Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Sep 16;7(1):183–192. doi: 10.1159/000541461
Abstract
Background
Glaucoma is an ocular disease with significant health burden. Despite the availability of many antiglaucoma drugs, a significant proportion of patients may experience worsening of the disease. Hence, there is a need for newer antiglaucoma drugs.
Summary
Natural and synthetic derivatives of cannabis plants have been studied in the treatment of glaucoma since the 1970s. This review describes the potential mechanisms of the cannabinoids in the treatment of glaucoma, summarizes the findings of clinical studies describing the efficacy of these compounds, and describes the adverse effects observed with the various cannabinoid formulations evaluated in clinical studies of glaucoma in healthy volunteers and patients. The implications of these findings in terms of the potential clinical status of cannabinoids in the treatment of glaucoma and the challenges involved have also been described.
Key Messages
Cannabinoids lower intraocular pressure. However, the effect is short-lived. There is also a lack of well-formulated ocular delivery system. The available evidence is inadequate to recommend the use of cannabinoids for the routine treatment of glaucoma.
Introduction
Glaucoma is a progressive neurological condition affecting the eyes and is characterized by elevated intraocular pressure (IOP), which can result in permanent vision loss [1]. Reducing aqueous humor formation and/or enhancing its drainage is the only treatment [2]. Glaucoma is one of the leading causes of permanent blindness; a recent estimate by the World Health Organization ranks glaucoma third in terms of causing blindness (8.5%), preceded by cataract (35.2%) and uncorrected refractive errors (20.3%) [3]. The prevalence of the relatively benign and chronic form, primary open-angle glaucoma (POAG), is 2.4% (95% confidence interval [CI], 2.0–2.8%) globally [4]; the absolute numbers are projected to be 68.56 million (95% CI, 59.99–79.98 million) worldwide [4]. It was estimated that 43.78 million POAG cases were undiagnosed in 2020, with 76.7% of those instances occurring in Asia and Africa [5]. Africa has the greatest POAG prevalence of any continent, with a frequency that is about twice as high as the global average [6].
By 2040, approximately 111.8 million people are estimated to be affected by glaucoma globally [7]. In India, the prevalence of POAG is higher than that of primary angle-closure glaucoma [8]. There may be a wide variation in the intra-country prevalence; for example, in the eastern part of India, the prevalence among the urban population is 2.10%, whereas that in the rural population is 1.45%. Moreover, a difference between genders has been noted, with more males developing the disease [8, 9]. In general, men are found to be more susceptible to POAG than women (relative risk [95% CI], 1.28 [1.12–1.45]) [4]. Some risk factors for developing glaucoma include age, family history, African-Caribbean ancestry, myopia, eye injury, and diabetes mellitus [10]. The prevalence increases with age, especially in people above 60 years of age, and ranges from 6.9% to 8.1% among those more than 40 years [10–12]. In low- and middle-income countries, the disease imposes high economic costs, especially in the late stages [13].
Many treatments have been proposed to reduce IOP, which remains the only modifiable risk factor. In 2020, the American Academy of Ophthalmology recommended topical drugs as the first-choice treatment for most newly diagnosed cases of POAG, rather than other methods of care [7]. Furthermore, dietary supplements could function as co-adjuvants in the therapeutic management of glaucoma; however, further evidence is needed to establish the clinical importance of these findings [14]. Although there are various antiglaucoma drugs and surgical techniques available to reduce IOP, a significant proportion of individuals continue to worsen even with appropriate therapy. Therapy that aims for more than merely reducing IOP would therefore be necessary [15]. Cannabinoids have been studied as a potential treatment for glaucoma since the early 1970s [15]. Cannabinoids are a broad class of chemical compounds that are produced artificially by pharmacological synthesis (synthetic cannabinoids) or obtained from the resins of secretory trichomes located on female flowers of Cannabis sativa (Cannabaceae family) [16, 17]. The leaves of Cannabis plants also contain a significant amount of cannabinoids, which are known as phytocannabinoids [18]. The flowers and leaves of Cannabis sativa, Cannabis indica, and Cannabis ruderalis are used to produce phytocannabinoids [19]. It is possible to extract around 113 distinct cannabinoids from Cannabis sativa; the most well-known and researched are tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabinol, which have significant psychotropic effects [20]. Cannabis sativa has the highest concentration of CBD; Cannabis indica is the primary source of THC, and Cannabis ruderalis has the lowest concentration of psychoactive cannabinoids [21]. These compounds engage with the body’s natural endocannabinoid receptors, cannabinoid receptors 1 and 2 (CB1 and CB2) [22]. In the human eye, cannabinoid receptors are expressed in the retina, ciliary body, iris, Schlemm’s canal, trabecular meshwork, and retinal pigment epithelium [16]. Because of the location and crucial anatomical distribution of cannabinoid receptors, cannabinoids can affect IOP by both reducing the generation of aqueous humor and enhancing its outflow [23]. Endogenous cannabinoids are eicosanoid neurotransmitters that influence a number of physiological and mental functions, including glucose metabolism, adipogenesis, and food intake [24]. The most researched endocannabinoids include anandamide and 2-arachidonoylglycerol because they appear to be involved in a number of diseases, including Parkinson’s and Alzheimer’s disease [25]. A recent study compared the plasma, aqueous humor, and tear levels of the endogenous cannabinoids among patients with POAG and those with cataract. A significantly lower plasma level of the endogenous cannabinoids was detected in the former group, with aqueous humor levels being undetectable and no significant difference in the tear levels between the groups [26].
Numerous retinal functions, including signal transduction, phototransduction, and IOP regulation, may be modulated by exogenous cannabinoids like THC, CBD, and synthetic preparations [16]. In addition to their ability to reduce IOP, cannabinoids have potentially useful neuroprotective effects on the central and peripheral nervous systems [27]. Multiple pharmacological actions of CBD have been described in the literature, including sedation, neuroprotection, antiepileptic, antiemetic, anti-inflammatory, and anxiolytic effects [28, 29]. THC and CBD are active components in cannabis-based medications [30]. In this review, we describe the mechanism of action of cannabinoids in glaucoma, the evidence regarding their usefulness for treating glaucoma, the adverse effects of various cannabinoid preparations evaluated for treating glaucoma, and the challenges involved in the use of these compounds.
Methodology
To obtain relevant information, we searched the PubMed and Embase databases for clinical studies in humans providing data on the efficacy and/or safety of cannabinoids in the treatment of glaucoma. The search period was from database inception to July 2024. We used the following search string to retrieve the articles: (“cannabinoid*”[Title/Abstract] OR “cannabis”[Title/Abstract] OR “marijuana”[Title/Abstract] OR “marihuana”[Title/Abstract] OR “dronabinol”[Title/Abstract]) AND (“glaucoma”[Title/Abstract] OR “intraocular pressure”[Title/Abstract]). Four hundred and twenty-eight articles were retrieved (182 from PubMed and 246 from Embase database); after removing duplicates, 293 papers were available. Two authors independently screened the title and abstract of these papers to identify the relevant articles. Twenty-six papers were selected for full-text reading. Any difference in the shortlisting of articles was resolved by consensus. All research papers published from the year 2000 onwards were considered. Prior to this period, only a few representative research papers have been presented in this review as these mainly evaluated inhalational use of the drug, which is unsuitable in the current therapeutic context. We screened the cross references and citations to identify any additional relevant publications. Studies involving animals and cell lines as well as review articles and other papers not describing primary research in humans were excluded. Seven papers, post the year 2000, describing clinical studies of marijuana/cannabinoids in healthy volunteers/patients were identified (Table 1).
Table 1.
Studies evaluating the efficacy of various cannabis compounds in healthy volunteers and patients with glaucoma
| Authors | Drug and formulation | Population | Aim of the study | Study design | Sample size | Results |
|---|---|---|---|---|---|---|
| Mosaed et al. [31] (2020) and [32] (2021) | 5.9 or 13.4 w/w % cannabis cigarette inhalation | Adult healthy subjects who are regular cannabis smokers | Evaluate the effect of inhaled cannabis on IOP | Prospective, randomized, double-blind, placebo-controlled study | 11 participants in test group, 3 in placebo group | Significant lowering of IOP compared with baseline but no consistent difference compared with placebo |
| Assess the correlation between plasma levels of THC plasma levels and reduction of IOP | Significant negative correlation between plasma THC levels and IOP. | |||||
| Hommer et al. [33] (2020) | Dronabinol 5 mg orally | Healthy volunteers | Does it alter optic nerve head blood flow and vascular autoregulation? | Randomized, placebo-controlled, double-blind, two‐way crossover | 12 in each group | Increase in optic nerve head blood flow without affecting IOP, OPP, or inducing psychoactive side effects |
| Plange et al. [34] (2007) | Dronabinol 7.5 mg orally | Healthy volunteers | Effect on retinal hemodynamics in healthy individuals | Self-experiment of medical doctors | 8 subjects | Increase in human retinal hemodynamics accompanied by (but not attributable to) a marked decrease in IOP. |
| Tomida et al. [35] (2006) | Δ-9-THC (5 mg) and CBD (20 and 40 mg) sublingual spray | Patients with ocular hypertension or early POAG | Effect on IOP; safety and tolerability | Randomized, double-blind, placebo-controlled, four-way crossover | 6 patients | Significant lowering of IOP compared with placebo at 2 h. CBD 40 mg produced a transient increase at 4 h. One patient experienced a transient and mild panic-like reaction with Δ-9-THC |
| Flach [36] (2002) | Oral THC 2.5 or 5 mg four times a day; Marijuana cigarettes containing 6 mg THC | Uncontrolled IOP while receiving maximally tolerated conventional glaucoma treatment | Effect on IOP and BP | Uncontrolled, unmasked, nonrandomized, prospective evaluation | 9 subjects | May reduce IOP but with the development of tolerance and systemic adverse effects |
| Porcella et al. [37] (2001) | WIN55212-2, a synthetic and selective CB1 receptor agonist (25 or 50 μg eyedrop) | Patients with bilateral glaucoma (>22 mm Hg) being treated with multiple topical drugs | Effect on IOP in patients with glaucoma resistant to conventional therapies | Self-controlled study (one eye treated) | 8 patients | Decrease in IOP within the first 30 minutes; maximum effect reached in the first 60 min |
| Tiedeman et al. [38] (1981) | Delta-1-THC derivatives BW146Y and BW29Y single oral dose | Ocular hypertensive patients | Effect on IOP and adverse effects | Single-dose, randomized, double-blind | BW146Y: 6 received placebo, 9 received 4 mg dose, 10 received 8 mg, 3 received 12 mg | BW146Y had a significant IOP-lowering effect independent of orthostatic BP changes, although some effects were observed. Both drugs produced mild subjective adverse effects |
| BW29Y: 6 received placebo, 5 received 5 mg dose, 5 received 10 mg | ||||||
| Merritt et al. [39] (1980) | Marihuana 900 mg (2% Δ-9-THC) inhalation | Patients with heterogeneous glaucomas | Effect on IOP and blood pressure | Prospective, single-arm study` | 18 subjects | Decrease in BP followed by IOP. Postural hypotension, tachycardia, palpitations, and alterations in mental status |
| Crawford et al. [40] (1979) | 2.8% THC inhalation | Systemic normotensive (N = 8) and hypertensive (N = 8) open-angle glaucoma patients | Relationship between simultaneous changes in heart rate, blood pressure, and IOP | Prospective, controlled study | 16 subjects | Increase in heart rate followed by a substantial decrease in systolic, diastolic, and IOP. The intensity and duration of the responses were greater in hypertensives |
Discussion
Potential Mechanisms of Cannabinoids in Glaucoma
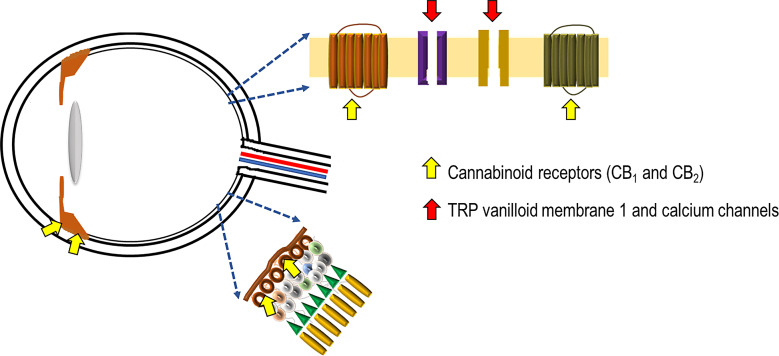
Multiple factors like oxidative stress, inflammation, and glutamatergic pathways play a role in the pathogenesis of glaucoma [27]. The presence of cannabinoid receptors CB1 and CB2 mRNA and protein has been recently demonstrated in the human ciliary body (Fig. 1) [30]. These receptors are class A (rhodopsin-like) G-protein coupled receptors and are present in the brain and some peripheral tissues, displaying psychoactive effects [30, 41]. THC acts on both receptors. It interacts with the CB1 receptors as a partial agonist, stimulates Gi/o proteins, and thereby inhibits adenylyl cyclase, thus hindering the conversion of adenosine triphosphate into cyclic adenosine monophosphate [42, 43]. The regulation of potassium channel activation is performed by these cyclic adenosine monophosphates, leading to a decline in aqueous humor production and an increase in its elimination, and subsequently, to a reduction in IOP [43–45]. In glaucoma, the death of retinal ganglion cells is observed, although its mechanism is not well understood [1]; it has been suggested that blocking of both anterograde and retrograde axonal transport results in scarcity of neurotrophic signals [46]. THC also further acts on other G-protein coupled receptors like GPR55 and GPR18 as an agonist [15]. GPR18 activation also results in lowering of IOP [47]. GPR55 and CB2 together regulate the neutrophil response, and GPR18, once activated by a metabolite of THC, contributes to cannabinoid-mediated retinal vasoactivity [48]. Furthermore, THC can operate as a COX-2-inducing cannabinoid, which lowers IOP by increasing COX-2 mRNA expression in a concentration- and time-dependent manner and phosphorylates p38 mitogen-activated protein kinase and p42/44 mitogen-activated protein kinase in a time-dependent manner [49]. This leads to the activation of peroxisome proliferator-activated receptor alpha and increases the outflow of aqueous humor [50]. The partial agonistic activity of THC at CB1 receptors is responsible for the psychotropic effects of cannabinoids and the cannabinoid-induced “tetrad” that encompasses analgesia, hypolocomotion, hypothermia, and catalepsy [15, 19, 30]. It was also found that there was a significant reduction in aqueous humor production in patients administered THC, and this response is considered to be mediated, at least partly, via the beta-adrenergic receptors in the eye, suggesting an adrenergic link to the CB1-mediated effects [16]. In some studies, when THC was administered to a neurally isolated eye, the reduction of IOP was diminished, which again suggests a possible sympathetic connection to the pressure-reducing action of cannabis [51]. Cannabidivarin and CBD, is gaining popularity because it has a lesser affinity for CB1 but can serve as an antagonist on CB1 receptors, modulating the psychoactive and other effects of THC [30]. The antagonistic effects of CBD can blunt the IOP-reducing effects of THC [47]. However, by raising the constitutional activity or “endocannabinoid tone” of CB1 receptors, CBD also has an indirect agonistic effect [50, 52]. Calcium flow across the plasma membrane is regulated by transient receptor potential ion channels [53]. CBD acts on transient receptor potential vanilloid member 1 channels located in the optic nerve head, suggesting that calcium handling may affect optic nerve function [53]. Activation of these channels results in retinal ganglion cell apoptosis [32]. Contraction of the ciliary muscle and decreased secretion from the ciliary process via reducing noradrenaline release are other processes that lower the IOP [54]. The synthetic CB1 receptor agonist WIN55212 significantly reduced IOP when administered topically [37]. Dronabinol is another synthetic cannabinoid that reduces IOP on oral consumption [34], although the mechanism is not completely understood; putatively, it enhances the blood flow via the iris, ciliary processes, and choroid while decreasing the production of aqueous humor; this is due to increased blood flow away from the anterior uvea, which in turn lowers the ultrafiltration pressure required for the generation of aqueous humor [55]. Inhalation of cannabis causes a decrease in blood pressure as well as IOP and an increase in heart rate. The decrease in intraocular effect is due to lowered blood pressure in the ciliary body vasculature due to the peripheral vasodilatory effects of marijuana [39].
Fig. 1.
Efficacy Data from Clinical Studies
The legalization of the medicinal use of cannabis and its derivatives in North America in the mid-1990s paved the way for formal clinical assessment of the products for various indications [56]. The regulatory approval in the USA and Canada, although with various restrictions and changing laws over time, led to the approval of different cannabinoids in various European countries [56]. Many early studies of cannabinoids in glaucoma assessed the effects of oral and intravenous administration on IOP, in addition to other systemic effects [57]. Although a decrease in IOP was observed, it was either accompanied by a fall in blood pressure or euphoria, depending on the route and compound assessed, both precluding long-term clinical use in glaucoma. However, the observations prompted further assessment of oral compounds and topical preparations, with varying degrees of success [58]. The modest benefits coupled with the legal/regulatory hurdles in widespread production and procurement are probably responsible for the limited number of studies available to date, with the former probably being more important. Table 1 provides a brief summary of the studies that assessed the efficacy of various cannabinoids.
The only study that evaluated the topical use of a synthetic cannabinoid was by Porcella et al. [37]. In their study, 25 μg and 50 μg doses produced a decrease in IOP by 15% and 23% in the first 30 min and by 20% and 31% after 60 min, respectively. It should be noted that the participants had glaucoma resistant to conventional therapy. At the end of 2 h, there was no statistically significant difference between the test and control eyes, indicating the short duration of action of the drug. In an uncontrolled, unblinded study conducted by Flach et al. [36] among 9 patients with open-angle glaucoma not responding to conventional treatment, 4 patients achieved the set target IOP over a treatment period of 1–9 months, the reduction in IOP being in the range of 1–5 mm Hg. Tomida et al. [35] conducted a placebo-controlled, 4-way crossover study to assess the effects of sublingual THC and CBD among 6 patients with ocular hypertension or early primary open-angle glaucoma. While the IOP reduction with CBD was not significantly different from that obtained with placebo, THC produced significant reductions (mean IOP reduction compared with baseline values, 6.55 vs. 5.17 mm Hg, respectively). However, this was a single-dose study with maximum IOP reductions seen at 6–12 h interval.
Safety Data from Clinical Studies
The description below focuses on the adverse effects of cannabis compounds in the treatment of glaucoma and excludes the possible harmful consequences, such as ganglion cell dysfunction [59], of cannabis and its products in the context of nonmedical use or abuse.
Early studies in the 1970s identified conjunctival hyperemia, decreased lacrimation, diplopia, impairment of accommodation, photophobia, nystagmus, and blepharospasm as adverse effects of topical, oral, systemic, and inhalational use of marijuana or THC [58]. Of note, on smoking 2% marijuana, the decrease in IOP was difficult to dissociate from the euphoria. An increase in the doses acutely increased the fall in IOP, but there was no significant prolongation of the duration of action. Meritt et al. [39] in their study of 900 mg marijuana cigarette containing approximately 2% THC among 18 patients with glaucoma, reported the occurrence of anxiety with tachycardia and palpitations in 8, postural hypotension in 5, alterations in sensorium (hunger, thirst, euphoria, drowsy, feeling cold) in 18, and bulbar conjunctival hyperemia and ptosis in 9. In fact, hypotension occurred even in a frequent user of marijuana for the past 5–6 years. However, the hypotensive events were corrected by placing the patient in a reclining position.
Tiedeman et al. [38] reported the occurrence of lightheadedness, constipation, and drowsiness with BW29Y (5 and 10 mg capsules); feeling lightheaded, dizzy, disoriented, or “drugged,” nausea, constipation, mildly sleepy, syncopal episode, orthostatic blood pressure drop, slight tachycardia, and slightly elevated body temperature with BW146Y (4, 8, and 12 mg capsules). Flach et al. [36] studied oral THC in various doses in 9 glaucoma patients for 3–36 weeks. In addition to the development of possible tolerance, all subjects experienced adverse effects that masked the therapeutic benefit from the patients’ perspective. Adverse effects included feeling dizzy, lightheaded, sleepy, dry mouth, confusion, weight increase, anxiety, depression, elation, and distortion of perception of varying severity. Plange et al. [34] in their study using oral dronabinol 7.5 mg on 8 healthy volunteers found no significant change in heart rate or blood pressure. In the study by Hommer et al. [33] of 5 mg of oral dronabinol in healthy volunteers, no adverse effects, including psychoactive effects, were observed nor any significant changes in heart or blood pressure.
Tomida et al. [35] in their study of THC and two doses of CBD sublingually in 6 patients reported oral pain/discomfort, dizziness, hypotension, nausea, panic, and photopsia with the former (3 patients); diastolic pressure increased, dizziness, disturbed attention with 20 mg CBD (2 patients); oral pain/discomfort, pharyngitis, bad taste, feeling hot, throat irritation with 40 mg CBD (5 patients); oral pain/discomfort, diastolic pressure increased, headache, hypoesthesia with placebo (2 patients). Majority of the events were mild, and there were no serious or severe AEs. Porcella et al. [37] did not report the safety aspect of the topical CB1 agonist in their study on patients with glaucoma.
Status of Cannabinoid Use in Glaucoma
Although the medical use of cannabis derivatives has been legalized in some countries, in many countries, for example, India, cannabis or its derivatives are not approved for any clinical indication. Cannabis is a “narcotic drug” as per Section 2 (xiv) of the Narcotic Drugs and Psychotropic Substances Act (NDPSA), 1985 [60]. Although dronabinol and nabilone, synthetic derivatives of THC, have been approved by the US FDA for chemotherapy-induced nausea and vomiting, there are currently no approved medical uses for these in India. Hence, even if any of the cannabis derivatives show a potential advantage over the existing antiglaucoma drugs, the results are likely to receive more scrutiny than any other new drug given the concerns regarding their misuse and abuse potential. Despite these potential hurdles, the main factor for such clinical use would be the availability of robust data supporting its use in glaucoma. The studies so far have not been able to provide evidence of an unequivocal benefit large enough to place cannabis derivatives as a second- or third-line option in the treatment of glaucoma.
In the absence of achievement of the target reduction in IOP in a patient with open-angle glaucoma, additional topical antiglaucoma drugs may be added; however, laser trabeculoplasty or surgical treatment should be considered if the patient requires three or more drugs, if not earlier [61, 62]. The mean IOP reductions with conventional medications range from 5.61 mm Hg for prostaglandin analogs to 2.49 mm Hg for carbonic anhydrase inhibitors [63]. The only study that evaluated a topical formulation of cannabinoid showed a significant acute reduction compared with placebo, although the overall reduction in both the groups was approximately 5 mm Hg [37]. Given these findings, it is to be assessed whether cannabinoids would be useful as an add-on drug in cases of resistant glaucoma. Also to be evaluated is the potential usefulness of the drug for acutely reducing IOP in angle-closure glaucoma, although the acute effect needs to be further established. The short duration of action of the topical formulation and the potential systemic adverse effects of non-topical formulations are additional limitations compared with the existing antiglaucoma drugs. Poor water solubility impairs ocular delivery of the drug on topical administration. Although special formulations with the potential to improve ocular delivery have been attempted, most of these are in the preclinical phase [64]. Given these issues, the lack of support expressed by various specialist groups a decade ago for use of any form of cannabinoids in glaucoma, due to lack of efficacy and potential adverse effects, seems to hold good even today [65–67].
Our review has certain limitations. The search for relevant publications was limited to two databases. We focused on clinical studies in healthy volunteers or patients and did not consider the effects of cannabinoids described in the context of nonmedical use, which may be relevant in considering the risk benefit ratio and possible adverse effects in the context of overdose/toxicity. Some clinical studies included in this review did not provide precise data to estimate the exact changes in the IOP following cannabinoid use as compared to the control group; hence, the data regarding mean changes in the IOP should be considered approximate; nonetheless, the lack of precise data is unlikely to alter the main conclusion of this review.
Conclusion
Cannabis and its derivatives have received wide attention for their potential usefulness in many diseases. Early studies of various cannabis derivatives and their formulations provided evidence of an IOP-lowering effect, prompting further investigation and clinical studies. However, to date, the evidence needed to establish it as part of treatment guidelines, even as an add-on drug, is inadequate. The short duration of action and lack of well-formulated ocular delivery are challenges that must be overcome. Despite the approval and use of cannabinoids in other therapeutic areas, such as spasticity in patients with multiple sclerosis, chronic pain, chemotherapy-induced nausea and vomiting, and cancer cachexia, their potential role for treating glaucoma is currently uncertain.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
N.J. and A.K. conceived the review topic; N.J. and H.M. drafted the manuscript; A.K. reviewed the manuscript for important intellectual content; all authors agree to be accountable for all aspects of the work.
Funding Statement
This study was not supported by any sponsor or funder.
References
- 1. Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–77. [DOI] [PubMed] [Google Scholar]
- 2. Jayaram H, Kolko M, Friedman DS, Gazzard G. Glaucoma: now and beyond. Lancet. 2023;402(10414):1788–801. [DOI] [PubMed] [Google Scholar]
- 3. Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–34. [DOI] [PubMed] [Google Scholar]
- 4. Zhang N, Wang J, Li Y, Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci Rep. 2021;11(1):13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J-P, Yu M-T, Xu B-L, Hua J-P, Jiang L-G, Wang J-T, et al. Analysis of retinal arteriolar and venular parameters in primary open angle glaucoma. Int J Ophthalmol. 2023;16(5):671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yimam W, Anbesaw T, Seid M, Kumar P, Wolie H. Knowledge about glaucoma among adults in Africa: a systematic review. BMC Ophthalmol. 2024;24(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prum BE, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, et al. Primary open-angle glaucoma preferred practice Pattern(®) guidelines. Ophthalmology. 2016;123(1):P41–111. [DOI] [PubMed] [Google Scholar]
- 8. Paul C, Sengupta S, Banerjee S, Choudhury S. Open-angle glaucoma in a rural and urban population in Eastern India—the Hooghly river glaucoma study. Indian J Ophthalmol. 2020;68(2):371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmad A, Ahmad SZ, Khalique N, Ashraf M, Alvi Y. Prevalence and associated risk factors of glaucoma in aligarh, India–a population based study. Delhi J Ophthalmol. 2020;31(1):36. [Google Scholar]
- 10. Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11):e11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palimkar A, Khandekar R, Venkataraman V. Prevalence and distribution of glaucoma in central India (glaucoma survey-2001). Indian J Ophthalmol. 2008;56(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garudadri C, Senthil S, Khanna RC, Sannapaneni K, Rao HBL. Prevalence and risk factors for primary glaucomas in adult urban and rural populations in the Andhra Pradesh eye disease study. Ophthalmology. 2010;117(7):1352–9. [DOI] [PubMed] [Google Scholar]
- 13. Pezzullo L, Streatfeild J, Simkiss P, Shickle D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv Res. 2018;18(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adornetto A, Rombolà L, Morrone LA, Nucci C, Corasaniti MT, Bagetta G, et al. Natural products: evidence for neuroprotection to Be exploited in glaucoma. Nutrients. 2020;12(10):3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindner T, Schmidl D, Peschorn L, Pai V, Popa-Cherecheanu A, Chua J, et al. Therapeutic potential of cannabinoids in glaucoma. Pharmaceuticals. 2023;16(8):1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cairns EA, Baldridge WH, Kelly MEM. The endocannabinoid system as a therapeutic target in glaucoma. Neural Plast. 2016;2016:e9364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centonze D, Rossi S, Finazzi-Agrò A, Bernardi G, Maccarrone M. The (Endo)Cannabinoid system in multiple sclerosis and amyotrophic lateral sclerosis. In: International review of neurobiology. Academic Press; 2007; p. 171–86. [DOI] [PubMed] [Google Scholar]
- 18. Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alves P, Amaral C, Teixeira N, Correia-da-Silva G. Cannabis sativa: much more beyond Δ9-tetrahydrocannabinol. Pharmacol Res. 2020;157:104822. [DOI] [PubMed] [Google Scholar]
- 20. Gülck T, Møller BL. Phytocannabinoids: origins and biosynthesis. Trends Plant Sci. 2020;25(10):985–1004. [DOI] [PubMed] [Google Scholar]
- 21. Happyana N, Kayser O. Monitoring metabolite profiles of cannabis sativa L. Trichomes during flowering period using 1H NMR-based metabolomics and real-time PCR. Planta Med. 2016;82(13):1217–23. [DOI] [PubMed] [Google Scholar]
- 22. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomida I, Pertwee RG, Azuara-Blanco A. Cannabinoids and glaucoma. Br J Ophthalmol. 2004;88(5):708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. López EM, Tagliaferro P, Onaivi ES, López-Costa JJ. Distribution of CB2 cannabinoid receptor in adult rat retina. Synapse. 2011;65(5):388–92. [DOI] [PubMed] [Google Scholar]
- 25. Fernández-Ruiz J, Romero J, Ramos JA. Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, huntington’s chorea, Alzheimer’s disease, and others. In: Pertwee RG, editor. Endocannabinoids. Cham: Springer International Publishing; 2015. p. 233–59. [DOI] [PubMed] [Google Scholar]
- 26. Angmo D, Warjri GB, Gowtham L, Velpandian T, Dada T. Endocannabinoids and cortisol in plasma, aqueous and tear samples of primary angle closure glaucoma versus controls. Eur J Ophthalmol. 2024;13:11206721241247419. [DOI] [PubMed] [Google Scholar]
- 27. Vallée A, Lecarpentier Y, Vallée J-N. Cannabidiol and the canonical WNT/β-Catenin pathway in glaucoma. Int J Mol Sci. 2021;22(7):3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12(4):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aychman MM, Goldman DL, Kaplan JS. Cannabidiol’s neuroprotective properties and potential treatment of traumatic brain injuries. Front Neurol. 2023;14:1087011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. An D, Peigneur S, Hendrickx LA, Tytgat J. Targeting cannabinoid receptors: current status and prospects of natural products. Int J Mol Sci. 2020;21(14):5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mosaed S, Liu JH, Minckler DS, Fitzgerald RL, Grelotti D, Sones E, et al. The effect of inhaled cannabis on intraocular pressure in healthy adult subjects; 2020. Vol. 1. [cited 2024 Apr 10]. Available from: https://www.touchophthalmology.com/glaucoma/journal-articles/the-effect-of-inhaled-cannabis-on-intraocular-pressure-in-healthy-adult-subjects/ [Google Scholar]
- 32. Mosaed S, Smith AK, Liu JHK, Minckler DS, Fitzgerald RL, Grelotti D, et al. The relationship between plasma tetrahydrocannabinol levels and intraocular pressure in healthy adult subjects. Front Med. 2021;8:736792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hommer N, Kallab M, Szegedi S, Puchner S, Stjepanek K, Bauer M, et al. The effect of orally administered dronabinol on optic nerve head blood flow in healthy subjects-A randomized clinical trial. Clin Pharmacol Ther. 2020;108(1):155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plange N, Arend KO, Kaup M, Doehmen B, Adams H, Hendricks S, et al. Dronabinol and retinal hemodynamics in humans. Am J Ophthalmol. 2007;143(1):173–4. [DOI] [PubMed] [Google Scholar]
- 35. Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma. 2006;15(5):349–53. [DOI] [PubMed] [Google Scholar]
- 36. Flach AJ. Delta-9-tetrahydrocannabinol (THC) in the treatment of end-stage open-angle glaucoma. Trans Am Ophthalmol Soc. 2002;100:215–24. [PMC free article] [PubMed] [Google Scholar]
- 37. Porcella A, Maxia C, Gessa GL, Pani L. The synthetic cannabinoid WIN55212-2 decreases the intraocular pressure in human glaucoma resistant to conventional therapies. Eur J Neurosci. 2001;13(2):409–12. [DOI] [PubMed] [Google Scholar]
- 38. Tiedeman JS, Shields MB, Weber PA, Crow JW, Cocchetto DM, Harris WA, et al. Effect of synthetic cannabinoids on elevated intraocular pressure. Ophthalmology. 1981;88(3):270–7. [DOI] [PubMed] [Google Scholar]
- 39. Merritt JC, Crawford WJ, Alexander PC, Anduze AL, Gelbart SS. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology. 1980;87(3):222–8. [DOI] [PubMed] [Google Scholar]
- 40. Crawford WJ, Merritt JC. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int J Clin Pharmacol Biopharm. 1979;17(5):191–6. [PubMed] [Google Scholar]
- 41. Schwitzer T, Schwan R, Angioi-Duprez K, Ingster-Moati I, Lalanne L, Giersch A, et al. The cannabinoid system and visual processing: a review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol. 2015;25(1):100–12. [DOI] [PubMed] [Google Scholar]
- 42. Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27(1):73–100. [DOI] [PubMed] [Google Scholar]
- 43. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–80. [DOI] [PubMed] [Google Scholar]
- 44. van der Horst J, Greenwood IA, Jepps TA. Cyclic AMP-dependent regulation of Kv7 voltage-gated potassium channels. Front Physiol. 2020;11:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Panchal SS, Mehta AA, Santani DD. Effect of potassium channel openers in acute and chronic models of glaucoma. Taiwan J Ophthalmol. 2016;6(3):131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang J, Liu X, Zhong Y. Rho/Rho-associated kinase pathway in glaucoma (Review). Int J Oncol. 2013;43(5):1357–67. [DOI] [PubMed] [Google Scholar]
- 47. Miller S, Daily L, Leishman E, Bradshaw H, Straiker A. Δ9-Tetrahydrocannabinol and cannabidiol differentially regulate intraocular pressure. Invest Ophthalmol Vis Sci. 2018;59(15):5904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. MacIntyre J, Dong A, Straiker A, Zhu J, Howlett SE, Bagher A, et al. Cannabinoid and lipid-mediated vasorelaxation in retinal microvasculature. Eur J Pharmacol. 2014;735:105–14. [DOI] [PubMed] [Google Scholar]
- 49. Rösch S, Ramer R, Brune K, Hinz B. R(+)-Methanandamide and other cannabinoids induce the expression of cyclooxygenase-2 and matrix metalloproteinases in human nonpigmented ciliary epithelial cells. J Pharmacol Exp Ther. 2006;316(3):1219–28. [DOI] [PubMed] [Google Scholar]
- 50. McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Green K, Bigger JF, Kim K, Bowman K. Cannabinoid action on the eye as mediated through the central nervous system and local adrenergic activity. Exp Eye Res. 1977;24(2):189–96. [DOI] [PubMed] [Google Scholar]
- 52. Miller S, Daily L, Ploss M, Greig I, Ross R, Rayana NP, et al. Evidence that cannabinoid CB1 receptors regulate intraocular pressure via two opposing mechanisms. Exp Eye Res. 2020;200:108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sappington RM, Sidorova T, Long DJ, Calkins DJ. TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest Ophthalmol Vis Sci. 2009;50(2):717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang MTM, Danesh-Meyer HV. Cannabinoids and the eye. Surv Ophthalmol. 2021;66(2):327–45. [DOI] [PubMed] [Google Scholar]
- 55. Green K, Wynn H, Padgett D. Effects of delta9-tetrahydrocannabinol on ocular blood flow and aqueous humor formation. Exp Eye Res. 1978;26(1):65–9. [DOI] [PubMed] [Google Scholar]
- 56. European Monitoring Centre for Drugs and Drug Addiction ; Medical use of cannabis and cannabinoids: questions and answers for policymaking. LU: Publications Office; 2018. [cited 2024 Feb 28]. Available from: https://data.europa.eu/doi/10.2810/979004 [Google Scholar]
- 57. Hepler RS, Petrus RJ. Experiences with administration of marihuana to glaucoma patients. In: Cohen S, Stillman RC, editors. The therapeutic potential of marihuana. Boston, MA, US: Springer; 1976. p. 63–75. [Google Scholar]
- 58. Green K. Marihuana and intraocular pressure. In: Nahas GG, Sutin KM, Harvey D, Agurell S, Pace N, Cancro R, editors. Marihuana and medicine. Totowa, NJ: Humana Press; 1999. p. 581–9. [Google Scholar]
- 59. Schwitzer T, Schwan R, Albuisson E, Giersch A, Lalanne L, Angioi-Duprez K, et al. Association between regular cannabis use and ganglion cell dysfunction. JAMA Ophthalmol. 2017;135(1):54–60. [DOI] [PubMed] [Google Scholar]
- 60. Chithra NK, Bojappen N, Vajawat B, Pai NM, Gowda GS, Moirangthem S, et al. Legalization of recreational cannabis: is India ready for it? Indian J Soc Psychiatry. 2023;39(4):325–31. [Google Scholar]
- 61. Jóhannesson G, Stille U, Taube AB, Karlsson M, Kalaboukhova L, Bergström A, et al. Guidelines for the management of open-angle glaucoma: national program area eye diseases, national working group glaucoma. Acta Ophthalmol. 2024;102(2):135–50. [DOI] [PubMed] [Google Scholar]
- 62.BMJ Publishing Group Ltd. BMA House TS. European Glaucoma Society Terminology and Guidelines for Glaucoma; Br J Ophthalmol. 2017(6):130–95. [Chapter 3]: Treatment principles and options Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; [Chapter 3] Treatment principles and options.4th ed101. [DOI] [PMC free article] [PubMed]
- 63.Open-angle glaucoma: treatment – UpToDate [Internet]. [cited 2024 Apr 9]. Available from: https://www.uptodate.com/contents/open-angle-glaucoma-treatment?topicRef=6909&source=see_link#H6891053
- 64. Saraiva SM, Martín-Banderas L, Durán-Lobato M. Cannabinoid-based ocular therapies and formulations. Pharmaceutics. 2023;15(4):1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grossman AL, Javitt MJ, Moster SJ, Grajewski AL; Pediatric Glaucoma Subcommittee of the American Glaucoma Society 2017–2018 . American glaucoma society position statement on cannabinoid use in pediatric glaucoma patients. Ophthalmol Glaucoma. 2019;2(6):365–6. [DOI] [PubMed] [Google Scholar]
- 66. Jampel H. American glaucoma society position statement: marijuana and the treatment of glaucoma. J Glaucoma. 2010;19(2):75–6. [DOI] [PubMed] [Google Scholar]
- 67. Buys YM, Rafuse PE. Canadian ophthalmological society policy statement on the medical use of marijuana for glaucoma. Can J Ophthalmol. 2010;45(4):324–6. [DOI] [PubMed] [Google Scholar]