Focus Article
Cannabinoid activation of
peroxisome proliferator-activated
receptors: an update and review of
the physiological relevance
Saoirse Elizabeth O’Sullivan∗
Since 2002, evidence has been building that cannabinoids, including endocannabinoids and endocannabinoid-like compounds, phytocannabinoids and synthetic cannabinoid ligands, bind to and activate the different isoforms of the nuclear receptors, peroxisome proliferator-activated receptors (PPARs; α, β, and γ ). This has been shown through the use of reporter gene assays, binding studies, the use of antagonists and knockout animals. Increasing use of tools to assess a potential role for PPAR activation in underpinning the physiological effects of cannabinoids means that a picture is emerging of the relevance of PPAR activation by cannabinoids. There is now evidence that activation of PPARα and γ mediate some of the anti-inflammatory, analgesic, neuroprotective, and cardiovascular effects of cannabinoids, sometimes in combination with activation of the more traditional target sites of action such as CB1, CB2, and TRPV1. There is also a role for PPARα activation by cannabinoids in some of their central effects including memory acquisition, reward processing, food intake and body weight regulation. Activation of PPARγ plays a role in the apoptotic effects of cannabinoids. However, much further work is required to fully establish the profile of cannabinoid compounds at all isoforms of the PPAR family and the relevance of this in normal physiology and pathological situations. © 2013 WILEY-VCH Verlag GmbH
& Co. KGaA,Weinheim.
How to cite this article:
WIREs Membr Transp Signal 2013, 2:17–25. doi: 10.1002/wmts.73
INTRODUCTION
Peroxisome proliferator-activated receptors (PPARs, three isoforms: α, δ, and γ ) are a family of nuclear receptors which heterodimerise with the retinoid X receptor (RXR), and bind to DNA sequences called PPAR response elements (PPRE), leading to changes in the transcription of target genes. Ligand binding to PPARs causes the recruitment of regulator proteins that bind to a third site on PPARs, and these are thought to modulate transactivation. PPAR target genes are primarily involved in the regulation of metabolism and energy homeostasis, cell differentiation and inflammation. The extensive research on PPARs, including their role in disease modulation, has been reviewed elsewhere.1–4 PPARs have large ligand binding domains and are relatively promiscuous receptors, capable of being activated by a large number of natural and synthetic ligands of different chemical structure. Endogenous activators of PPARs include the unsaturated fatty acids linolenic acid, linoleic acid, petroselenic acid and arachidonic acid, with EC50 values in the 2–20 μM range.5 The eicosanoids 15-deoxy-Al2,14- prostaglandin J2 (15d-PGJ2) and 8S-HETE interact with PPARs with an EC50 of around 500 nM.5 By contrast, most synthetic ligands of PPARs have EC50sin the low nanomolar range.6
Focus Article wires.wiley.com/mts
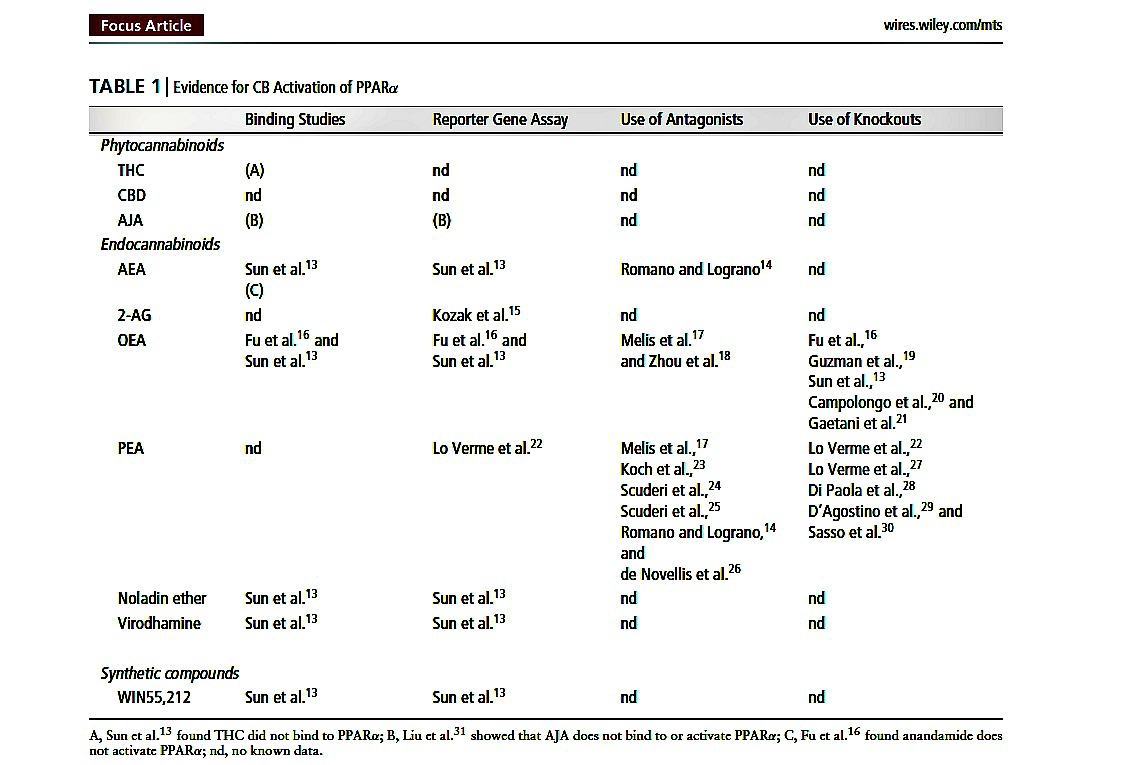
TABLE 1 (below) Evidence for CB Activation of PPARα
As endocannabinoids are fatty acid derivatives, it is not surprising that an increasing body of evidence has shown that endocannabinoids and other cannabinoid compounds also activate PPARs. This review will present the literature implicating a role for cannabinoid activation of PPARs and discuss the physiological effects of cannabinoids which might be mediated through PPAR activation.
REVIEW OF THE EVIDENCE OF PPAR
ACTIVATION BY CANNABINOIDS
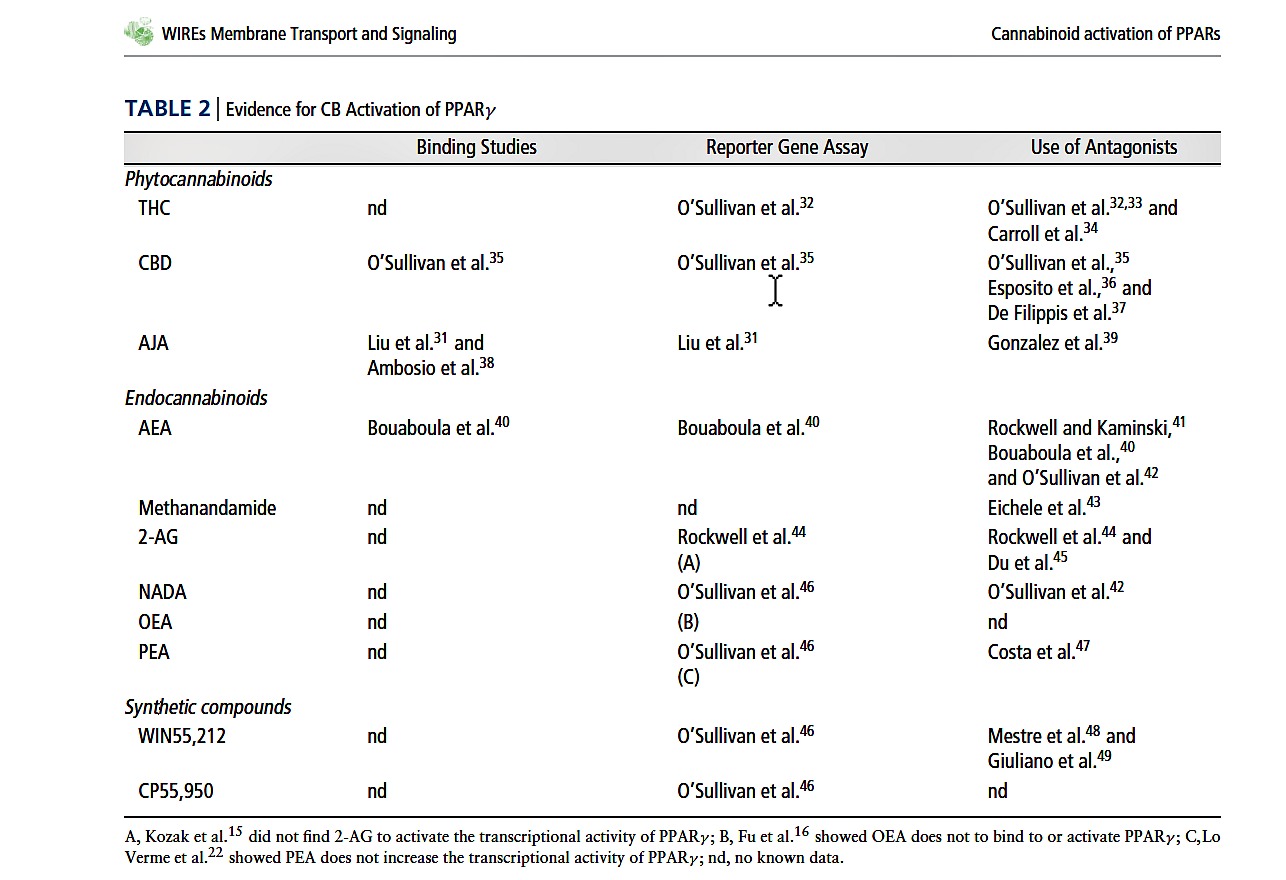
A summary of the current data supporting the activation of PPAR nuclear receptors by cannabinoids is provided in Table 1 (PPARα) and Table 2 (PPARγ ). These tables do not include studies where a role for endocannabinoid activation of PPARs has been implicated after administration of fatty acid amide hydrolase (FAAH) inhibitors7–10 or endocannabinoid uptake inhibitors11,12 to increase local endocannabinoids levels, but where the activating ligand has not been specifically identified.
PHYTOCANNABINOIDS
The phytocannabinoids 9-tetrahydrocannabinol (THC), cannabidiol (CBD) and ajulemic acid (a synthetic analogue of a tetrahydrocannabinol metabolite, AJA) can all bind to, increase the transcriptional activity of, and have effects that are inhibited by an antagonist of PPARγ (see Table 2). By contrast, two studies investigating the potential activity of phytocannabinoids at PPARα found that THC does not bind to PPARα,13 and that AJA does not bind to or activate PPARα.31 Few studies have investigated the effects of other novel phytocannabinoids on PPAR activity, but we showed that tetrahydrocannabivarin (THCV) does not increase the transcriptional activity of PPARγ .33 Thus activation of PPARγ is not universal to all phytocannabinoids, and as yet, there is no evidence that phytocannabinoids activate PPARα.
There is now strong evidence that the endocannabinoid-like compounds oleoylethanolamine (OEA) and palmitoylethanolamide (PEA) activate PPARα, as shown through binding studies, reporter gene assays, the use of antagonists and also the absence of responses to these compounds in PPARα knockout mice (see Table 1). Recently, it has been shown that OEA is transported to the nucleus and PPARα by fatty acid binding proteins.50 2AG, noladin ether and virodhamine have also been implicated in PPARα activation, and some, but not all studies, have shown that anandamide (AEA) activates PPARα, (see Table 1). This suggests, thus far, that most endocannabinoids are activators of this isoform of the PPAR family.
Despite the fact that AEA binds to both PPARα13 and PPARγ ,40 few studies have probed this as a mechanism of action for the physiological effects of AEA, which is also true for 2-AG. Lesser known endocannabinoids such as N-arachidonyl-dopamine (NADA), virodhamine and noladin ether might also have activity at PPARs,13,42 but again, this requires further investigation to establish how much of the effects of endocannabinoids other than OEA and PEA are mediated by PPARs.
Some studies have shown that metabolites of 2-AG are PPAR activators. Raman and colleagues51 showed that 15-deoxy-12,31-PGJ(2)- glycerol ester activates PPARγ in a reporter gene assay, and Kozak and colleagues15 showed that 15-hydroxyeicosatetraenoic acid glyceryl ester increases the transcriptional activity of PPARα. Whether the same is true of metabolites of other endocannabinoids remains to be established.
It is worth pointing out that a study by Lenman & Fowler52 showed that PPARγ ligands inhibited FAAH activity in rat brain homogenates and glioma cells. Thus part of the physiological effects of PPAR agonists may be through upregulation of local endocannabinoid levels by inhibiting their degradation.
SYNTHETIC CANNABINOIDS
Few studies have investigated the potential for synthetic cannabinoid compounds to activate PPARs. WIN55212 has been shown to bind to and activate the transcriptional activity of both PPARα and PPARγ , and to have effects that are antagonized by PPARγ antagonists (see Tables 1 and 2). CP55,950 also increases the transcriptional activity of PPARγ .33
PEROXISOME
PROLIFERATOR-ACTIVATED
RECEPTORS δ
PPARδ is the least investigated of the three PPAR isoforms and there is little information on the effects of cannabinoids at PPARδ. Fu et al.16 showed that OEA activates the transcriptional activity of PPARδ, but no further studies have examined this. AJA,31 2-AG metabolites,15 and PEA22 do not activate PPARδ. However, there may be some interactions between the endocannabinoid system and PPARδ, as Yan et al.53 showed that silencing PPARδ significantly increases CB1 receptor expression, and that over expression of PPARδ significantly reduces CB1 receptor expression.
PHYSIOLOGICAL RESPONSES TO
CANNABINOIDS MEDIATED BY PPARs
Since the first indication that cannabinoids can interact with PPARs, an important task has been to establish how much of the physiological effects of cannabinoids are mediated through activation of nuclear receptors, particularly since their affinity for PPARs tends to be in the micromolar range (although this is not dissimilar to the affinity of other endogenous ligands, see Ref 5). Fortunately, many cannabinoid-based studies have now included the use of tools to assess a role for PPAR activation.
Below is a summary of the evidence for PPAR activation as a mechanism of action for cannabinoids in some of the more commonly recognized physiological effects of cannabinoids; anti-inflammatory actions, neuroprotection, analgesia, and effects on memory reward, the cardiovascular system and metabolism.
ANTI-INFLAMMATORY EFFECTS
One study has shown that the anti-inflammatory effects of CBD on intestinal inflammation in lipopolysachharide (LPS)-treated mice are PPARγ –mediated.37 The anti-inflammatory effects of AJA are also suggested to be a result of PPARγ activation since AJA inhibits the promoter activity of the pro-inflammatory cytokine, interleukin (IL)-8, in a PPARγ -dependent manner.31 However, other studies report that the anti-inflammatory effects of AJA are not PPARγ mediated.54,55 The effects of AJA could be due to increased production of lipoxin A4, the endogenous pro-resolving and anti-inflammatory eicosanoid.56 More recently, AJA was found to inhibit skin fibrosis in mice overexpressing transforming growth factor β, which was sensitive to PPARγ antagonism.39
Rockwell&Kaminski41 found that AEA inhibits the secretion of the pro-inflammatory cytokine, IL-2, in a CB1/CB2receptor-independent manner that could also be prevented by a PPARγ antagonist. 2-AG also inhibits IL-2 secretion through the suppression of pro-inflammatory transcription factors, sensitive to PPARγ antagonism.44 More recently, 2-AG was found to decrease the expression of COX-2 in response to IL1β or LPS, which were sensitive to PPARγ antagonism.45 The 2-AG metabolite 15-deoxy-12,31-PGJ(2)-glycerol ester also has anti-inflammatory actions mediated by PPARγ ,51 so the effects of 2-AG on PPARγ may be due to both activation by 2-AG itself and/or by its metabolites.
Both OEA and PEA have anti-inflammatory actions in 12-O-tetradecanoylphorbol-13-acetateinduced and carregeenan-induced edema that were absent in PPARα knock-out mice.22 Similarly, PEA decreases intestinal inflammation induced by ischemia/reperfusion injury that was reduced in PPARα knock-out mice.28 However, PEA has also been shown to have a protective role in a model of contact dermatitis that was mediated by TRPV1 but not PPARα.57
Upregulation of local endocannabinoid levels by either FAAH inhibition (URB597) or inhibition of the AEA transporter (AM404) significantly potentiated the circulating cytokine response to LPS in rats, and this effect was sensitive to antagonism of CB1, CB2,TRPV1 and PPARγ .11
Thus activation of both PPARγ (AJA, CBD, AEA, and 2-AG) and PPARα (OEA and PEA) underpins some of the anti-inflammatory effects of cannabinoids.
NEUROPROTECTION
OEA reduces infarct volume after cerebral artery occlusion in mice, which is absent in PPARα knockout mice.13 Similarly, Zhou et al.18 showed that OEA improves neurological dysfunction, reduces infarct size and brain edema after cerebral artery occlusion, which is inhibited by PPARα antagonists. Importantly, this effect could be observed when OEA was administered within 1 h of reperfusion. Several studies have now shown that PEA also has neuroprotective effects that are mediated by PPARα; PEA protects against excitotoxicity in hippocampal cultures which is blocked by a PPARα but not PPARγ antagonist,23 blunts the expression of pro-inflammatory molecules in response to β-amyloid in astrocytes in a PPARα-dependent, PPARγ -independent manner,24 and decreases infiltrating astrocytes in hippocampal slices treated with β-amyloid, sensitive to PPARα, but not PPARγ , antagonism.25 CBD also protects against β-amyloid neurotoxicity in rats, but this is through activation of PPARγ .36 THC similarly has neuroprotective effects in a cell culture model of Parkinson’s disease that was not inhibited by CB1, but was inhibited by a PPARγ antagonist.34 In a model of multiple sclerosis, increasing local levels of endocannabinoids by inhibiting their uptake using UCM707, had neuroprotective effects against excitotoxicity which could be inhibited by CB1, CB2, and PPARγ antagonism.12
Thus activation of both PPARγ (CBD, THC, and endocannabinoid upregulation) and PPARα (OEA and PEA) underpins some of the neuroprotective effects of cannabinoids, in combination with activation of more traditional sites such as CB1 and CB2. Several studies have shown that the neuroprotective effects of PEA are not mediated by PPARγ .
ANALGESIA
PEA has analgesic effects in vivo in several different models of pain behavior, which are absent in PPARα knock-out mice.22,30 A PEA analog, palmitoylallylamide, reduces hypersensitivity in neuropathic pain that was inhibited by CB1, CB2, and PPARα antagonists.58 By contract, Costa et al.47 found that the analgesic effects of PEA, also in a model of neuropathic pain, involved CB1,TRPV1, and PPARγ (but not PPARα or CB2), and de Novellis et al.26 found that the analgesic effects of centrally administered PEA are mediated by CB1,TRPV1 and PPARα (PPARγ not examined).
Upregulation of local endocannabinoid levels by inhibition of FAAH with URB597 induces analgesia in an inflammatory pain model, and this was inhibited by a PPARα antagonist but not a CB1antagonist7 or a PPARγ antagonist.8 In the Jhaveri study, URB597 increased local levels of AEA and 2-AG, so either ligand could be activating PPARα, although there is possibly more evidence to suggest that AEA is the ligand responsible (see Table 1). Interestingly, Jhaveri and colleagues also showed that COX2 inhibition increased local PEA levels and caused analgesia that was inhibited by a PPARα antagonist. Another FAAH inhibitor, ST4070, reduces neuropathy, increases AEA and 2-AG levels, and is sensitive to CB1,TRPV1, and PPARα antagonism.59
In summary, activation of both PPARγ (PEA) and PPARα (PEA, upregulation of local endocannabinoids) underpins some of the analgesic effects of cannabinoids, sometimes in addition to activation of CB1, CB2, and TRPV1.
APOPTOSIS
In many different cancer cell lines, WIN55212 (hepatoma HepG2 cells49) and methanandamide (cervical carcinoma cells (HeLa and C33A) and lung carcinoma cells (A549)43) induce apoptosis which can be inhibited with a PPARγ antagonist or by silencing of PPARγ . However, the anti-proliferative effect of the endocannabinoid noladin ether on prostate carcinoma cells was not found to be PPARγ -mediated.60
MEMORY
Mazzola et al.9 showed that memory acquisition in rats is enhanced by the FAAH inhibitor URB597, which was sensitive to PPARα antagonism. Campologno et al.20 showed that OEA administration also has a central memory enhancing effect which was absent in PPARα-null mice. More recently, chronic PEA administration was found to protect against the memory deficits induced by β-amyloid in an Alzheimer’s disease model, which was absent in PPARα-null mice.29 Together, these studies suggest that OEA, PEA and elevated endogenous levels of AEA have positive effects on memory through PPARα activation.
REWARD
Upregulation of local endocannabinoids by URB597, or administration of OEA and PEA, inhibits neuronal responses in the reward area of the brain to nicotine (but not cocaine or morphine61), which was sensitive to both CB1 and PPARα antagonism.17,61 Interestingly, some of the effects of OEA and PEA mediated by activation of PPARα were through nongenomic stimulation of tyrosine kinases.17 A similar effect on nicotine reward mediated by PPARα was seen by the same authors in response to methyl OEA, a long-lasting form of OEA, or to PPARα agonists.62
CARDIOVASCULAR SYSTEM
We have shown that THC causes time-dependent, PPARγ -dependent vasorelaxation in rat isolated arteries (the aorta and superior mesenteric artery) that is dependent on nitric oxide (NO) and hydrogen peroxide (H2O2) production, and superoxide dismutase (SOD) activity.32 Furthermore, in vitro THC enhances vasodilator responses in isolated arteries, which was also inhibited by a PPARγ antagonist.46 A similar time-dependent and PPARγ sensitive vasorelaxant response was seen in response to CBD35 and the endocannabinoids AEA and NADA, but not PEA.63
Interestingly, Romano and Lograno14 recently showed a similar time-dependent vasorelaxant response to AEA and PEA in the bovine ophthalmic artery that could be inhibited by a PPARα, but not PPARγ , antagonist. In a viral model ofmultiple sclerosis,WIN55212 suppressed the increase in intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM) in brain endothelium, sensitive to PPARγ antagonism, but not CB1 or CB2 antagonism.48 In a similar study, an analogue of OEA (OPA) decreased the expression of VCAM and ICAM and monocyte adhesion in response to inflammation in human umbilical endothelial cells, and this appeared to be mediated by PPARα.64
In summary, activation of PPARγ (CBD, THC, AEA, NADA, WIN55212) and PPARα (OPA, AEA, PEA) in vascular cells partly mediates the positive effects of cannabinoids in the vasculature.
METABOLISM
Fu et al.16,65 showed that the appetite-suppressing and weight-reducing effects of OEA were absent in PPARα knock-out mice, and that daily treatment with OEA reduced serum cholesterol levels in rat and mouse models of obesity. Guzman et al.19 also showed that the stimulatory effect of OEA on lipolysis in vivo was absent in PPARα knock-out mice. A number of structural analogues of OEA with a high affinity for PPARα cause similar reductions in food intake.66 More recent work has shown that the anorexic effects of OEA are mediated centrally by oxytocin signaling, which was absent in PPARα knock-out mice.21 Surprisingly, no studies have examined whether the metabolic effects of cannabinoids other than OEA are mediated by activation of PPARs.
NO ROLE FOR PPAR-ACTIVATION
There are several studies which have not found a role for PPAR-activation to underpin the effects of cannabinoids in their experimental model. For example, we have not found a role for either PPARα or PPARγ in mediating the effects of THC, CBD, AEA, 2-AG on intestinal permeability.67,68 Similarly, the effects of OEA in inhibiting upper GI transit69 or in reducing gastric emptying70 are not mediated by PPARα. Some behavioral effects of AEA71 and methanandamide17 are not mediated by PPARα.
CONCLUSION
This review of the current literature suggests that many of the well recognized physiological responses to cannabinoids are at least partly mediated by the activation of PPARs, but this area still requires further research. There are still many cannabinoids compounds whose activity at PPAR remains unknown. For example, there is little known about the effects, if any, of phytocannabinoids on PPARα, there is still almost no data on any potential role for the activation of PPARδ by either endo- or phytocannabinoids, and the effects of some of the lesser known endocannabinoids at PPARs are not known. The majority of evidence for a role for a physiological response via PPAR activation by cannabinoids comes from studies using OEA and PEA, primarily at PPARα, but further work is required to establish how much of the effects of AEA and 2-AG are mediated by nuclear receptors. From a therapeutic point of view, the beneficial effects of PPAR ligands are well recognized in a range of disorders including type II diabetes, cancer, hyperlipidaemia, atherosclerosis, metabolic syndrome, neurodegenerative disorders and nicotine addiction. However, some drugs which target PPARs such as the thiazolidinediones (TZDs), have been associated with severe side effects including heart failure leading to their withdrawal from the market. The excellent tolerability profile of cannabinoids, combined with their known activity at PPARα and γ , warrants further investigation into their potential utility in diseases where PPARs are known to be beneficial.
Conflict of interest: The author declare no conflict of interest.
∗Correspondence to: Saoirse.osullivan@nottingham.ac.uk
School of Graduate Entry Medicine and Health, University of
Nottingham, Royal Derby Hospital, Derby, UK
REFERENCES
1. Menendez-Gutierrez MP, Roszer T, Ricote M. Biology
and therapeutic applications of peroxisome
proliferator-activated receptors. Curr Top Med Chem
2012, 12:548–584.
2. Friedland SN, Leong A, Filion KB, Genest J, Lega
IC, Mottillo S, Poirier P, Reoch J, Eisenberg MJ.
The cardiovascular effects of peroxisome
proliferator-activated receptor agonists. Am J Med
2012, 125:126–133.
3. Poulsen LL, Siersbæk M. Mandrup S.PPARs: Fatty acid
sensors controlling metabolism. Semin Cell Dev Biol
2012, 23:631–639. [Epub ahead of print].
4. Neher MD, Weckbach S, Huber-Lang MS, Stahel PF.
New insights into the role of peroxisome proliferator-activated receptors in regulating the inflammatory response after tissue injury. PPAR Res 2012,
2012:728461.
5. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely
GB, Koble CS, Devchand P, Wahli W, Willson TM,
Lenhard JM, et al. Fatty acids and eicosanoids regulate
gene expression through direct interactions with peroxisome
proliferator-activated receptors α and γ . Proc
Natl Acad Sci U S A 1997, 94:4318–4323.
6. Seimandi M, Lemaire G, Pillon A, Perrin A, Carlavan I,
Voegel JJ, Vignon F, Nicolas JC, Balaguer P. Differential
responses of PPARα, PPARδ, and PPARγ reporter
cell lines to selective PPAR synthetic ligands. Anal
Biochem 2005, 344:8–15.
7. Sagar DR, Kendall DA, Chapman V. Inhibition of fatty
acid amide hydrolase produces PPAR-α-mediated analgesia
in a rat model of inflammatory pain. Br
J Pharmacol 2008, 155:1297–1306.
8. Jhaveri MD, Richardson D, Robinson I, Garle MJ,
Patel A, Sun Y, Sagar DR, Bennett AJ, Alexander SP,
Kendall DA, et al. Inhibition of fatty acid amide hydrolase
and cyclooxygenase-2 increases levels of endocannabinoid
related molecules and produces analgesia
via peroxisome proliferator-activated receptor-α in
a model of inflammatory pain. Neuropharmacology
2008, 55:85–93.
9. Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas
M, Tanda G, Drago F, Cadet JL, Goldberg SR,
Yasar S. Fatty acid amide hydrolase (FAAH) inhibition
enhances memory acquisition through activation
of PPAR-α nuclear receptors. Learn Mem 2009,
16:332–337.
10. Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL,
Yasar S, Goldberg SR, PistisM. Effects of fatty acid
amide hydrolase inhibition on neuronal responses
to nicotine, cocaine and morphine in the nucleus
accumbens shell and ventral tegmental area: involvement
of PPAR-α nuclear receptors. Addict Biol 2010,
15:277–288.
11. Roche M, Kelly JP, O’Driscoll M, Finn DP. Augmentation
of endogenous cannabinoid tone modulates
lipopolysaccharide-induced alterations in circulating
cytokine levels in rats. Immunology 2008,
125:263–271.
12. Loría F, Petrosino S, Hernang ´omez M,Mestre L, Spagnolo
A, Correa F, DiMarzo V, Docagne F, Guaza C. An
endocannabinoid tone limits excitotoxicity in vitro and
in a model of multiple sclerosis. Neurobiol Dis 2010,
37:166–176 [Epub October 6, 2009].
13. Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K,
Murphy SP, Kendall DA, Bennett AJ. Cannabinoid activation
of PPAR α; a novel neuroprotective mechanism.
Br J Pharmacol 2007, 152:734–743.
14. Romano MR, Lograno MD. Involvement of the peroxisome
proliferator-activated receptor (PPAR) α
in vascular response of endocannabinoids in the
bovine ophthalmic artery. Eur J Pharmacol 2012,
683:197–203.
15. Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE,
DuBois RN, Brash AR, Marnett LJ. 15-Lipoxygenase
metabolism of 2-arachidonylglycerol. Generation of a
peroxisome proliferator-activated receptor α agonist.
J Biol Chem 2002, 277:23278–23286.
16. Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A,
Rodríguez De Fonseca F, Rosengarth A, Luecke H,
Di Giacomo B, Tarzia G, et al. Oleylethanolamide regulates
feeding and body weight through activation of
the nuclear receptor PPAR-α. Nature 2003, 425:90–93.
17. Melis M, Pillolla G, Luchicchi A,Muntoni AL, Yasar S,
Goldberg SR, Pistis M. Endogenous fatty acid
ethanolamides suppress nicotine-induced activation of
mesolimbic dopamine neurons through nuclear receptors.
J Neurosci 2008, 28:13985–13994.
18. Zhou Y, Yang L, Ma A, Zhang X, Li W, Yang W,
Chen C, Jin X. Orally administered oleoylethanolamide
protects mice from focal cerebral ischemic injury by
activating peroxisome proliferator-activated receptor α.
Neuropharmacology 2012, 63:242–249.
19. Guzm´an M, Lo Verme J, Fu J, Oveisi F, Bl´azquez C,
Piomelli D. Oleoylethanolamide stimulates lipolysis by
activating the nuclear receptorperoxisome proliferatoractivated
receptor α (PPAR-α). J Biol Chem 2004,
279:27849–27854.
20. Campolongo P, Roozendaal B, Trezza V, Cuomo V,
Astarita G, Fu J, McGaugh JL, Piomelli D. Fat-induced
satiety factor oleoylethanolamide enhances memory
consolidation. Proc Natl Acad Sci U S A 2009,
106:8027–8031.
21. Gaetani S, Fu J, Cassano T, Dipasquale P, Romano A,
Righetti L, Cianci S, Laconca L, Giannini E, Scaccianoce
S, et al. The fat-induced satiety factor
oleoylethanolamide suppresses feeding through central
release of oxytocin. J Neurosci 2010, 30:8096–8101.
22. Lo Verme J, Fu J, Astarita G, La Rana G, Russo R,
Calignano A, Piomelli D. The nuclear receptor peroxisome
proliferator-activated receptor-α mediates the
anti-inflammatory actions of palmitoylethanolamide.
Mol Pharmacol 2005, 67:15–19.
23. Koch M, Kreutz S, B¨ ottger C, Benz A, Maronde
E, Ghadban C, Korf HW, Dehghani F. Palmitoylethanolamide
protects dentate gyrus granule cells
via peroxisome proliferator-activated receptor-α. Neurotox
Res 2011, 19:330–340.
24. Scuderi C, Esposito G, Blasio A, Valenza M, Arietti P,
Steardo L, Jr. Carnuccio R, De Filippis D, Petrosino S,
Iuvone T, et al. Palmitoylethanolamide counteracts
reactive astrogliosis induced by β-amyloid peptide.
J Cell Mol Med 2011, 15:2664–2674.
25. Scuderi C, Valenza M, Stecca C, Esposito G, Carrat
`u MR, Steardo L. Palmitoylethanolamide exerts
neuroprotective effects in mixed neuroglial cultures
and organotypic hippocampal slices via peroxisome
proliferator-activated receptor-α. J Neuroinflammation
2012, 9:49.
26. de Novellis V, Luongo L, Guida F, Cristino L, Palazzo
E, Russo R, Marabese I, D’Agostino G, Calignano A,
Rossi F, et al. Effects of intra-ventrolateral periaqueductal
grey palmitoylethanolamide on thermoceptive
threshold and rostral ventromedial medulla cell activity.
Eur J Pharmacol 2012, 676:41–50.
27. Lo Verme J, Russo R, La Rana G, Fu J, Farthing J,
MattaceRaso G, Meli R, Hohmann A, Calignano A,
Piomelli D. Rapid broad-spectrum analgesia through
activation of peroxisome proliferatoractivated receptoralpha.
J Pharmacol Exp Ther 2006 319:1051–1061.
28. Di Paola R, Impellizzeri D, Torre A, Mazzon E, Cappellani
A, Faggio C, Esposito E, Trischitta F, Cuzzocrea
S. Effects of palmitoylethanolamide on intestinal injury
and inflammation caused by ischemia-reperfusion in
mice. J Leukoc Biol 2012, 91:911–920. (Epub ahead
of print; April 2, 2012)
29. D’Agostino G, Russo R, Avagliano C, Cristiano C,
Meli R, Calignano A. Palmitoylethanolamide protects
against the amyloid-β25-35-induced learning and memory
impairment in mice, an experimental model of
alzheimer disease. Neuropsychopharmacology 2012.
doi: 10.1038/npp.2012, 37:1784–1792.
30. Sasso O, Russo R, Vitiello S, Raso GM, D’Agostino G,
Iacono A, Rana GL, Vall´ee M, Cuzzocrea S, Piazza PV,
et al. Implication of allopregnanolone in the antinociceptive
effect of N-palmitoylethanolamide in acute or
persistent pain. Pain 2012, 153:33–41.
31. Liu J, Li H, Burstein SH, Zurier RB, Chen JD. Activation
and binding of peroxisome proliferator-activated
receptor γ by synthetic cannabinoid ajulemic acid. Mol
Pharmacol 2003, 63:983–992.
32. O’Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA,
Randall MD. Novel time-dependent vascular actions
of δ9-tetrahydrocannabinol mediated by peroxisome
proliferator-activated receptor γ . Biochem Biophys Res
Commun 2005, 337:824–831. [Epub September 29,
2009].
33. O’Sullivan SE, Bennett AJ, Kendall DA, Randall MD.
Cannabinoids and peroxisome proliferator-activated
receptor γ (PPARg). Proceedings of the International
Cannabinoid Research Society, 2006.
34. Carroll CB, Zeissler ML, Hanemann CO, Zajicek JP.
(9) -tetrahydrocannabinol ((9) -THC) exerts a direct
neuroprotective effect in a human cell culture model
of Parkinson’s disease. Neuropathol Appl Neurobiol
2012, 38:535–547.
35. O’Sullivan SE, Sun Y, Bennett AJ, Randall MD,
Kendall DA. Time-dependent vascular actions of
cannabidiol in the rat aorta. Eur J Pharmacol 2009,
612:61–68.
36. Esposito G, Scuderi C, Valenza M, Togna GI, Latina V,
De Filippis D, Cipriano M, Carrat `u MR, Iuvone T,
Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation
and promotes hippocampal neurogenesis
through PPARγ involvement. PLoS One 2011,
6:e28668.
37. De Filippis D, Esposito G, Cirillo C, Cipriano M, De
Winter BY, Scuderi C, Sarnelli G, Cuomo R, Steardo L,
De Man JG, et al. Cannabidiol reduces intestinal inflammation
through the control of neuroimmune axis. PLoS
One 2011, 6:e28159.
38. Ambrosio AL, Dias SM, Polikarpov I, Zurier RB,
Burstein SH, Garratt RC. Ajulemic acid, a synthetic
nonpsychoactive cannabinoid acid, bound to
the ligand binding domain of the human peroxisome
proliferator-activated receptor gamma. J Biol Chem
2007, 282:18625–18633.
39. Gonzalez EG, Selvi E, Balistreri E, Akhmetshina A,
Palumbo K, Lorenzini S, Lazzerini PE, Montilli C, Capecchi
PL, Lucattelli M, et al. Synthetic cannabinoid
ajulemic acid exerts potent antifibrotic effects in experimental
models of systemic sclerosis. Ann Rheum Dis
2012, 71:1545–1551. doi:10.1136/annrheumdis-2011-
200314.
40. Bouaboula M, Hilairet S, Marchand J, Fajas L, Le
Fur G, Casellas P. Anandamide induced PPARγ transcriptional
activation and 3T3-L1 preadipocyte differentiation.
Eur J Pharmacol 2005, 517:174–811.
41. Rockwell CE, Kaminski NE. A cyclooxygenase metabolite
of anandamide causes inhibition of interleukin-2
secretion in murine splenocytes. J Pharmacol Exp Ther
2004, 311:683–690.
42. O’Sullivan SE, Kendall DA, Randall MD. Timedependent
vascular effects of endocannabinoids mediated
by peroxisome proliferator-activated receptor γ
(PPARγ ). PPAR Res 2009, 2009:425289.
43. Eichele K, Ramer R, Hinz B. R(+)-methanandamideinduced
apoptosis of human cervical carcinoma
cells involves a cyclooxygenase-2-dependent pathway.
Pharm Res 2009, 26:346–355.
44. Rockwell CE, Snider NT, Thompson JT, Vanden
Heuvel JP. Kaminski NE.Interleukin-2 suppression by
2-arachidonyl glycerol is mediated throughperoxisome
proliferator-activated receptor γ independently of
cannabinoid receptors 1 and 2. Mol Pharmacol 2006,
70:101–111.
45. Du H, Chen X, Zhang J, Chen C. Inhibition of COX-2
expression by endocannabinoid 2-arachidonoylglycerol
is mediated via PPAR-γ . Br J Pharmacol 2011,
163:1533–1549.
46. O’Sullivan SE, Kendall DA, Randall MD. Further characterization
of the time-dependent vascular effects of
δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 2006,
317:428–438.
47. Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G.
The endogenous fatty acid amide, palmitoylethanolamide,
has anti-allodynic and anti-hyperalgesic effects
in a murine model of neuropathic pain: involvement of
CB(1), TRPV1 and PPARγ receptors and neurotrophic
factors. Pain 2008, 139:541–550.
48. Mestre L, Docagne F, Correa F, Loría F, Hernang ´omez
M, Borrell J, Guaza C. A cannabinoid agonist interferes
with the progression of a chronic model of multiple
sclerosis by downregulating adhesion molecules. Mol
Cell Neurosci 2009, 40:258–266.
49. Giuliano M, Pellerito O, Portanova P, Calvaruso G,
Santulli A, De Blasio A, Vento R, Tesoriere G. Apoptosis
induced in HepG2 cells by the synthetic cannabinoid
WIN: involvement of the transcription factor PPARγ .
Biochimie 2009, 91:457–465.
50. Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG.
Fatty acid-binding proteins transport N-acylethanolamines
to nuclear receptors and are targets of endocannabinoid
transport inhibitors. J Biol Chem 2012,
287:3415–3424.
51. Raman P, Kaplan BL, Thompson JT, Vanden Heuvel
JP, Kaminski NE. 15-Deoxy-δ12,14-prostaglandin J2-
glycerol ester, a putative metabolite of 2-arachidonyl
glycerol, activates peroxisome proliferator activated
receptor γ . Mol Pharmacol 2011, 80:201–209.
52. Lenman A, Fowler CJ. Interaction of ligands for the
peroxisome proliferator-activated receptor γ with
the endocannabinoid system. Br J Pharmacol 2007,
151:1343–1351.
53. Yan ZC, Liu DY, Zhang LL, Shen CY, Ma QL,
Cao TB, Wang LJ, Nie H, Zidek W, Tepel M, et al.
Exercise reduces adipose tissue via cannabinoid receptor
type 1 which is regulated by peroxisome proliferatoractivated
receptor-δ. Biochem Biophys Res Commun
2007, 354:427–433 [Epub January 9, 2007].
54. Johnson DR, Stebulis JA, Rossetti RG, Burstein SH,
Zurier RB. Suppression of fibroblast metalloproteinases
by ajulemic acid, a nonpsychoactive cannabinoid acid.
J Cell Biochem 2007, 100:184–190.
55. Parker J, Atez F, Rossetti RG, Skulas A, Patel R,
Zurier RB. Suppression of human macrophage
interleukin-6 by a nonpsychoactive cannabinoid acid.
Rheumatol Int 2008, 28:631–635.
56. Zurier RB, Sun YP, George KL, Stebulis JA, Rossetti
RG, Skulas A, Judge E, Serhan CN. Ajulemic
acid, a synthetic cannabinoid, increases formation of
the endogenous proresolving and anti-inflammatory
eicosanoid, lipoxin A4. FASEB J 2009, 23:1503–1509.
57. Petrosino S, Cristino L, Karsak M, Gaffal E, Ueda N,
T¨ uting T, Bisogno T, De Filippis D, D’Amico
A, Saturnino C, et al. Protective role of palmitoylethanolamide
in contact allergic dermatitis. Allergy
2010, 65:698–711.
58. Wallace VC, Segerdahl AR, Lambert DM, Vandevoorde
S, Blackbeard J, Pheby T, Hasnie F, Rice AS. The
effect of the palmitoylethanolamide analogue, palmitoylallylamide
(L-29) on pain behaviour in rodent models
of neuropathy. Br J Pharmacol 2007, 151:1117–1128.
59. Caprioli A, Coccurello R, Rapino C, Di Serio S,
Di Tommaso M, Vertechy M, Vacca V, Battista N,
Pavone F, Maccarrone M, et al. The novel reversible
fatty acid amide hydrolase inhibitor ST4070 increases
endocannabinoid brain levels and counteracts neuropathic
pain in different animal models. J Pharmacol
Exp Ther 2012, 342:188–195.
60. Nithipatikom K, Isbell MA, Endsley MP, Woodliff JE,
Campbell WB. Anti-proliferative effect of a putative
endocannabinoid, 2-arachidonylglyceryl ether in
prostate carcinoma cells. Prostaglandins Other Lipid
Mediat 2011, 94:34–43.
61. Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL,
Yasar S, Goldberg SR, PistisM. Effects of fatty acid
amide hydrolase inhibition on neuronal responses
to nicotine, cocaine and morphine in the nucleus
accumbens shell and ventral tegmental area: involvement
of PPAR-α nuclear receptors. Addict Biol 2010,
15:277–288.
62. Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi
A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes
C, et al. Blockade of nicotine reward and reinstatement
by activation of α-type peroxisome proliferatoractivated
receptors. Biol Psychiatry 2011, 69:633–641.
63. O’Sullivan SE, Kendall DA, Randall MD. Time-dependent
vascular effects of Endocannabinoids mediated by
peroxisome proliferator-activated receptor γ (PPARγ ).
PPAR Res 2009, 2009:425289.
64. Chen C, Jin X, Meng X, Zheng C, Shen Y, Wang Y.
Inhibition of TNFα-induced adhesion molecule
expression by (Z)-(S)-9-octadecenamide, N-(2-
hydroxyethyl,1-methyl). Eur J Pharmacol 2011, 660:
305–309.
65. Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamide,
an endogenous PPAR-α agonist, lowers
body weight and hyperlipidemia in obese rats. Neuropharmacology
2005, 48:1147–1153.
66. Astarita G, Di Giacomo B, Gaetani S, Oveisi F, Compton
TR, Rivara S, Tarzia G, Mor M, Piomelli D. Pharmacological
characterization of hydrolysis-resistant
analogs of oleoylethanolamide with potent anorexiant
properties. J Pharmacol Exp Ther 2006, 318:563–570.
67. Alhamoruni A, Lee AC, Wright KL, Larvin M, O’Sullivan
SE. Pharmacological effects of cannabinoids on the
Caco-2 cell culture model of intestinal permeability.
J Pharmacol Exp Ther 2010, 335:92–102.
68. Alhamoruni A, Wright KL, Larvin M, O’Sullivan SE.
Cannabinoids mediate opposing effects on
inflammation-induced intestinal permeability. Br
J Pharmacol 2012, 165:2598–2610.
69. Cluny NL, Keenan CM, Lutz B, Piomelli D, Sharkey
KA. The identification of peroxisome proliferatoractivated
receptor α-independent effects of
oleoylethanolamide on intestinal transit in mice. Neurogastroenterol
Motil 2009, 21:420–429.
70. Aviello G, Matias I, Capasso R, Petrosino S, Borrelli
F, Orlando P, Romano B, Capasso F, Di Marzo V,
Izzo AA. Inhibitory effect of the anorexic compound
oleoylethanolamide on gastric emptying in control and
overweight mice. J Mol Med (Berl) 2008, 86:413–422.
71. Panlilio LV, Mazzola C, Medalie J, Hahn B, Justinova
Z, Drago F, Cadet JL, Yasar S, Goldberg SR.
Anandamide-induced behavioral disruption through a
vanilloid-dependent mechanism in rats. Psychopharmacol
(Berl) 2009, 203:529–538.