between CBD and ACh in the central nervous system, particularly during the onset and progression of the disease. Thus, it is crucial to conduct additional mechanistic studies to explore the specific biochemical pathways through which CBD affects ACh levels and cholinergic function, including receptor interactions and subsequent signaling cascades. Furthermore, there is an urgent need for clinical trials to assess the safety and efficacy of CBD in AD patients, with a focus on its effects on cognitive decline, behavioral symptoms, and ACh-related biomarkers. Only through these clinical trials can objective measures of success and potential adverse effects be assessed.
Acknowledgments
Authors are thankful to ThriftMaster Holding Group for providing the inhalant CBD for this study.
Author Contributions
Conceptualization: B.B. (Babak Baban), L.P.W. and J.C.Y. Methodology: H.K., B.B. (Babak Baban), D.C.H., K.M.D., K.V., L.P.W., J.C.M. and É.L.S.; Formal analysis: B.B. (Babak Baban), S.E.N., É.L.S., H.K., B.B. (Bidhan Bhandari), W.M., H.M.R. and J.G.; Investigation: S.E.N., J.G. and A.P.; Resources: B.B. (Babak Baban), K.M.D., K.V., A.V.T.J. and D.C.H.; Data curation: B.B. (Bidhan Bhandari), W.M., A.V.T.J. and H.M.R.; Writing and original draft preparation: B.B. (Babak Baban), L.P.W., J.C.M., J.C.Y. and A.P.; Writing, review and editing: B.B. (Babak Baban), K.M.D., D.C.H., K.V. and A.P.; Supervision: B.B. (Babak Baban), L.P.W. and D.C.H.; Project administration: L.P.W., H.K., É.L.S., S.E.N., A.V.T.J. and B.B. (Babak Baban); Funding acquisition: B.B. (Babak Baban) and L.P.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All mouse experiments were conducted in accordance with the principles of the ‘Three Rs’ (Replacement, Reduction, and Refinement) and were approved by the Institutional Animal Care and Use Committee (IACUC) at Augusta University (Augustsa, GA, USA) (Protocol# 2011-0062). Procedures were designed to minimize discomfort and distress to the animals, and all animal care practices adhered to the highest standards of humane treatment as outlined by the NIH (National Institute of Helath, Bethesda, Maryland, USA) guidelines.
Informed Consent Statement
Not applicable. No human involvement.
Data Availability Statement
Original data are available upon request, please send requests to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Khodadadi H., Salles É.L., Jarrahi A., Costigliola V., Khan M.B., Yu J.C., Morgan J.C., Hess D.C., Vaibhav K., Dhandapani K.M., et al. Cannabidiol Ameliorates Cognitive Function via Regulation of IL-33 and TREM2 Upregulation in a Murine Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2021;80:973–977. doi: 10.3233/JAD-210026. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar A., Irwin M., Singh A., Riccetti M., Singh A. Alzheimer’s disease: The silver tsunami of the 21st century. Neural Regen. Res. 2016;11:693–697. doi: 10.4103/1673-5374.182680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Flier W.M., de Vugt M.E., Smets E.M.A., Blom M., Teunissen C.E. Towards a future where Alzheimer’s disease pathology is stopped before the onset of dementia. Nat. Aging. 2023;3:494–505. doi: 10.1038/s43587-023-00404-2. [DOI] [PubMed] [Google Scholar]
- 4.Ameen T.B., Kashif S.N., Abbas S.M.I., Babar K., Ali S.M.S., Raheem A. Unraveling Alzheimer’s: The promise of aducanumab, lecanemab, and donanemab. Egypt. J. Neurol. Psychiatry Neurosurg. 2024;60:72. doi: 10.1186/s41983-024-00845-5. [DOI] [Google Scholar]
- 5.Bhunia S., Kolishetti N., Arias A.Y., Vashist A., Nair M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front. Pharmacol. 2022;13:989717. doi: 10.3389/fphar.2022.989717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin I., Kipnis J. Learning and memory, and the immune system. Learn Mem. 2013;20:601–606. doi: 10.1101/lm.028357.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marques R.E., Marques P.E., Guabiraba R., Teixeira M.M. Exploring the Homeostatic and Sensory Roles of the Immune System. Front. Immunol. 2016;7:125. doi: 10.3389/fimmu.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halder N., Lal G. Cholinergic System and Its Therapeutic Importance in Inflammation and Autoimmunity. Front. Immunol. 2021;12:660342. doi: 10.3389/fimmu.2021.660342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., Zhang X., Shi P., Yuan J., Jia Q., Pi C., Chen T., Xiong L., Chen J., Tang J., et al. α7 Nicotinic acetylcholine receptor: A key receptor in the cholinergic anti-inflammatory pathway exerting an antidepressant effect. J. Neuroinflammation. 2023;20:84. doi: 10.1186/s12974-023-02768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sam C., Bordoni B. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2024. Physiology, Acetylcholine. [PubMed] [Google Scholar]
- 11.Changeux J.P. Discovery of the First Neurotransmitter Receptor: The Acetylcholine Nicotinic Receptor. Biomolecules. 2020;10:547. doi: 10.3390/biom10040547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofuoglu M., Mooney M. Cholinergic functioning in stimulant addiction: Implications for medications development. CNS Drugs. 2009;23:939–952. doi: 10.2165/11310920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajmohan R., Reddy P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimers Dis. 2017;57:975–999. doi: 10.3233/JAD-160612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreira N.C.D.S., Lima J.E.B.F., Marchiori M.F., Carvalho I., Sakamoto-Hojo E.T. Neuroprotective Effects of Cholinesterase Inhibitors: Current Scenario in Therapies for Alzheimer’s Disease and Future Perspectives. J. Alzheimer’s Dis. Rep. 2022;6:177–193. doi: 10.3233/ADR-210061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallowitsch-Puerta M., Pavlov V.A. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007;80:2325–2329. doi: 10.1016/j.lfs.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Oliveira J., Kucharska E., Garcez M.L., Rodrigues M.S., Quevedo J., Moreno-Gonzalez I., Budni J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells. 2021;10:2581. doi: 10.3390/cells10102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zieneldien T., Kim J., Sawmiller D., Cao C. The Immune System as a Therapeutic Target for Alzheimer’s Disease. Life. 2022;12:1440. doi: 10.3390/life12091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z.R., Huang J.B., Yang S.L., Hong F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules. 2022;27:1816. doi: 10.3390/molecules27061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanciu G.D., Luca A., Rusu R.N., Bild V., Chiriac B.S.I., Solcan C., Bild W., Ababei D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules. 2020;10:40. doi: 10.3390/biom10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii T., Mashimo M., Moriwaki Y., Misawa H., Ono S., Horiguchi K., Kawashima K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017;8:1085. doi: 10.3389/fimmu.2017.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong L., Nutt S.L., Seillet C. Innate lymphoid cells: More than just immune cells. Front. Immunol. 2022;13:1033904. doi: 10.3389/fimmu.2022.1033904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klose C.S.N., Artis D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020;30:475–491. doi: 10.1038/s41422-020-0323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts L.B., Schnoeller C., Berkachy R., Darby M., Pillaye J., Oudhoff M.J., Parmar N., Mackowiak C., Sedda D., Quesniaux V., et al. Acetylcholine production by group 2 innate lymphoid cells promotes mucosal immunity to helminths. Sci. Immunol. 2021;6:eabd0359. doi: 10.1126/sciimmunol.abd0359. [DOI] [PubMed] [Google Scholar]
- 25.Kim J., Ryu S., Kim H.Y. Innate Lymphoid Cells in Tissue Homeostasis and Disease Pathogenesis. Mol. Cells. 2021;44:301–309. doi: 10.14348/molcells.2021.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baban B., Malik A., Bhatia J., Yu J.C. Presence and Profile of Innate Lymphoid Cells in Human Breast Milk. JAMA Pediatr. 2018;172:594–596. doi: 10.1001/jamapediatrics.2018.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willinger T. Metabolic Control of Innate Lymphoid Cell Migration. Front. Immunol. 2019;10:2010. doi: 10.3389/fimmu.2019.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meininger I., Carrasco A., Rao A., Soini T., Kokkinou E., Mjösberg J. Tissue-Specific Features of Innate Lymphoid Cells. Trends Immunol. 2020;41:902–917. doi: 10.1016/j.it.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Baban B., Braun M., Khodadadi H., Ward A., Alverson K., Malik A., Nguyen K., Nazarian S., Hess D.C., Forseen S., et al. AMPK induces regulatory innate lymphoid cells after traumatic brain injury. JCI Insight. 2021;6:e126766. doi: 10.1172/jci.insight.126766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Q., Seillet C., Belz G.T. Shaping Innate Lymphoid Cell Diversity. Front. Immunol. 2017;8:1569. doi: 10.3389/fimmu.2017.01569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ignacio A., Breda C.N.S., Camara N.O.S. Innate lymphoid cells in tissue homeostasis and diseases. World J. Hepatol. 2017;9:979–989. doi: 10.4254/wjh.v9.i23.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeung S.S., Ho Y.S., Chang R.C. The role of meningeal populations of type II innate lymphoid cells in modulating neuroinflammation in neurodegenerative diseases. Exp. Mol. Med. 2021;53:1251–1267. doi: 10.1038/s12276-021-00660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung I.T.H., Sankar P., Zhang Y., Robison L.S., Zhao X., D’Souza S.S., Salinero A.E., Wang Y., Qian J., Kuentzel M.L., et al. Activation of group 2 innate lymphoid cells alleviates aging-associated cognitive decline. J. Exp. Med. 2020;217:e20190915. doi: 10.1084/jem.20190915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S., van de Pavert S.A. Innate Lymphoid Cells in the Central Nervous System. Front. Immunol. 2022;13:837250. doi: 10.3389/fimmu.2022.837250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamuladze T., Kipnis J. Type 2 immunity in the brain and brain borders. Cell Mol. Immunol. 2023;20:1290–1299. doi: 10.1038/s41423-023-01043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y., Lim C.S. Understanding the Modulatory Effects of Cannabidiol on Alzheimer’s Disease. Brain Sci. 2021;11:1211. doi: 10.3390/brainsci11091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z.Q., Zhang J.J., Kong N., Zhang G.Y., Ke P., Han T., Su D.F., Liu C. Autophagy is Involved in Neuroprotective Effect of Alpha7 Nicotinic Acetylcholine Receptor on Ischemic Stroke. Front. Pharmacol. 2021;12:676589. doi: 10.3389/fphar.2021.676589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han B., Li X., Hao J. The cholinergic anti-inflammatory pathway: An innovative treatment strategy for neurological diseases. Neurosci. Biobehav. Rev. 2017;77:358–368. doi: 10.1016/j.neubiorev.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Barichello T. The role of innate lymphoid cells (ILCs) in mental health. Discov. Ment. Health. 2022;2:2. doi: 10.1007/s44192-022-00006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grigg J.B., Shanmugavadivu A., Regen T., Parkhurst C.N., Ahmed A., Joseph A.M., Mazzucco M., Gronke K., Diefenbach A., Eberl G., et al. Antigen-presenting innate lymphoid cells orchestrate neuroinflammation. Nature. 2021;600:707–712. doi: 10.1038/s41586-021-04136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Si Y., Zhang Y., Zuloaga K., Yang Q. The role of innate lymphocytes in regulating brain and cognitive function. Neurobiol. Dis. 2023;179:106061. doi: 10.1016/j.nbd.2023.106061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khodadadi H., Salles É.L., Alptekin A., Mehrabian D., Rutkowski M., Arbab A.S., Yeudall W.A., Yu J.C., Morgan J.C., Hess D.C., et al. Inhalant Cannabidiol Inhibits Glioblastoma Progression Through Regulation of Tumor Microenvironment. Cannabis Cannabinoid Res. 2023;8:824–834. doi: 10.1089/can.2021.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhandari B., Chagas H.I.S., Naeini S.E., Chagas P.S., Rogers H.M., Gouron J., Khan A., Maciel L.M., Seyyedi M., MacKinnon N.J., et al. Cannabidiol reverses fentanyl-induced addiction and modulates neuroinflammation. bioRxiv. 2024 doi: 10.1101/2024.07.20.604441. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data are available upon request, please send requests to the corresponding author.