Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Oct 28;19(1):20241075. doi: 10.1515/med-2024-1075
Abstract
Background
Parkinson’s disease (PD) is primarily known as a motor disorder; however, its debilitating non-motor symptoms have a significant impact on patients’ quality of life. The current standard treatment, l-DOPA, is used to relieve motor symptoms, but prolonged use is often associated with severe side effects. This creates an urgent need for effective alternatives targeting both motor and non-motor symptoms.
Objectives
Over the past decade, Cannabis sativa and its cannabinoids have been widely studied across various health conditions. Among these compounds, cannabidiol (CBD), a non-psychoactive component, is garnering growing interest due to its multi-targeted pleiotropic properties. This work aims to provide a comprehensive overview of CBD’s efficacy in PD.
Methods
This review compiles data on both motor and non-motor symptoms of PD, integrating results from preclinical animal studies and available clinical trials.
Results
Preclinical research has demonstrated promising results regarding CBD’s potential benefits in PD; however, the total number of clinical trials is limited (with only seven studies to date), making it difficult to draw definitive conclusions on its efficacy.
Conclusions
While preclinical findings suggest that CBD may have therapeutic potential in PD, the limited number of clinical trials highlights the need for further research. This review emphasizes the gaps that need to be addressed in future studies to fully understand CBD’s role in treating both motor and non-motor symptoms of PD.
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by the degeneration of dopaminergic neurons in the substantia nigra pars compacta. It affects 4.5 million people worldwide, with a prevalence of 1% of the population over 60 years old; it is the second most common neurodegenerative disease [1], accounting for more than 90% of sporadic cases, and is associated with a high lifetime cost and impairment [2]. PD is generally defined as a movement disorder characterized by several common manifestations, such as abnormal posture, bradykinesia, resting tremor, and rigidity; however, other non-motor symptoms (e.g., sleep impairment, depression, and pain) are also prominent comorbidities [3].
The pathophysiology of idiopathic PD is still not fully understood, but environmental and genetic variables are known to be involved in the disease. Head injury, age, obesity, sedentary lifestyle, rural life, and herbicide/insecticide exposure have all been linked to PD in several studies; however, smokers and coffee users have a lower risk of developing PD [4]. The pathophysiology of this disease involves multiple molecular and cellular dysfunctions, including mitochondrial dysfunction, oxidative stress, alpha-synuclein misfolding and aggregation, calcium homeostasis dysregulation, and neuroinflammation [5]. These changes may result in the death of dopaminergic neurons in the nigrostriatal pathway, which is the most severe feature of PD [6].
The main standard of medical therapy to improve the symptoms of dopamine deficiency is the dopamine precursor levodopa. However, while levodopa is highly efficacious, especially when combined with DOPA decarboxylase blocking agents such as carbidopa, which inhibit the metabolic degradation of levodopa in the peripheral nervous system before it is released into the central nervous system, chronic use may lead to reduced efficacy and difficulty in titration as the therapeutic window narrows [7]. Furthermore, antiparkinsonian treatment accounts for 22–58% of the direct costs for PD patients worldwide [1]. As a result, there is an urgent need to discover more effective and tolerable alternative options with fewer side effects to slow neurodegeneration and improve patients’ quality of life.
Recently, derivatives extracted from Cannabis (Cannabis sativa L.) have attracted the focus of several studies and regulations. Apart from delta-9-tetrahydrocannabinol (Δ9-THC), which is known for its psychotropic effects, there are other non-psychotropic phytocannabinoids, and cannabidiol (CBD) is the most well-known of them because it is non-intoxicating and has several advantageous pharmacological properties. Given the growing body of literature on the clinical use of CBD, PD is one of the movement disorders of interest to researchers seeking to discover its possible therapeutic potential due to its growing popularity and favorable side effect profile [7].
In Europe, Gowers described the beneficial use of Cannabis indica tincture for treating PD for the first time in 1888. A century later, Cannabis has gained traction as a potential PD therapy [8]. Patients with PD have been found to express high levels of cannabinoid receptors (CBRs) [9]; thus, Parkinsonian models have recently been used to study the effects of the endocannabinoid system (ECS) on basal ganglia functioning and corticostriatal processing. However, there is still a lack of clinical data on the benefits of cannabinoids in patients with PD [10].
Several lines of evidence show that dopamine (DA) depletion results in a significant rearrangement of the striatal ECS. There is a strong interaction between the endocannabinoid and dopaminergic systems through specific G protein-coupled cannabinoid receptors (CB1 and CB2) in the basal ganglia. This interaction is involved in both the control of synaptic function and the regulation of motor behavior [11]. Despite progress in the molecular understanding of how cannabinoids (CBs) interact with DA, the clinical impact of CB therapies on the motor symptoms of PD is still unclear [12].
Several recent studies have investigated the role of CBD in reducing tremors and movement impairment caused by PD. According to a theory, CBD’s possible therapeutic effects in movement disorders such as PD are thought to be due to neuroprotection and a reduction in dopaminergic neuron degeneration [13].
This work presents a comprehensive review of the literature on the use of CBD in PD. The structure of this review begins with an examination of the safety and tolerability of the CBD molecule, followed by its interaction with other cannabinoids to modulate the ECS. The main objective of this review is to present essentially all the animal and clinical studies that have used CBD to treat both motor (including l-DOPA-induced dyskinesia [LID]) and non-motor symptoms of PD and highlight gaps to be filled in future research.
2. CBD: a phytocannabinoid with a pharmacological profile of interest
C. sativa plants contain more than 500 phytochemical compounds, at least 113 of which are identified as phytocannabinoids [14]. Among this mixture of phytocannabinoids, the compound that has gained attention in recent decades, in addition to Δ9-THC, is CBD, which is known as one of the main nonpsychotropic molecules of cannabis. This phytocannabinoid constitutes up to 40% of some plant extracts [2]. CBD was originally isolated in 1940 by Adams et al. [15], after which Mechoulam and Shvo determined its structure 23 years later [16]. The CBD concentration varies greatly depending on the plant’s genotype, the growing environment, and the part used to make the extract [17]. The (−) CBD isomer is the primary naturally occurring component of C. sativa. To date, CBD is recognized as a pleiotropic compound that acts on multiple targets; however, its molecular pharmacology needs to be further investigated.
In terms of CBRs, CBD has a low affinity for both CB1 and CB2 receptors [2,18,19]. According to Jones and colleagues, CBD showed no CB1 receptor agonist activity but was able to inhibit cannabinoid agonists in vitro by acting as an inverse agonist for both receptors CB1 and CB2 [20]. It also acts as an indirect agonist at CB1R by increasing endogenous levels of anandamide (AEA) via fatty acid amide hydrolase (FAAH) blockade [21,22]. CBD is thought to have no impact on how 2-AG interacts with either CB1Rs or CB2Rs [23,24]. However, CBD acts as a negative allosteric modulator of CB1R by decreasing the efficacy and potency of 2-AG at that receptor [25]. Regarding the impact of CBD on non-cannabinoid targets, it has been demonstrated that CBD acts as an agonist of transient receptor potential vanilloid channel type 2 (TRPV-2) and an antagonist of transient receptor potential for melastatin (TRPM8) [26,27]. Additionally, according to de Petrocellis et al., CBD can indirectly agonist potential vanilloid channel type 1 (TRPV-1) receptors by increasing the levels of anandamide, an endogenous agonist of TRPV-1 [21]. In terms of dopamine neurotransmission, CBD inhibits the dopaminergic receptor [28], thereby increasing endogenous levels of dopamine [29] and acting as a negative allosteric modulator of the dopaminergic receptor D2 [30]. As a result, it is suggested that CBD may have an effect on dopaminergic neurotransmission in the basal ganglia.
Under various experimental conditions, CBD reduces the production of several molecules, such as prostaglandin E2 [31], reactive oxygen species, and inducible nitric oxide synthase [13,32,33,34] and influences the production of numerous pro-inflammatory molecules [35,36,37]. The anti-inflammatory and antioxidant properties of CBD may explain most of its neuroprotective effects. Nevertheless, the specific pathways and mechanisms through which CBD exerts its effects in PD and other neurodegenerative disorders are still under investigation.
3. CBD safety and tolerability
Due to its potential medical uses, CBD is currently the focus of significant investigations, especially after the Food and Drug Administration (FDA)-approved Epidiolex® in 2018 for the treatment of infantile refractory epilepsy syndromes (Dravet and Lennox-Gastaut). However, CBD is not completely risk-free. In preclinical research, adverse effects of CBD on animals included hypotension, changes in organ weight, decreased spermatogenesis, neurotoxicity of the central nervous system, hepatocellular damage, developmental toxicity, and embryo-fetal death, but these toxic effects were essentially observed at high doses and above-recommended levels [38]. Clinical investigations have shown that CBD can cause liver issues, diarrhea, fatigue, vomiting, somnolence, and more importantly, CBD-induced drug-drug interactions [38]. The first comprehensive review and meta-analysis of the side effects and tolerability of CBD across all medical indications revealed that only children with epilepsy were susceptible to pneumonia, respiratory depression and aspiration, sedative effect, lower appetite, and abnormal liver function. After excluding research on pediatric epilepsy, diarrhea was the sole unfavorable effect connected to CBD therapy [39]. However, while the side effects of CBD are generally less severe compared to Δ9-THC, they should not be overlooked.
In an open-label study on PD, 100 mg/mL Epidiolex® was given to patients at doses ranging from 5 to 20–25 mg/kg per day for 10–15 days. According to the study’s findings, the side effects of Epidiolex were generally minor, and none were severe [40]. However, co-administration of clobazam with Epidiolex leads to a threefold increase in the active metabolite of clobazam, consequently increasing the risk of side effects such as excessive sedation [38]. An interesting review published in 2019 discusses CBD interactions with other drugs and the resulting side effects (for more details, see [41]). CYP450 enzymes play a crucial role in the metabolism and biotransformation of most endogenous and xenobiotic compounds. CBD has been shown to interact with several isoforms of these enzymes, including 3A4, 2C9, 2C19, 1A2, 2C8, 2B6, and 2E1 [41]. For instance, this phenomenon highlights the importance of monitoring potential drug interactions, particularly in elderly patients, where medication management is already complex and this demographic is often characterized by comorbidities, polypharmacy, and physiological changes that affect pharmacokinetics and drug tolerability [42].
According to the existing evidence from clinical trials, CBD is generally well tolerated and has few major side effects compared to Δ9-THC; nevertheless, interactions with other drugs should be carefully monitored, particularly in vulnerable populations such as the elderly and patients with chronic diseases.
4. Modulation of the ECS with cannabinoids in PD
The ECS is an endogenous system that consists of cannabinoid receptors (CB1 and CB2), their ligands (N-arachidonoyl ethanolamine [AEA or anandamide] and 2-arachidonoylglycerol [2-AG]), the enzymes that synthesize (N-arachidonoyl phosphatidyl ethanol phospholipase D [NAPE-PLD] and diacylglycerol lipase) and metabolize them (FAAH and monoacylglycerol lipase) [43]. During the course of PD, the ECS exhibits neurochemical modifications, with CB1 receptors downregulated in the early stages and upregulated (together with CB2 receptors) with a higher endocannabinoid tone in the intermediate/late stages of the disease, according to animal and human studies [44,45,46].
The preclinical research performed over the past 20 years has demonstrated that cannabinoids (both natural and synthetic) can control neurochemical changes in the glutamatergic and GABAergic systems caused by decreased dopamine levels by activating and/or inhibiting CB1/2 receptors [34,45,46,47,48]. The anatomical and functional complexity of CB1 receptors and the distribution of endocannabinoids in several subareas of the basal ganglia are also discussed in these studies. Moreover, preclinical research suggests that CB1 receptor agonists and antagonists, as well as drugs that modulate endocannabinoid metabolism, could be useful in the treatment of this disorder [34,45,46,47,48].
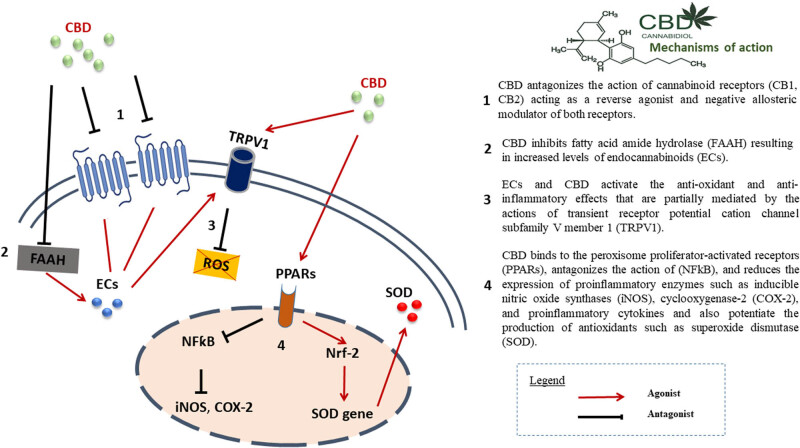
Several mechanisms (Figure 1) have been implicated in the action of cannabinoids in PD. These include antioxidant, anti-excitotoxic, and anti-inflammatory properties (mediated not only by activation of CB1R but also CB2R), inhibition of anandamide hydrolysis and their actions on other receptors, including modulation of the TRPV1 receptor channel and G protein-coupled receptor 55 (GPR55) among others [34,45,46,47].
Figure 1.
Generally, cannabinoids act at two levels of basal ganglia function: glutamatergic/dopaminergic synaptic neurotransmission and corticostriatal plasticity, both of which are important in LID [10]. Additionally, activation of the ECS may result in neuroprotective effects via direct receptor-independent mechanisms [49], activation of anti-inflammatory cascades in glial cells via CB2Rs [34], and anti-glutamatergic anti-excitotoxic effects [47].
In fact, cannabinoids were shown to affect catecholaminergic and dopaminergic systems in early animal experiments [50,51]. Dopaminergic pathways, including the striatum, contain significant levels of CB1Rs, anandamide, and 2-AG, which regulate dopaminergic transmission via retrograde feedback mechanisms at presynaptic glutamate and GABAergic nerve terminals [52]. In the GABAergic system, it has been suggested that by blocking GABA uptake into the lateral part of the globus pallidus internus (GPi), cannabinoid agonists enhance GABAergic transmission in the indirect loop of the basal ganglia. In the glutamatergic system, the activation of CB1R at glutamate synapses inhibits the excitation of N-methyl-d-aspartate (NMDA) and AMPA receptors on dopamine neurons, which leads to the inhibition of excitation. Both methods may play a role in preventing dyskinesia [53].
The primary thalamo-cortical output region within the basal ganglia, particularly the medial section of the GPi, displays a high concentration of CB-1 receptors [54]. The presence of CB2Rs on human nigrostriatal dopaminergic neurons [11] shows that the ECS has direct modulatory effects on dopaminergic transmission [46]. Although nigrostriatal neurons do not express CB1R [55], they are regulated by the ECS [47,56] through CB1Rs which are expressed on GABAergic, glutamatergic, and opioidergic neurons [57]. Furthermore, interactions with various endocannabinoids have been reported for TRP channels expressed on dopaminergic neurons [58,59].
CB1R activation in the midbrain has also been shown to increase acetylcholine release, thereby ameliorating the local cholinergic deficit observed in PD [60]. Furthermore, cannabis interactions with the serotonergic system may have an impact on LID; denervation of striatal dopamine neurons causes a shift in the conversion of levodopa to dopamine from dopamine to serotonin neurons, resulting in non-physiological pulsatile dopamine release (false messengers) [61].
The concentration of CB receptors on the target structure largely determines the activating effects of a ligand with low CB receptor affinity, such as Δ9-THC. This may explain why CB ligands have such a wide range of effects, as the concentration of CB receptors varies so much in different brain areas. Moreover, the relative sensitivity of GABAergic and glutamatergic neurons to CB1-R agonists varies among species [62].
In terms of corticostriatal plasticity, experimental in vivo PD studies have shown that the ECS appears to influence corticostriatal synaptic plasticity. CB1R agonists reduce abnormal dopamine-mediated corticostriatal long-term potentiation and promote long-term depression in LID conditions, rendering glutamatergic synapses less responsive to future stimulation. This antidyskinetic effect is reversed by CB1R inhibition [46].
Investigations of PD animal models and human tissues from PD patients revealed an increase in ECS activity [63], with overexpression of CBRs [64,65], accumulation of cannabinoid receptor agonists [66,67], and a decrease in their breakdown [68]. In an animal model, chronic levodopa substitution reversed this ECS adaptation [69].
Overall, preclinical research has shown that cannabinoids can modulate the ECS, influence neurochemical changes in different systems, and potentially offer neuroprotective effects in PD. These findings suggest that ECS modulation could be a promising therapeutic approach. However, it is important to recognize that the translation of these preclinical findings to clinical practice remains uncertain.
5. Preclinical data on the use of CBD in PD
CBD has been studied extensively in preclinical investigations for a variety of neurodegenerative disorders [70]. The neuroprotective properties of CBD do not appear to depend on the direct activation of CB1 receptors [71], even though CB2 receptor involvement has been documented in specific pathological conditions [72]. The direct activity of CBD at these CBRs is still under debate. However, CBD could facilitate AEA-mediated effects by lowering FAAH activity, which is an indirect route [21].
The first preclinical study addressing CBD’s properties in PD was published by Lastres-Becker and his colleagues in 2005. The study revealed that administering 3 mg/kg CBD daily for 2 weeks reduced the depletion of dopamine and tyrosine hydroxylase in the striatum of rats injected with 6-hydroxydopamine (6-OHDA) immediately after lesion induction. The neuroprotective effect is likely mediated by cannabinoid receptor-independent antioxidant and anti-inflammatory properties [34]. However, when the treatment began one week later, there was no effect. Increased expression of Cu2+/Zn-superoxide dismutase mRNA, a key enzyme in endogenous oxidative stress responses, was associated with the effects of CBD [73].
In contrast, a 2016 study showed that 5 mg/kg CBD for 5 weeks did not affect dopaminergic neuron loss or motor impairments caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [74]. Another investigation in the same year tested different doses of CBD (15, 30, and 60 mg/kg, for 3 days) in a mouse model of LID by giving l-DOPA for 21 days after receiving 6-OHDA treatment. Although CBD alone had no impact, when it was combined with capsazepine (CPZ) (a TRPV-1 antagonist) at a dose of 30 mg/kg, it resulted in a remarkable reduction in involuntary movements induced by l-DOPA. Furthermore, this combination significantly reduced the levels of the pro-inflammatory markers cyclooxygenase-2 (COX-2) and nuclear factor-kappa B (NF-κB). CB1 and peroxisome proliferator-activated type gamma (PPAR) receptor antagonists prevented all of these effects. Thus, the anti-inflammatory effects mediated by CB1 and PPAR receptors are thought to be responsible for the neuroprotective effects of CBD (when combined with a TRPV-1 antagonist) [75]. CB2R activation, but not CB1R activation, may have neuroprotective effects on PD, which could be due to an anti-inflammatory effect [64,73]. CB2R agonists protected mice from MPTP-induced nigrostriatal degeneration by decreasing microglial activation and infiltration, according to rodent PD model research [76]. More recently, Wang and colleagues have demonstrated that the administration of 100 mg/kg CBD by oral gavage for 14 days alleviates PD symptoms in the MPTP mouse model. The study reported an improvement of cognitive behaviors, an increase in DA, 5-HT, and IL-10 levels, and a decrease in several apoptosis and neuroinflammation markers [77].
Drug-induced catalepsy tests, which are often used to assess motor deficits due to changes in striatal function, were employed in two investigations [78,79]. The first study aimed to test the effects of acute CBD pretreatment (5, 15, 30, or 60 mg/kg) on catalepsy caused by haloperidol (a D2 receptor antagonist), l-nitro-N-arginine (a selective nitric oxide synthase [NOS] inhibitor), and WIN55,212 (a CB1 receptor agonist) in male Swiss mice. CBD reduced the increase in catalepsy time caused by all three medications in a dose-dependent manner [78]. In the second study, rats were given reserpine (an irreversible inhibitor of vesicular monoamine transporters 1 and 2 or VMAT1/2) to cause motor impairments and cognitive abnormalities to imitate both tardive dyskinesia and PD. In the discriminative avoidance task, CBD doses of 0.5 or 5 mg/kg considerably reduced reserpine-induced catalepsy and chewing movements (but not locomotor activity), and the lowest dose also significantly improved reserpine-induced memory/learning deficits [79].
CBD has been shown to protect neurons in both cell tissue and animal models of PD in vitro. CBD enhanced the survival, differentiation, and expression of axons (GAP-43) and the synaptic proteins synaptophysin and synapsin I in a cellular model of PD in PC12 and SH-SY5Y cells treated with 1-methyl-4-phenylpyridinium (MPP+) [80]. After incubation with lipopolysaccharide (LPS), CBD enhances cell viability and decreases microglial activation [81,82]. Since chronic inflammation is a prominent hallmark of PD and dopaminergic neurons are particularly sensitive to glial activation [83], the anti-inflammatory properties of CBD may contribute to its neuroprotective potential in PD [2]. In a cell culture model of PD, differentiated SH-SY5Y neuroblastoma cells were exposed to three toxins that model PD-associated biochemical abnormalities: reduced mitochondrial activity by MPP+, free radical production by paraquat, and inhibition of the ubiquitin‒proteasome system by lactacystin. MPP+ and lactacystin administration resulted in substantial upregulation of CB1 receptors. In this model, no protective effects of CBD (0.01–1.0 M) were identified [49].
In these animal and cellular models of motor, biochemical, and cognitive symptoms, the preclinical data discussed above suggest that CBD generally alleviates PD-related symptoms. However, the majority of studies have employed a single model of motor symptoms, with only a few examining additional elements of PD. Furthermore, the overall volume of investigations is still limited, and their methodology varies in terms of CBD dosage.
6. Clinical trials on the use of CBD in PD
To date, 17 clinical studies using Cannabis or its derivatives to treat PD have been reported in the literature (Appendix). Seven studies utilized CBD as the main drug of interest, six studies used pure CBD [60,84,85,86,87,88], and one study used a mixture of 1.25 mg CBD and 2.5 mg Δ9-THC (Cannador®) [89].
6.1. Motor symptoms
Among all the clinical trials that have been conducted thus far investigating the usefulness of CBD in PD patients (four studies) concluded that no improvement was noted regarding the effect of CBD on the severity of motor symptoms, as evaluated by the Unified Parkinson Disease Rating scale (UPDRS) [84,86,88,89]. The details of each study are summarized in Table 1.
Table 1.
Studies with CBD addressing motor symptoms in PD
| Symptom | Design | Sample size | Duration | Primary outcome | Application form | Dosage | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Motor symptoms | Double-blind, randomized, placebo-controlled crossover study | 17 | 2 × 4 weeks, 2 weeks wash-out phase | UPDRS | Orally | 2.5 mg THC + 1.25 mg CBD (Cannador) or placebo | No positive effect compared with placebo | Carroll et al. [89] |
| Open-label pilot | 6 | 4 weeks | UPDRS total | Orally | 150–400 mg/day CBD or Placebo | CBD did not worsen the motor function and decreased the total scores of UPDRS | Zuardi et al. [165] | |
| Randomized, double-blind, placebo-controlled study | 21 | 6 weeks | UPDRS total | Orally | 1. Group: CBD 75 mg/day | UPDRS unchanged | Chagas et al. [60] | |
| 2. Group: CBD 300 mg/day | ||||||||
| 3. Placebo group | ||||||||
| Phase II/III, randomized double-blind, placebo-controlled clinical trial | 33 | 14 weeks | UPDRS | Orally | 75 or 300 mg/day CBD or placebo | No significant difference between groups | de Almeida et al. [86] |
6.2. Pain
In medicine, pain is a complex and multifaceted notion, making it particularly challenging to comprehend and treat therapeutically. Pain is assumed to be a combination of a subjective psychophysical experience, objective sensory neurophysiology, and emotional response to stressful stimuli [90]. In PD, chronic pain is one of the most common non-motor symptoms in PD patients, affecting 60–80% of individuals [91]. According to a study of nearly 2,000 PD patients, chronic pain cannot be explained uniquely by peripheral variables because central causes appear to play a significantly greater role than previously thought [92].
For the management of chronic pain, a range of analgesic medicines have been created and widely used. Standard medical therapy for persistent neuropathic pain includes antiepileptic medications, selective serotonin reuptake inhibitors (SSRIs), nonsteroidal anti-inflammatory drugs (NSAIDs), and in refractory situations, opioids, all of which have different efficacies and distinct long-term side effects [7].
Natural cannabinoids have emerged as strong candidates for pain therapy in the face of rising opiate misuse rates [43]. Although cannabis has been used to alleviate pain since 5000 BC, little is known about its mechanisms of action [93]. There are still some doubts about whether cannabis can help with specific forms of pain [94]. Currently, nabiximols mouth spray (Sativex®), an oromucosal spray containing a 1:1 ratio of CBD and Δ9-THC, is the only approved cannabis-based treatment for MS-related spasticity and neuropathic pain in some countries[95]. CBD has been shown to help with pain alleviation in several preclinical studies [96,97,98]; however, recent clinical trials evaluating the efficacy of CBD alone in other forms of pain are reporting heterogeneous results [99,100,101,102], with the lack of those focusing on Parkinson-related forms of pain.
According to our literature search, one study has assessed pain in PD patients (Table 2) using Cannador® with 2.5 mg of Δ9-THC and 1.25 mg of CBD [89]. This double-blind randomized, placebo-controlled crossover study in 2004 reported no positive effect on pain assessed by the McGill Pain Scale in 17 patients. Therefore, evaluating CBD’s efficacy treatment exclusively in this population could be of interest.
Table 2.
Studies with CBD addressing pain symptoms in PD
| Symptom | Design | Sample size | Duration | Primary outcome | Application form | Dosage | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Pain | Double-blind, randomized, placebo-controlled crossover study | 17 | 2 × 4 weeks, 2 weeks wash-out phase | McGill Pain Score | Orally | 2.5 mg THC + 1.25 mg CBD (Cannador) or placebo | No positive effect on pain | Carroll et al. [89] |
6.3. Depression
Depression is one of the most commonly reported neuropsychiatric disorders in PD and it is widely acknowledged that 40–50% of patients with PD experience clinically significant depressive disorders [103]. Several studies suggest that depression can occur at any stage of the disease; importantly, affective disorders often precede the onset of motor symptoms, on average 4–6 years before the diagnosis of PD [104]. These disorders may have a long-term or recurrent course and therefore contribute to the alteration of the quality of life of PD patients [105].
CBD is hypothesized to affect depression due to its capacity to target and modulate serotonin and norepinephrine brain neurotransmission as well as its active binding to 5HT-1A receptors [106]. Furthermore, CBD promotes synaptic plasticity and neurogenesis, both of which are important in the development and treatment of depression [21,52,106]. The conclusions of certain animal models have been positive and encouraging [107,108]. However, only a few clinical studies involving individual patients with a history of depression who successfully tried CBD products have been reported [107,109]. These studies should be approached with caution. CBD use has been linked to a number of negative side effects, including increased depression and even suicidal thoughts [41]. Depression and suicidal ideation are listed as possible side effects of the FDA-approved form of CBD Epidiolex’s box insert [110].
According to our literature search, there was one clinical trial (Table 3) in which CBD was administered to 10 depressed PD patients in a study including 33 patients with rapid eye movement disorder for 14 weeks, but no positive effect on depression was noted [86]. As a result, more large-scale trials are required to evaluate both the potential antidepressant benefits of CBD as well as its long-term efficacy and safety.
Table 3.
Studies with CBD addressing depression symptoms in PD
| Symptom | Design | Sample size | Duration | Primary outcome | Application form | Dosage | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Depression | Double-blind, randomized, placebo-controlled study | 10/33 | 14 weeks | Zung Self-Rating Depression Scale (SDS) | Orally | CBD 75 mg/day or 300 mg/day or placebo | No significant effect between groups | de Almeida et al. [86] |
6.4. Sleep disturbances
Sleep disorders affect 50–70 million people in the United States, resulting in millions of physician visits each year. There are numerous reviews and studies on the effects of cannabis and cannabinoids on sleep; a meta-analysis revealed significant improvement in sleep quality in 8 trials and in sleep disturbance in three trials, but these improvements were regarded as minor [95,111,112,113].
Human investigations on the effects of CBD on sleep disorders are limited and modest in number [60,88,114,115]. Sleep problems, such as poor sleep quality, insomnia, restless legs syndrome, and rapid eye movement sleep behavior disorder, are more common in PD patients [116]. Sleep problems in PD patients, similar to other non-motor symptoms, appear to be related to dopaminergic neurotoxicity and subsequent neurochemical abnormalities in areas of the brain involved in the regulation of the sleep-wake cycle mediated by cholinergic, GABAergic, and serotonergic neurons [60]. The use of benzodiazepines and, less commonly, melatonin and cholinesterase inhibitors to treat sleep disorders in this population is limited because of side effects. Benzodiazepines are associated with somnolence and impaired motor coordination, which may worsen sleep and motor symptoms in PD patients, as well as tolerance, abuse/dependence, and withdrawal symptoms. The administration of melatonin can cause somnolence and psychosis, which can increase sleep and psychotic symptoms, whereas cholinesterase inhibitors might cause gastrointestinal problems, anorexia, and bradycardia [60,117]. Studies about CBD’s influence on sleep are still in their early stages and it is assumed to influence sleep because endocannabinoids have been proven to play a role in the circadian rhythm [88,118,119].
According to our literature search, there are four studies investigating CBD on sleep quality in PD patients (Table 4). The first study was conducted in 2004 by Carroll et al. in a double-blind randomized placebo-controlled crossover study in 17 PD patients using Cannador® (2.5 mg Δ9-THC + 1.25 mg CBD). The study concluded that no positive effect on sleep was detected by the visual analog sleep scale [89]. However, in 2014, Chagas et al. confirmed that 4 PD patients with REM sleep disorder experienced rapid and substantial reductions in agitation, limb movements, and nightmares, with pure CBD [60]. The third study was the first randomized, double-blind, placebo-controlled clinical trial by de Almeida et al. evaluating CBD’s effect on sleep disorders in PD patients. They concluded that prolonged use of CBD did not produce any significant difference from the placebo for primary outcomes [86]. More recently, the same results were observed in an exploratory study of phase II/III clinical trial assessing the effect of 75–300 mg of CBD in PD patients with restless leg syndrome and REM sleep disorder [87]. Therefore, there is a need for large-scale trials to assess the efficacy of CBD in the treatment of sleep disorders and its long-term safety, with the recommendation of investigating the addictive potential of CBD in long-term studies.
Table 4.
Studies with CBD addressing sleep disturbance symptoms in PD
| Symptom | Design | Sample size | Duration | Primary outcome | Application form | Dosage | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Sleep | Double-blind, randomized, placebo-controlled crossover study | 17 | 2 × 4 weeks, 2 weeks wash-out phase | Visual analog sleep scale | Orally | 2.5 mg THC + 1.25 mg CBD (Cannador) or placebo | No positive effect on sleep | Carroll et al. [89] |
| Observational case series | 4 | 6 weeks | PD patients with REM sleep disorder | Orally | 75 or 300 mg CBD | Reduction of agitation, limb movements | Chagas et al. [84] | |
| Clinical assessments not mentioned | And nightmares in all patients (symptoms returned after interrupted treatment) | |||||||
| Phase II/III, randomized double-blind, placebo-controlled clinical trial | 33 | 14 weeks | Frequency of nights with RBDa, CGIb | Orally | 75 or 300 mg CBD or placebo | CBD showed no difference to placebo for primary outcomes | de Almeida et al. [86] | |
| Significant improvement in average sleep satisfaction with 300 mg was noted | ||||||||
| Phase II/III, a parallel, double-blind, placebo-controlled clinical trial | 6/18 | 14 weeks | Restless Legs Syndrome Rating Scale | Orally | 75 or 300 mg CBD or placebo | No reduction in the severity of RLSc in PD patients with RBDa | de Almeida et al. [87] |
aRapid eye movement sleep behavior disorder.
bClinical Global Impression scale.
cRestless leg syndrome.
6.5. Psychosis
Psychosis is very common in people with PD, affecting almost one-third of individuals, especially in the later stages of the disease [116]. Antiparkinsonian drugs, dopaminergic neurotoxicity, and Lewy body pathology all appear to play a role in the pathogenesis of psychosis in PD. Treatment of psychosis in PD patients is complicated and remains a therapeutic challenge, as it usually involves lowering or eliminating antiparkinsonian drugs (which might worsen symptoms) and/or adding conventional antipsychotics to the regimen (which can worsen motor symptoms). Atypical antipsychotics, such as clozapine, are not linked to worsening motor symptoms, but they can have serious hematological, cardiovascular, and neurological adverse effects [88,120,121].
Several reviews [120,122,123,124,125] have shown that animal and human studies consistently suggest that CBD has antipsychotic effects and is well tolerated in general. However, there are few published clinical trials including CBD given to psychotic patients, and most of them had small sample sizes and short durations, focused on symptomatic or first-episode patients, and did not particularly address negative/cognitive symptoms [117].
The mechanisms underlying the antipsychotic activity of CBD are not yet understood, but CBD has shown a pattern of activation of Fos-immunoreactive neurons similar to that of the atypical antipsychotic clozapine but different from that of the typical antipsychotic haloperidol, with activation of limbic but not motor areas [126]. The antipsychotic effects of CBD appear to be mediated through the ECS via the inhibition of FAAH and subsequent increase in anandamide levels, as well as the activation of vanilloid TRPV1 and serotonin 5-HT1A receptors [22,120].
According to our literature search, there was one study assessing the effect of CBD on psychosis in PD patients (Table 5). This open-label pilot study demonstrated that 6 PD patients had a significant decrease in their psychotic symptoms[88]. The size of these studies is often small, and the follow-up is either brief or limited to a single event. The number of accessible research is quite restricted, and the majority of them are published as individual case reports. As a result, large-scale clinical research is required to determine CBD’s long-term efficacy and safety.
Table 5.
Studies with CBD addressing psychosis symptoms in PD
| Symptom | Design | Sample size | Duration | Primary outcome | Application form | Dosage | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Psychosis | Open-label pilot study | 6 | 4 weeks | Brief Psychiatric | Orally | 150 mg CBD starting dose, weekly dosage increase by 150 mg depending on clinical response | Significant decrease of psychotic symptoms | Zuardi et al. [88] |
| Rating Scale, Parkinson | ||||||||
| Psychosis | ||||||||
| Questionnaire |
6.6. Anxiety
Anxiety disorders encompass a wide range of symptoms and manifestations, all of which can negatively affect one’s quality of life and capacity to perform daily tasks. These disorders are the most common mental condition with a lifetime prevalence of 29% in the general population, and the most common psychological symptoms in people with PD affecting nearly 67% of PD patients [127,128]. Although psychotherapy, SSRIs/serotonin-norepinephrine reuptake inhibitors, benzodiazepines, monoamine oxidase inhibitors, and/or tricyclic antidepressants are usually the mainstays of treatment for this group of disorders, all of these pharmacologic solutions have highly variable rates of efficacy as well as their own risk of side effects. In PD, first-line anxiolytic drugs (typically SSRIs) have low efficacy in PD patients and might worsen motor symptoms [85,116,117]. As a result, there is a growing demand for alternative pharmacologic therapies that can provide more consistent relief with fewer side effects. Consequently, CBD has lately been identified as a promising treatment option for anxiety disorders by researchers [7,127].
CBD has been shown to be a potent activator of the 5-HT1A and 5-HT2A receptors, both of which are targets of existing anxiolytic drugs such as buspirone for the treatment of generalized anxiety disorder [129]. Overall, CBD’s promise as an effective treatment for anxiety-related disorders is supported by some of the best data currently available on CBD-related research. However, as most studies have had small sample sizes and have largely investigated only acute treatment with CBD for anxiety, further research is needed to confirm this evidence and determine whether CBD is a viable option for chronic therapy in anxiety-related conditions [7].
According to our literature search, there was one study investigating the effect of CBD in PD patients with anxiety symptoms (Table 6). A randomized, double-blinded, placebo-controlled, crossover clinical trial with a total of 24 PD patients was conducted by de Faria in 2020. The findings revealed that a dose of 300 mg of pure CBD attenuated the anxiety experimentally induced by the Simulated Public Speaking Test (SPST) [130].
Table 6.
Studies with CBD addressing anxiety symptoms in PD
| Symptom | Design | Sample size | Duration | Primary outcome | Application form | Dosage | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Anxiety | Randomized, double-blind, placebo-controlled crossover | 24 | Two experimental sessions within a 15-day interval | 1. 2 SPSTa sessions | Orally | 1. CBD group: 300 mg of pure CBD dissolved in corn oil | CBD attenuated the anxiety experimentally induced by the SPSTa | De Faria et al. [85] |
| 2. UPDRSb | 2. Placebo group: corn oil capsules | |||||||
| 3. VAMSc | ||||||||
| 4.SSPSd | ||||||||
| 5. Blood pressure and heart rate |
aSimulated Public Speaking Test.
bUnified Parkinson Disease Rating Scale.
cVisual Analog Mood Scales.
dSelf- Statements during Public Speaking Scale.
6.7. CBD and quality of life in PD patients
The non-motor PD symptoms mentioned above (psychosis, anxiety, depression, sleep disturbance, and pain) are associated with PD patients’ quality of life (QOL). These symptoms are significant predictors of declining QOL [84,117]. However, few studies have examined the impact of pharmacological therapies on PD patients’ QOL, and the results are inconclusive.
The positive impact of CBD on the quality of life of PD patients may be due to its therapeutic effects on non-motor symptoms. CBD differs from traditional drugs, which usually target specific sites of action to treat specific conditions by acting on a broader range of conditions simultaneously and through different yet unidentified mechanisms of action [71,129]. The multi-target properties of CBD may make it more useful than other drugs for people with PD, as the pathophysiology of PD (and other disorders) is generally multifactorial.
According to our literature search, three studies investigated the effect of CBD on quality of life in PD patients (Table 7). The first study in 2004 with Cannador® (2.5 mg Δ9-THC + 1.25 mg CBD) revealed no positive effect on quality of life. However, in 2014, Chagas et al. conducted an explorative, randomized double-blind, placebo-controlled study for 6 weeks in 21 PD patients and concluded that CBD (300 mg) significantly improved QOL [84,89]. Conversely, a recent study concluded that even doses of 75 or 300 mg CBD had no significant effect on quality of life as a secondary outcome [86].
Table 7.
Studies with CBD addressing quality of life in PD
| Symptom | Design | Sample size | Duration | Primary outcome | Application form | Dosage | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| QOL | Double-blind, randomized, placebo-controlled crossover study | 17 | 2 × 4 weeks, 2 weeks wash-out phase | PDQ-39a | Orally | 2.5 mg THC + 1.25 mg CBD (Cannador) or placebo | No positive effect on quality of life | Carroll et al. [89] |
| Explorative, randomized, double-blind, placebo-controlled | 21 | 6 weeks | PDQ-39a | Orally | 75 or 300 mg CBD or placebo | Significant improvement of quality of life (300 mg CBD group) | Chagas et al. [84] | |
| Randomized double-blind, placebo-controlled clinical trial | 33 | 14 weeks | PDQ-39a | Orally | 75 or 300 mg CBD or placebo | No significant effect between groups | de Almeida et al. [86] |
aParkinson’s Disease Questionnaire-39.
7. Effects of CBD on LID
7.1. LID: a brief introduction
l-3,4-Dihydroxyphenylalanine (l-DOPA), the amino acid precursor of dopamine, is still the mainstay treatment for PD motor symptoms. However, the development of debilitating motor problems such as LIDs limits its long-term efficacy. The LID is a collection of debilitating involuntary movements that includes chorea, ballism, dystonia, and, to a lesser extent, myoclonus (a rapid and involuntary muscular contraction that occurs primarily in the extremities) [131].
Treatment with l-DOPA in the early stages of PD causes “positive” plasticity, which leads to long-term symptom relief [132,133]. However, as the disease progresses, l-DOPA-induced clinical relief disappears. l-DOPA impairs striatum-dependent learning capabilities [134,135] and has a deleterious impact on cortical plasticity [136]. LIDs are linked to corticostriatal overactivity as well as molecular alterations in the basal ganglia. Striatal neurons combine cortical and thalamic inputs to adjust the basal ganglia’s output, making them important in movement selection and adaptive motor control [137]. The pathogenesis of LID has also been linked to increased glutamatergic neurotransmission [138]. The currently available pharmacological treatment for LID is amantadine, a non-competitive antagonist of NMDA-type glutamate receptors [139].
Preclinical studies have demonstrated that drugs targeting various neurotransmitters, such as noradrenaline, acetylcholine, serotonin, adenosine, and nitric oxide, can reduce LID [140,141,142].
7.2. Modulation of the ECS to treat LID
There is a growing body of evidence indicating that ECS is involved in dyskinesia. Several studies have investigated whether ECS modulation could be used to help people with l-DOPA-induced aberrant involuntary movements (AIMs) [2]. Some dysregulated metabolites, including AEA and 2-AG, were found in the striatum of dyskinetic rats according to a recent study [143]. Also, in hemiparkinsonian rats, the intrastriatal injection of both endocannabinoids before l-DOPA treatment delayed the start of LID, according to the same study [143].
Modifications of the CB1 and CB2 receptors suggest a potential therapeutic target for the active period of LID [144]. In support of this theory, the CB1 agonist nabilone has been shown to reduce LID in MPTP-lesioned non-human primates treated with l-DOPA [145]. In hemiparkinsonian rats, small dosages of HU-210 (a synthetic CB1 agonist) significantly attenuated l-DOPA and apomorphine-induced contralateral rotations [146]. In neurotoxic 6-OHDA-lesioned rats that were repeatedly administered levodopa, the cannabinoid agonist WIN-55,212-2 provided antidyskinetic effects, and its activity was reversed by the CB1 antagonist rimonabant SR141716A [147]. In MPTP-lesioned marmosets, coadministration of l-DOPA (8 mg/kg) and the CB1 receptor antagonist rimonabant (1 and 3 mg/kg) reduced the severity of LID without reducing the antiparkinsonian effect of l-DOPA [67]. This treatment also reduced LID in rats, with partial preservation of dopaminergic cells [148]. In MPTP-treated rhesus monkeys, CB1 blockade increased the l-DOPA response to motor impairment [149]. When TRPV1 receptors were blocked, FAAH inhibitors were found to have anti-dyskinetic effects, suggesting that CB1 and TRPV1 receptors may play opposing roles in LID [48].
In LID therapy, CB1 receptor agonists as adjuvants are theoretically more favorable than DA substitutes. CB1 receptor stimulation reduces striatal glutamate release, inhibits D1-receptor-mediated effects, and increases GABA levels in the lateral or external segment of the globus pallidus, all of which help to reduce LID [150]. However, there are conflicting results regarding the effects of CB1 activation on LID pathogenesis. When hemiparkinsonian mice lacking CB1 receptors were given l-DOPA, they developed mild dyskinesia rather than severe dyskinesia, according to Pérez-Rial and colleagues [151].
In summary, LID alleviation following CB1 pharmacological manipulation has been described in the majority of publications [2]. Blocking CB1 receptors is presumably only beneficial in specific circumstances, such as when CB1 receptor antagonists are administered at low doses in moderate parkinsonism, when patients are intolerant to dopamine therapy, or when they are in the late stages of PD [152,153]. Although these results were obtained from treatments with varying specificities and different animal models, they demonstrated that CB1 antagonists have no conclusive effect on treating PD symptoms [154].
Many investigations have confirmed the importance of CB1 in LID using nonselective agonists of CB receptors as a pharmacological tool but have ignored the potential function of CB2 in the pathophysiology of this condition [2].
7.3. CBD on LID: preclinical studies
To the best of our knowledge, the effect of CBD on LID has been studied in two preclinical studies [75,155]. The first investigation showed that CBD (10, 30, and 60 mg/kg) had no anti-dyskinetic effect. However, both of the two studies have confirmed the anti-dyskinetic effect of CBD when combined with TRPV-1 antagonist CPZ in hemiparkinsonian mice chronically treated with l-DOPA.
When CB1 and TRPV-1 receptors are coexpressed in the same cell or in close neuron-neuron/neuron-glia contacts, mutual modulation is thought to occur (between CB1 and TRPV-1) [156,157]. Activating CB1 and TRPV-1 receptors have shown conflicting effects on excitatory and inhibitory neurotransmission in hippocampal neurons [158]. The simultaneous activation of CB1 and TRPV-1 has different effects on intracellular calcium concentrations [159]. According to one theory to explain these findings, TRPV-1 activation by either AEA (indirectly boosted by CBD) or CBD enhances LID or impairs the positive effects mediated by other pathways, such as the stimulation of CB1 and PPAR receptors. The favorable results observed with the administration of a powerful FAAH inhibitor and TRPV-1 antagonist (arachidonoyl serotonin) support this idea since the particular increase in AEA levels (in combination with TRPV-1 receptor antagonism) reduced LID manifestations [75]. AM251 reduced the antidyskinetic effects of CPZ + CBD on limb and orofacial LID, but GW9662, a PPAR antagonist, uniquely inhibited the antidyskinetic effect on axial AIMs, corroborating these results. As a result, direct (or indirect) activation of CB1 and PPAR (together with TRPV-1 inhibition) could be a potential method for alleviating LID [2].
7.4. Clinical studies on the effects of CBD on LID
To the best of our knowledge, no clinical investigation has been conducted with the explicit goal of evaluating CBD’s effects on LID reduction in PD patients.
Only three randomized controlled clinical trials investigated the efficacy of other cannabinoids rather than CBD (nabilone, THC/CBD, rimonabant) in alleviating LID symptoms. Two of them concluded that no changes or improvements have been noticed neither with rimonabant nor with THC/CBD mixture treatments [89,160]. However, a study with nabilone (smoked) showed a reduction in LID severity and its duration in seven PD patients [161]. In a survey of 339 PD patients in the Czech Republic, 25% reported taking cannabis with meals in an oral formulation of fresh or dried leaves, 46% reported mild or significant relief from PD symptoms in general, 31% reported improvement of rest tremor, 45% reported relief from bradykinesia, and 14% reported improvement of LID [162]. However, after being treated with oral doses of CBD (100–600 mg/day over 6 weeks) along with standard medication, a preliminary open pilot study showed an aggravation of parkinsonian symptoms in 2 PD patients with dystonic movement disorders [163].
These few studies that have examined the effects of cannabis/cannabinoids on motor dysfunctions associated with PD (such as LID) have produced contradictory results (for more details, see Appendix).
8. Conclusion
The above-cited preclinical studies collectively suggest that CBD is a promising candidate for the treatment of various health conditions which is due to the fact that it lacks psychoactive effects and is a potent anti-inflammatory and antioxidant compound. In the context of clinical trials, it is challenging to derive definitive conclusions about the efficacy of CBD as a treatment for PD, given that only seven studies have been conducted thus far. Nevertheless, the current body of research is constrained by several limitations. A notable limitation is the insufficient number of large-scale, high-quality randomized controlled trials examining the efficacy and safety of CBD in the treatment of PD. The majority of existing studies are relatively small and have various methodological shortcomings, with considerable heterogeneity in terms of study design, CBD dosages, routes of administration, and outcome measures. Furthermore, the majority of patients diagnosed with PD are of an advanced age. Consequently, age-related factors, including comorbidities, polypharmacy, and alterations in pharmacokinetics and pharmacodynamics, can potentially impact the efficacy of CBD treatment. Moreover, the impact of sex differences on the effectiveness and safety of CBD has not been sufficiently explored, and male and female patients may exhibit variability in their response to treatment. Additionally, many studies do not stratify results based on disease stage, which limits the ability to ascertain the efficacy of CBD at different stages of the disease.
To address the aforementioned limitations, it is proposed that several avenues for future investigation be explored. The necessity for large-scale, double-blind, placebo-controlled trials is paramount for the evaluation of the efficacy of CBD in treating both motor and non-motor symptoms of PD. Longitudinal studies are essential to investigate the long-term effects of CBD on human health, including an assessment of its safety, efficacy, and potential disease-modifying properties. The establishment of standardized protocols for CBD dosage, administration routes, and outcome measures will facilitate greater comparability between studies and help to reduce heterogeneity. To gain a deeper understanding of the mechanisms through which CBD interacts with the ECS and other neurotransmitter systems in the context of neurodegenerative disorders, further mechanistic studies are required. An investigation into the potential advantages of combining CBD with other pharmacological interventions, such as l-DOPA, may elucidate synergistic effects that could enhance therapeutic efficacy. Furthermore, future studies should concentrate on age-specific analyses to address age-related factors and guarantee applicability to the typical demographic affected by PD. It would be beneficial to include sex-based analyses to identify any differential effects of CBD between male and female patients. Ultimately, stratifying patients based on the stages of PD will facilitate the determination of the efficacy and safety of CBD at various stages of disease progression, thereby providing more precise guidance for its use in clinical practice.
Notably, due to CBD’s well-documented safety profile in patients, it may represent a promising add-on therapy with the potential to alleviate some symptoms. Accordingly, we believe that more extensive, well-designed preclinical and clinical research addressing motor and non-motor symptoms of this disease is required to establish the true effects of CBD.
Abbreviations
- ABC
- Activities of balance confidence
- ADL scale
- Activities of daily living
- BAI
- Beck Anxiety Inventory
- BDNF
- Brain-derived neurotrophic factor
- BPRS
- Brief Psychiatric Rating Scale
- CGI-I
- Clinical Global Impression Improvement
- ESS
- Epworth Sleepiness Scale
- FAB
- Frontal assessment battery
- FSS
- Fatigue Severity Scale
- GNDS
- Guy’s Neurological Status Scale
- IPAQ
- International Physical Activities Questionnaire
- H1-MRS
- Proton magnetic resonance scans
- H&Y scale
- Hoehn & Yahr
- LID
- (l-DOPA)-induced dyskinesias
- MMSE
- Mini-mental status examination
- NHP
- Nottingham Health Profile
- NR
- Nonreported
- NSAID
- Nonsteroidal anti-inflammatory drug
- PAS
- Parkinson Anxiety Scale
- PDQ-39
- Parkinson’s Disease Questionnaire-39
- PPQ
- Parkinson Psychosis Questionnaire
- PRI
- Pain Rating Index
- PSQI
- Pittsburgh Sleep Quality Index
- PSS
- PD Sleep Scale
- RBD
- Rapid eye movement sleep behavior disorder
- RBDSQ
- REM Sleep Behavior Disorder Screening Questionnaire
- RLS
- Restless leg syndrome
- SDS
- Zung Self-Rating Depression Scale
- SPST
- Simulated Public Speaking Test
- SSPS
- Self- Statements during Public Speaking Scale
- THC
- Tetrahydrocannabinol
- UPDRS
- Unified Parkinson Disease Rating Scale
- UKU
- Udvalg for kliniske undersøgelser
- VAS
- Visual Analog Scale
- VAMS
- Visual Analog Mood Scales
Appendix. Total number of conducted studies with cannabis/cannabinoids in PD, randomized controlled, and uncontrolled trials
Table A1.
Randomized controlled trials
| References | Study design | Sample size | Study duration | Type of the drug and route of administration | Measures | Main findings | Adverse events |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Disease duration | |||||||
| Sieradzan et al. [161] | Randomized, double-blind, placebo-controlled | N = 7 | Single exposure of nabilone or placebo; half of dose administered 12 h and 1 h, respectively, before acute levodopa challenge test; crossover after 2 weeks | 1. Group: smoke Nabilone (0.03 mg/kg) + l-DOPA (0.03 mg/kg) 2. placebo: l-DOPA (0.03 mg/kg) | 1. Rush Dyskinesia Disability Scale | Reduction in LID severity and duration | Mild sedation, “floating sensation,” dizziness, hyperacusis, partial disorientation, and formed visual hallucinations |
| Age: NR | 2. H&Y | No changes were observed in the severity of PD symptom | |||||
| Crossover study | Dd: NR | 3. Modified Webster Scale | |||||
| Carroll et al. [89] | Randomized, double-blind, placebo-controlled Crossover study | N= 17 Age: 67 years Dd: 14 years | 4 weeks | 1. Group: Cannador (1.25 mg CBD and 2.5 mg THC) 2. Placebo: synthetic oil capsules | 1.UPDRS | No improvement in LID severity duration, motor Symptoms, quality of life, pain or sleep | Mild physical and psychological symptoms |
| 2. Rush scale | |||||||
| 3. Bain scale | |||||||
| 4. Tablet arm drawing task | |||||||
| 5. ADL scale | |||||||
| 6. PDQ-39 | |||||||
| 7. On-off diaries | |||||||
| 8. Category rating scales | |||||||
| Mesnage et al. [160] | Randomized, double-blind, placebo-controlled study | N = 24Age: NRDd: NR | 16 days | 1. Rimonabant (20 mg/day, n = 4) for 16 d | 1. UPDRS | No change in motor impairment or LID in neither | None |
| 2. SR 48692, (180 mg/day, n = 4) for 9d | |||||||
| 3. SR 142801 (200 mg/day, n = 4) for 19 d | 2. Standardized videotape | On- nor Off-state | |||||
| 4. placebo (n = 12) | |||||||
| Chagas et al. [60] | Randomized, double-blind, placebo-controlled study | N= 21 | 6 weeks | 1. Group: CBD 75 mg/day | 1. UPDRS total | Improvement of PDQ-39 with 300 mg CBD; UPDRS unchanged | None |
| Age: 65.52 years | 2. Group: CBD 300 mg/day | 2. PDQ-39 | |||||
| 3. UKU | |||||||
| Dd: 8.28 years | 3. Group: placebo (corn oil) | 4. Plasma BDNF levels | |||||
| 5. H1-MRS | |||||||
| de Faria et al. [130] | Randomized, double-blind, placebo-controlled | N= 24 | Two experimental sessions within a 15-day interval | 1. CBD group: 300 mg of pure CBD dissolved in corn oil | 1.2 SPST sessions | Improvement of anxiety and tremor in CBD vs placebo group | NR |
| 2. UPDRS | |||||||
| Age: NR | 3. VAMS | ||||||
| Crossover study | 2. Placebo group: corn oil capsules | 4. SSPS | |||||
| Dd: NR | 5. Blood pressure and heart rate | ||||||
| 6. Accelerometer | |||||||
| de Almeida et al. [86] | Phase II/III, randomized double-blind, placebo-controlled clinical trial | N= 33 | 14 weeks | CBD 75 or 300 mg/day placebo (corn oil) CBD dissolved in corn oil | 1. CGI | CBD showed no difference to placebo for primary outcomes Significant improvement in average sleep satisfaction with 300 mg was noted | Epigastric pain |
| Age: 57 years Dd: 9 years | 2. PSQI 3. ESS 4. RBDSQ 5. PDSS 6. BAI 7. SDS 8. PAS 9. PDQ-39 10. UPDRS | Headache Drowsiness Sadness Dizziness | |||||
| de Almeida et al. [87] | Phase II/III, randomized double-blind, placebo-controlled clinical trial | N= 18 | 14 weeks | 75 or 300 mg/day CBD or placebo | Restless Legs Syndrome Rating Scale | No reduction in the severity of RLS in PD patients with RBD | NR |
Table A2.
Uncontrolled studies
| References | Study design | Sample size | Study duration | Type of the drug and route of administration | Measures | Main findings | Adverse events |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Disease duration | |||||||
| Frankel et al. [164] | Case series | N = 5 | NR | 1. Smoked cannabis (1 g with 2.9% THC) | Webster scale | No improvement of tremor | Drowsiness or mild euphoria |
| Age: NR | |||||||
| Dd: NR | |||||||
| 2. Diazepam 5 mg orally | Mild unsteadiness occurred after diazepam | ||||||
| 3. l-DOPA/Carbidopa 250 mg/25 mg orally (Sinemet 275) | |||||||
| 4. Apomorphine 1.5 mg subcutaneously | |||||||
| Venderová et al. [162] | Open-label pilot | N = 85 of 339 | — | 84 patients with ½ teaspoon cannabis orally; 1 patient by inhalation | Anonymous questionnaire sent by mail | 31% reported improvement of rest tremor, 45% of bradykinesia and 14% of LID | Unspecified |
| Age: 65.7 yearsDd: 8.5 yeasr | |||||||
| 52.9% daily | |||||||
| Zuardi et al. [165] | Open-label pilot | N= 6 | 4 weeks | 1st week:150 mg/day | 1.BPRS | Improvement of psychiatric plus and minus symptoms, mild improvement | None |
| 2nd week:250 mg/day | 2. PPQ | ||||||
| Age: 58.8 ± 14.9 years | 3rd week: 325 mg/day | 3. UPDRS total | |||||
| 4th week: 400 mg/day | 4. CGI-I | ||||||
| Dd: 10.6 ± 3.7 years | CBD dissolved in corn oil | 5. MMSE | |||||
| Placebo group: corn oil capsules | 6. FAB | ||||||
| Chagas et al. [60] | Case series | 4 patients with RBD | 6 weeks | 75 or 300 mg/day CBD | REM Sleep behavior disorder questionnaire | Significant improvements in sleep symptoms; Well tolerated | NR |
| Finseth et al. [166] | Patient survey | N = 9 | — | Past or current use of Cannabis | Self-administered questionnaire via e-mail | Benefits in mood (56%), sleep (56%), motor symptoms (22%), and quality of life (22%) | None |
| Age: 49–75 years | |||||||
| Dd: 2–11 years | |||||||
| Lotan et al. [167] | Case series | N = 22 | Smoked cannabis for at least 2 months | Smoked cannabis, 0.5 g (with 2.9% THC) |
|
Improvement of tremor and bradykinesia and some non-motor symptoms | Short-term: hypoglycemia, dizzinessLong-term: somnolence, drowsiness, palpitations, bad taste |
| Age: 65 ± 10.2 yearsDd: 7.3 ± 4.8 years | |||||||
| Shohet et al. [168] | Open-label, observational | N = 20 | Cannabis use for at least 10 weeks (median 14 weeks) | Vaporizer or smoking Cannabis (1 g), anticholinergic agents, dopamine agonists, amantadine, and rasagiline | 1. UPDRS | Improvement in mean motor and pain scores in 18PD patients | NR |
| 2. PRI | |||||||
| 3. Short-form McGill Pain Questionnaire 4.VAS | |||||||
| Age: 62.4 ± 9 years | |||||||
| Dd: 6.8 ± 3.5 years | |||||||
| Control: 12 Age: 70 ± 7.2 years | |||||||
| Dd: 6 ± 2.6 years | |||||||
| Yust-Katz et al. [169] | Open-label | N = 114 | — | Medical cannabis (MC), anti- Parkinson medication, pain killers (paracetamol, NSAID), opiates (Tramadol, Oxycodone) | 1. UPDRS | MC significantly alleviated pain | NR |
| Age: 68 ± 9.9 years | 2. McGill test | No significant difference in different pain medications (pain killers vs opiates vs MC) | |||||
| Dd: 6 ± 6 years | 3. Structured questionnaire for pain typ | ||||||
| Balash et al. [170] | Retrospective observational telephone survey | N = 47 | Patients treated for at least 3 months with MC | Most means of cannabis administration was Smoking flowers and leaves or oil ingestion daily dose 0.9 ± 0.5 g | Structured questionnaire | Improvement in pain, sleep, mood, and significant reduction of falls were reported by a significant percentage of patients. | Cough (34.9%), anxiety, confusion, and hallucinations |
| Age: 64.2 ± 10.8 years | |||||||
| Dd: 10.8 ± 8.3 years | |||||||
| Kindred et al. [171] | Open anonymous web-based survey | N = 453 | — | Most reported routes of administration: smoking cannabis edible | Self-reported scales: GNDS, NHP, ABC, FSS, IPAQ | Improvement on mood, memory, fatigue, and obesity status, and reduction of prescribed medication | NR |
| Age: 61.1 ± 9.5 years | |||||||
| Smoked + edibles |
Rows in bold emphasis indicate studies using CBD.
Footnotes
Funding information: The authors state no funding involved.
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its final version. EH: idea of the article, literature search, data analysis, writing – original draft. SR: review and editing. GH: review and editing. KN: review and editing.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- [1].Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, Adsuar JC, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17:939–53. 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed]
- [2].Junior NCF, dos-Santos-Pereira M, Guimarães FS, Del Bel E. Cannabidiol and cannabinoid compounds as potential strategies for treating parkinson’s disease and l-DOPA-induced dyskinesia. Neurotox Res. 2020;37:12–29. 10.1007/s12640-019-00109-8. [DOI] [PubMed]
- [3].Parkinson J. An essay on the shaking palsy. J Neuropsychiatry Clin Neurosci. 2002;14:223–36. [DOI] [PubMed]
- [4].Elbaz A, Tranchant C. Epidemiologic studies of environmental exposures in Parkinson’s disease. J Neurol Sci. 2007;262:37–44. 10.1016/j.jns.2007.06.024. [DOI] [PubMed]
- [5].Reglodi D, Renaud J, Tamas A, Tizabi Y, Socías SB, Del-Bel E, et al. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol. 2017;155:120–48. 10.1016/j.pneurobio.2015.10.004. [DOI] [PubMed]
- [6].Michel PP, Hirsch EC, Hunot S. Understanding dopaminergic cell death pathways in parkinson disease. Neuron. 2016;90:675–91. 10.1016/j.neuron.2016.03.038. [DOI] [PubMed]
- [7].Fiani B, Sarhadi KJ, Soula M, Zafar A, Quadri SA. Current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurol Sci. 2020;41:3085–98. 10.1007/s10072-020-04514-2. [DOI] [PubMed]
- [8].Gowers WR. A manual of diseases of the nervous system. London: J. A. Churchill; 1886.
- [9].Giuffrida R, Vingerhoets FJG, Bogousslavsky J, Ghika J. Pain in Parkinson’s disease. Rev Neurol (Paris). 2005;161:407–18. 10.1016/s0035-3787(05)85070-2. [DOI] [PubMed]
- [10].Buhmann C, Mainka T, Ebersbach G, Gandor F. Evidence for the use of cannabinoids in Parkinson’s disease. J Neural Transm. 2019;126:913–24. 10.1007/s00702-019-02018-8. [DOI] [PubMed]
- [11].García MC, Cinquina V, Palomo-Garo C, Rábano A, Fernández-Ruiz J. Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson’s disease. Neurosci Lett. 2015;587:1–4. 10.1016/j.neulet.2014.12.003. [DOI] [PubMed]
- [12].Bougea A, Koros C, Simitsi A-M, Chrysovitsanou C, Leonardos A, Stefanis L. Medical cannabis as an alternative therapeutics for Parkinsons’ disease: Systematic review. Complement Ther Clin Pract. 2020;39:101154. 10.1016/j.ctcp.2020.101154. [DOI] [PubMed]
- [13].Iuvone T, Esposito G, De Filippis D, Scuderi C, Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther. 2009;15:65–75. 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed]
- [14].Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J Nat Prod. 2016;79:324–31. 10.1021/acs.jnatprod.5b00949. [DOI] [PubMed]
- [15].Adams R, Hunt M, Clark JH. Structure of cannabidiol, a product isolated from the marihuana extract of minnesota Wild Hemp. I. J Am Chem Soc. 1940;62:196–200. 10.1021/ja01858a058. [DOI]
- [16].Mechoulam R, Shvo Y, Hashish. I. The structure of cannabidiol. Tetrahedron. 1963;19:2073–8. 10.1016/0040-4020(63)85022-x. [DOI] [PubMed]
- [17].Fischedick JT, Hazekamp A, Erkelens T, Choi YH, Verpoorte R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 2010;71:2058–73. 10.1016/j.phytochem.2010.10.001. [DOI] [PubMed]
- [18].Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332:569–77. 10.1124/jpet.109.159145. [DOI] [PMC free article] [PubMed]
- [19].McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–53. 10.1111/bph.12944. [DOI] [PMC free article] [PubMed]
- [20].Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–23. 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed]
- [21].Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–52. 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed]
- [22].Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed]
- [23].Pertwee RG. Endocannabinoids and their pharmacological actions. Handb Exp Pharmacol. 2015;231:1–37. 10.1007/978-3-319-20825-1_1. [DOI] [PubMed]
- [24].Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–84. 10.1038/nrn1247. [DOI] [PubMed]
- [25].Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805. 10.1111/bph.13250. [DOI] [PMC free article] [PubMed]
- [26].De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–94. 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed]
- [27].Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–8. 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed]
- [28].Pandolfo P, Silveirinha V, dos Santos-Rodrigues A, Venance L, Ledent C, Takahashi RN, et al. Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur J Pharmacol. 2011;655:38–45. 10.1016/j.ejphar.2011.01.013. [DOI] [PubMed]
- [29].Murillo-Rodríguez E, Palomero-Rivero M, Millán-Aldaco D, Mechoulam R, Drucker-Colín R. Effects on sleep and dopamine levels of microdialysis perfusion of cannabidiol into the lateral hypothalamus of rats. Life Sci. 2011;88:504–11. 10.1016/j.lfs.2011.01.013. [DOI] [PubMed]
- [30].Bloom AS, Hillard CJ. Cannabinoids, neurotransmitter receptors and brain membranes. Marihuana ’84: Proceedings of the Oxford Symposium on Cannabis: 9th International Congress of Pharmacology, 3rd Satellite Symposium on Cannabis/Edited by DJ Harvey; Assistant Editors Sir William Paton, GG Nahas; 1985.
- [31].Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, et al. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:294–9. 10.1007/s00210-004-0871-3. [DOI] [PubMed]
- [32].Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci Lett. 2006;399:91–5. 10.1016/j.neulet.2006.01.047. [DOI] [PubMed]
- [33].Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–73. 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed]
- [34].Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol Dis. 2005;19:96–107. 10.1016/j.nbd.2004.11.009. [DOI] [PubMed]
- [35].Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z. Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem. 2010;285:1616–26. 10.1074/jbc.M109.069294. [DOI] [PMC free article] [PubMed]
- [36].Li K, Feng J, Li Y, Yuece B, Lin X, Yu L, et al. Anti-inflammatory role of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas. 2013;42:123–9. 10.1097/MPA.0b013e318259f6f0. [DOI] [PubMed]
- [37].Watzl B, Scuderi P, Watson RR. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int J Immunopharmacol. 1991;13:1091–7. 10.1016/0192-0561(91)90160-9. [DOI] [PubMed]
- [38].Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol. 2019;17:974–89. 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed]
- [39].Chesney E, Oliver D, Green A, Sovi S, Wilson J, Englund A, et al. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45:1799–806. 10.1038/s41386-020-0667-2. [DOI] [PMC free article] [PubMed]
- [40].Leehey MA, Liu Y, Hart F, Epstein C, Cook M, Sillau S, et al. Safety and tolerability of cannabidiol in Parkinson disease: An open label, dose-escalation study. Cannabis Cannabinoid Res. 2020;5:326–36. 10.1089/can.2019.0068. [DOI] [PMC free article] [PubMed]
- [41].Brown JD, Winterstein AG. Potential adverse drug events and drug-drug interactions with medical and consumer cannabidiol (CBD) use. J Clin Med. 2019;8:E989. 10.3390/jcm8070989. [DOI] [PMC free article] [PubMed]
- [42].Velayudhan L, McGoohan K, Bhattacharyya S. Safety and tolerability of natural and synthetic cannabinoids in adults aged over 50 years: A systematic review and meta-analysis. PLOS Med. 2021;18:e1003524. 10.1371/journal.pmed.1003524. [DOI] [PMC free article] [PubMed]
- [43].Reddy V, Grogan D, Ahluwalia M, Salles ÉL, Ahluwalia P, Khodadadi H, et al. Targeting the endocannabinoid system: a predictive, preventive, and personalized medicine-directed approach to the management of brain pathologies. EPMA J. 2020;11:217–50. 10.1007/s13167-020-00203-4. [DOI] [PMC free article] [PubMed]
- [44].Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000;14:1432–8. 10.1096/fj.14.10.1432. [DOI] [PubMed]
- [45].Giacoppo S, Mandolino G, Galuppo M, Bramanti P, Mazzon E. Cannabinoids: New promising agents in the treatment of neurological diseases. Molecules. 2014;19:18781–816. 10.3390/molecules191118781. [DOI] [PMC free article] [PubMed]
- [46].Stampanoni Bassi M, Sancesario A, Morace R, Centonze D, Iezzi E. Cannabinoids in Parkinson’s disease. Cannabis Cannabinoid Res. 2017;2:21–9. 10.1089/can.2017.0002. [DOI] [PMC free article] [PubMed]
- [47].Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol. 2009;156:1029–40. 10.1111/j.1476-5381.2008.00088.x. [DOI] [PMC free article] [PubMed]
- [48].Morgese MG, Cassano T, Cuomo V, Giuffrida A. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson’s disease: role of CB1 and TRPV1 receptors. Exp Neurol. 2007;208:110–9. 10.1016/j.expneurol.2007.07.021. [DOI] [PMC free article] [PubMed]
- [49].Carroll CB, Zeissler M-L, Hanemann CO, Zajicek JP. Δ9-tetrahydrocannabinol (Δ9-THC) exerts a direct neuroprotective effect in a human cell culture model of Parkinson’s disease. Neuropathol Appl Neurobiol. 2012;38:535–47. 10.1111/j.1365-2990.2011.01248.x. [DOI] [PubMed]
- [50].Garriott JC, King LJ, Forney RB, Hughes FW. Effects of some tetrahydrocannabinols on hexobarbital sleeping time and amphetamine induced hyperactivity in mice. Life Sci. 1967;6:2119–28. 10.1016/0024-3205(67)90232-9. [DOI] [PubMed]
- [51].Howes J, Osgood P. The effect of delta9-tetrahydrocannabinol on the uptake and release of 14C-dopamine from crude striatal synaptosoma; preparations. Neuropharmacology. 1974;13:1109–14. 10.1016/0028-3908(74)90060-4. [DOI] [PubMed]
- [52].Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–21. 10.1054/plef.2001.0341. [DOI] [PubMed]
- [53].Covey DP, Mateo Y, Sulzer D, Cheer JF, Lovinger DM. Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology. 2017;124:52–61. 10.1016/j.neuropharm.2017.04.033. [DOI] [PMC free article] [PubMed]
- [54].Sierra S, Luquin N, Rico AJ, Gomez-Bautista V, Roda E, Dopeso-Reyes IG, et al. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct. 2015;220(5):2721–38. 10.1007/s00429-014-0823-8. [DOI] [PMC free article] [PubMed]
- [55].Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, et al. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–18. 10.1016/S0306-4522(03)00070-8. [DOI] [PubMed]
- [56].Fernández-Ruiz J, Hernández M, Ramos JA. Cannabinoid–dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e72–91. 10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed]
- [57].van der Stelt M, Di Marzo V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol. 2003;480:133–50. 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed]
- [58].Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97:3655–60. 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed]
- [59].Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114:13–33. 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed]
- [60].Chagas MHN, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther. 2014;39:564–6. 10.1111/jcpt.12179. [DOI] [PubMed]
- [61].Espay AJ, Morgante F, Merola A, Fasano A, Marsili L, Fox SH, et al. Levodopa-induced dyskinesia in Parkinson disease: Current and evolving concepts. Ann Neurol. 2018;84:797–811. 10.1002/ana.25364. [DOI] [PubMed]
- [62].Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed]
- [63].Fernández-Ruiz J, Moreno-Martet M, Rodríguez-Cueto C, Palomo-Garo C, Gómez-Cañas M, Valdeolivas S, et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br J Pharmacol. 2011;163:1365–78. 10.1111/j.1476-5381.2011.01365.x. [DOI] [PMC free article] [PubMed]
- [64].Gómez-Gálvez Y, Palomo-Garo C, Fernández-Ruiz J, García C. Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:200–8. 10.1016/j.pnpbp.2015.03.017. [DOI] [PubMed]
- [65].Lastres-Becker I, Cebeira M, de Ceballos ML, Zeng BY, Jenner P, Ramos JA, et al. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur J Neurosci. 2001;14:1827–32. 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed]
- [66].Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000;14:1432–8. 10.1096/fj.14.10.1432. [DOI] [PubMed]
- [67].van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, et al. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005;19:1140–2. 10.1096/fj.04-3010fje. [DOI] [PubMed]
- [68].Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, et al. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–7. 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed]
- [69].Maccarrone M, Gubellini P, Bari M, Picconi B, Battista N, Centonze D, et al. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem. 2003;85:1018–25. 10.1046/j.1471-4159.2003.01759.x. [DOI] [PubMed]
- [70].Aymerich MS, Aso E, Abellanas MA, Tolon RM, Ramos JA, Ferrer I, et al. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem Pharmacol. 2018;157:67–84. 10.1016/j.bcp.2018.08.016. [DOI] [PubMed]
- [71].Fernández-Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid. Br J Clin Pharmacol. 2013;75:323–33. 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed]
- [72].Castillo A, Tolón MR, Fernández-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis. 2010;37:434–40. 10.1016/j.nbd.2009.10.023. [DOI] [PubMed]
- [73].García-Arencibia M, González S, de Lago E, Ramos JA, Mechoulam R, Fernández-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134:162–70. 10.1016/j.brainres.2006.11.063. [DOI] [PubMed]
- [74].Celorrio M, Rojo-Bustamante E, Fernández-Suárez D, Sáez E, Estella-Hermoso de Mendoza A, Müller CE, et al. GPR55: A therapeutic target for Parkinson’s disease. Neuropharmacology. 2017;125:319–32. 10.1016/j.neuropharm.2017.08.017. [DOI] [PubMed]
- [75].Dos-Santos-Pereira M, da-Silva CA, Guimarães FS, Del-Bel E. Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: Possible mechanism of action. Neurobiol Dis. 2016;94:179–95. 10.1016/j.nbd.2016.06.013. [DOI] [PubMed]
- [76].Price DA, Martinez AA, Seillier A, Koek W, Acosta Y, Fernandez E, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29:2177–86. 10.1111/j.1460-9568.2009.06764.x. [DOI] [PMC free article] [PubMed]
- [77].Wang L, Wu X, Yang G, Hu N, Zhao Z, Zhao L, et al. Cannabidiol alleviates the damage to dopaminergic neurons in 1-Methyl-4-Phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease mice via regulating neuronal apoptosis and neuroinflammation. Neuroscience. 2022;498:64–72. 10.1016/j.neuroscience.2022.06.036. [DOI] [PubMed]
- [78].Gomes FV, Del Bel EA, Guimarães FS. Cannabidiol attenuates catalepsy induced by distinct pharmacological mechanisms via 5-HT1A receptor activation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:43–7. 10.1016/j.pnpbp.2013.06.005. [DOI] [PubMed]
- [79].Peres FF, Levin R, Suiama MA, Diana MC, Gouvêa DA, Almeida V, et al. Cannabidiol prevents motor and cognitive impairments induced by reserpine in rats. Front Pharmacol. 2016;7:343. 10.3389/fphar.2016.00343. [DOI] [PMC free article] [PubMed]
- [80].Santos NAG, Martins NM, Sisti FM, Fernandes LS, Ferreira RS, Queiroz RHC, et al. The neuroprotection of cannabidiol against MPP+ -induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson’s disease. Toxicol Vitro. 2015;30:231–40. 10.1016/j.tiv.2015.11.004. [DOI] [PubMed]
- [81].Janefjord E, Mååg JLV, Harvey BS, Smid SD. Cannabinoid effects on β amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell Mol Neurobiol. 2014;34:31–42. 10.1007/s10571-013-9984-x. [DOI] [PMC free article] [PubMed]
- [82].Martín-Moreno AM, Reigada D, Ramírez BG, Mechoulam R, Innamorato N, Cuadrado A, et al. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol. 2011;79:964–73. 10.1124/mol.111.071290. [DOI] [PMC free article] [PubMed]
- [83].Herrero M-T, Estrada C, Maatouk L, Vyas S. Inflammation in Parkinson’s disease: role of glucocorticoids. Front Neuroanat. 2015;9:32. 10.3389/fnana.2015.00032. [DOI] [PMC free article] [PubMed]
- [84].Chagas MHN, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J Psychopharmacol. 2014;28:1088–98. 10.1177/0269881114550355. [DOI] [PubMed]
- [85].de Faria SM, de Morais Fabrício D, Tumas V, Castro PC, Ponti MA, Hallak JE, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a simulated public speaking test in patients with Parkinson’s disease. J Psychopharmacol. 2020;34:189–96. 10.1177/0269881119895536. [DOI] [PubMed]
- [86].de Almeida CMO, Brito MMC, Bosaipo NB, Pimentel AV, Tumas V, Zuardi AW, et al. Cannabidiol for rapid eye movement sleep behavior disorder. Mov Disord. 2021;36:1711–5. 10.1002/mds.28577. [DOI] [PubMed]
- [87].de Almeida CMO, Brito MMC, Bosaipo NB, Pimentel AV, Sobreira-Neto MA, Tumas V, et al. The effect of cannabidiol for restless legs syndrome/Willis-Ekbom disease in Parkinson’s disease patients with REM sleep behavior disorder: A post hoc exploratory analysis of phase 2/3 clinical trial. Cannabis Cannabinoid Res. 2023;8:374–8. 10.1089/can.2021.0158. [DOI] [PubMed]
- [88].Zuardi A, Crippa J, Hallak J, Pinto J, Chagas M, Rodrigues G, et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol. 2009;23:979–83. 10.1177/0269881108096519. [DOI] [PubMed]
- [89].Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, et al. Cannabis for dyskinesia in Parkinson disease: A randomized double-blind crossover study. Neurology. 2004;63:1245–50. 10.1212/01.WNL.0000140288.48796.8E. [DOI] [PubMed]
- [90].Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed]
- [91].Beiske AG, Loge JH, Rønningen A, Svensson E. Pain in Parkinson’s disease: Prevalence and characteristics. Pain. 2009;141:173–7. 10.1016/j.pain.2008.12.004. [DOI] [PubMed]
- [92].Nègre-Pagès L, Regragui W, Bouhassira D, Grandjean H, Rascol O, DoPaMiP Study Group. Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord. 2008;23:1361–9. 10.1002/mds.22142. [DOI] [PubMed]
- [93].Zuardi AW. History of cannabis as a medicine: a review. Rev Bras Psiquiatr. 2006;28:153–7. 10.1590/S1516-44462006000200015. [DOI] [PubMed]
- [94].Alles SRA, Smith PA. Etiology and pharmacology of neuropathic pain. Pharmacol Rev. 2018;70:315–47. 10.1124/pr.117.014399. [DOI] [PubMed]
- [95].Koppel BS, Brust JCM, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556–63. 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed]
- [96].Maione S, Piscitelli F, Gatta L, Vita D, De Petrocellis L, Palazzo E, et al. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol. 2011;162:584–96. 10.1111/j.1476-5381.2010.01063.x. [DOI] [PMC free article] [PubMed]
- [97].Toth CC, Jedrzejewski NM, Ellis CL, Frey WH. Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain. 2010;6:16. 10.1186/1744-8069-6-16. [DOI] [PMC free article] [PubMed]
- [98].De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160:136–50. 10.1097/j.pain.0000000000001386. [DOI] [PMC free article] [PubMed]
- [99].Zubcevic K, Petersen M, Bach FW, Heinesen A, Enggaard TP, Almdal TP, et al. Oral capsules of tetra-hydro-cannabinol (THC), cannabidiol (CBD) and their combination in peripheral neuropathic pain treatment. Eur J Pain. 2023;27:492–506. 10.1002/ejp.2072. [DOI] [PubMed]
- [100].Alaia MJ, Hurley ET, Vasavada K, Markus DH, Britton B, Gonzalez-Lomas G, et al. Buccally absorbed cannabidiol shows significantly superior pain control and improved satisfaction immediately after arthroscopic rotator cuff repair: A placebo-controlled, double-blinded, randomized trial. Am J Sports Med. 2022;50:3056–63. 10.1177/03635465221109573. [DOI] [PubMed]
- [101].Chrepa V, Villasenor S, Mauney A, Kotsakis G, Macpherson L. Cannabidiol as an alternative analgesic for acute dental pain. J Dent Res. 2024;103:235–42. 10.1177/00220345231200814. [DOI] [PMC free article] [PubMed]
- [102].Cuñetti L, Manzo L, Peyraube R, Arnaiz J, Curi L, Orihuela S. Chronic pain treatment with cannabidiol in kidney transplant patients in Uruguay. Transplant Proc. 2018;50:461–4. 10.1016/j.transproceed.2017.12.042. [DOI] [PubMed]
- [103].Reijnders JSAM, Ehrt U, Weber WEJ, Aarsland D, Leentjens AFG. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord. 2008;23:183–9. quiz 313. 10.1002/mds.21803. [DOI] [PubMed]
- [104].Ishihara L, Brayne C. A systematic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol Scand. 2006;113:211–20. 10.1111/j.1600-0404.2006.00579.x. [DOI] [PubMed]
- [105].Marsh L. Depression and Parkinson’s disease: Current knowledge. Curr Neurol Neurosci Rep. 2013;13:409. 10.1007/s11910-013-0409-5. [DOI] [PMC free article] [PubMed]
- [106].Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–43. 10.1007/s11064-005-6978-1. [DOI] [PubMed]
- [107].Hegazy O, Platnick H. Cannabidiol (CBD) for treatment of neurofibromatosis-related pain and concomitant mood disorder: a case report. Cureus. 2019;11:e6312. 10.7759/cureus.6312. [DOI] [PMC free article] [PubMed]
- [108].Xu C, Chang T, Du Y, Yu C, Tan X, Li X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ Toxicol Pharmacol. 2019;70:103202. 10.1016/j.etap.2019.103202. [DOI] [PubMed]
- [109].Pinto JV, Crippa JAS, Ceresér KM, Vianna-Sulzbach MF, Silveira Júnior ÉdeM, Santana da Rosa G, et al. Cannabidiol as an adjunctive treatment for acute bipolar depression: A pilot study: Le cannabidiol comme traitement d’appoint de la dépression bipolaire aiguë: une étude pilote. Can J Psychiatry. 2024;69:242–51. 10.1177/07067437231209650. [DOI] [PMC free article] [PubMed]
- [110].Oberbarnscheidt T, Miller NS. The impact of cannabidiol on psychiatric and medical conditions. J Clin Med Res. 2020;12:393–403. 10.14740/jocmr4159. [DOI] [PMC free article] [PubMed]
- [111].Abrams DI. The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med. 2018;49:7–11. 10.1016/j.ejim.2018.01.003. [DOI] [PubMed]
- [112].Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–8. 10.1016/j.psyneuen.2014.11.002. [DOI] [PubMed]
- [113].Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA. 2015;313:2456–73. 10.1001/jama.2015.6358. [DOI] [PubMed]
- [114].Kaul M, Zee PC, Sahni AS. Effects of cannabinoids on sleep and their therapeutic potential for sleep disorders. Neurotherapeutics. 2021;18:217–27. 10.1007/s13311-021-01013-w. [DOI] [PMC free article] [PubMed]
- [115].Linares IMP, Guimaraes FS, Eckeli A, Crippa ACS, Zuardi AW, Souza JDS, et al. No acute effects of cannabidiol on the sleep-wake cycle of healthy subjects: A randomized, double-blind, placebo-controlled, crossover study. Front Pharmacol. 2018;9:315. 10.3389/fphar.2018.00315. [DOI] [PMC free article] [PubMed]
- [116].WHO. Neurological disorders: Public health challenges. 2006. https://www.who.int/publications-detail-redirect/9789241563369 (accessed March 24, 2022).
- [117].Crippa JAS, Hallak JEC, Zuardi AW, Guimarães FS, Tumas V, dos Santos RG. Is cannabidiol the ideal drug to treat non-motor Parkinson’s disease symptoms? Eur Arch Psychiatry Clin Neurosci. 2019;269:121–33. 10.1007/s00406-019-00982-6. [DOI] [PubMed]
- [118].Chagas MHN, Crippa JAS, Zuardi AW, Hallak JEC, Machado-de-Sousa JP, Hirotsu C, et al. Effects of acute systemic administration of cannabidiol on sleep-wake cycle in rats. J Psychopharmacol. 2013;27:312–6. 10.1177/0269881112474524. [DOI] [PubMed]
- [119].Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of Delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol. 2004;24:305–13. 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed]
- [120].Fakhoury M. Could cannabidiol be used as an alternative to antipsychotics. J Psychiatr Res. 2016;80:14–21. 10.1016/j.jpsychires.2016.05.013. [DOI] [PubMed]
- [121].Thanvi BR, Lo TCN, Harsh DP. Psychosis in Parkinson’s disease. Postgrad Med J. 2005;81:644–6. 10.1136/pgmj.2004.032029. [DOI] [PMC free article] [PubMed]
- [122].Zuardi AW, Rodrigues JA, Cunha JM. Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology (Berl). 1991;104:260–4. 10.1007/BF02244189. [DOI] [PubMed]
- [123].Zuardi AW, Crippa JAS, Hallak JEC, Bhattacharyya S, Atakan Z, Martin-Santos R, et al. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des. 2012;18:5131–40. 10.2174/138161212802884681. [DOI] [PubMed]
- [124].Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015;162:153–61. 10.1016/j.schres.2015.01.033. [DOI] [PubMed]
- [125].Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Braz J Psychiatry. 2008;30:271–80. 10.1590/s1516-44462008000300015. [DOI] [PubMed]
- [126].Guimarães VMC, Zuardi AW, Del Bel EA, Guimarães FS. Cannabidiol increases Fos expression in the nucleus accumbens but not in the dorsal striatum. Life Sci. 2004;75:633–8. 10.1016/j.lfs.2004.01.015. [DOI] [PubMed]
- [127].Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12:825–36. 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed]
- [128].Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317–25. 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed]
- [129].Ibeas Bih C, Chen T, Nunn AVW, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730. 10.1007/s13311-015-0377-3. [DOI] [PMC free article] [PubMed]
- [130].de Faria SM, de Morais Fabrício D, Tumas V, Castro PC, Ponti MA, Hallak JE, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. J Psychopharmacol. 2020;34:189–96. 10.1177/0269881119895536. [DOI] [PubMed]
- [131].Nutt JG. Levodopa-induced dyskinesia: review, observations, and speculations. Neurology. 1990;40:340–5. 10.1212/wnl.40.2.340. [DOI] [PubMed]
- [132].Cenci MA, Ohlin KE, Rylander D. Plastic effects of L-DOPA treatment in the basal ganglia and their relevance to the development of dyskinesia. Parkinsonism Relat Disord. 2009;15(Suppl 3):S59–63. 10.1016/S1353-8020(09)70782-5. [DOI] [PubMed]
- [133].Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain. 2009;132:309–18. 10.1093/brain/awn322. [DOI] [PubMed]
- [134].Cools R, Lewis SJG, Clark L, Barker RA, Robbins TW. L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson’s disease. Neuropsychopharmacology. 2007;32:180–9. 10.1038/sj.npp.1301153. [DOI] [PubMed]
- [135].Feigin A, Ghilardi MF, Carbon M, Edwards C, Fukuda M, Dhawan V, et al. Effects of levodopa on motor sequence learning in Parkinson’s disease. Neurology. 2003;60:1744–9. 10.1212/01.wnl.0000072263.03608.42. [DOI] [PubMed]
- [136].Cenci MA, Konradi C. Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res. 2010;183:209–33. 10.1016/S0079-6123(10)83011-0. [DOI] [PMC free article] [PubMed]
- [137].Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson’s disease. Prog Brain Res. 2010;183:275–97. 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed]
- [138].Ahmed I, Bose SK, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G, et al. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain. 2011;134:979–86. 10.1093/brain/awr028. [DOI] [PubMed]
- [139].Ossola B, Schendzielorz N, Chen S-H, Bird GS, Tuominen RK, Männistö PT, et al. Amantadine protects dopamine neurons by a dual action: reducing activation of microglia and inducing expression of GDNF in astroglia [corrected]. Neuropharmacology. 2011;61:574–82. 10.1016/j.neuropharm.2011.04.030. [DOI] [PMC free article] [PubMed]
- [140].Blandini F. Adenosine receptors and L-DOPA-induced dyskinesia in Parkinson’s disease: potential targets for a new therapeutic approach. Exp Neurol. 2003;184:556–60. 10.1016/S0014-4886(03)00402-3. [DOI] [PubMed]
- [141].Del-Bel E, Padovan-Neto FE, Bortolanza M, Tumas V, Aguiar AS, Raisman-Vozari R, et al. Nitric oxide, a new player in L-DOPA-induced dyskinesia? Front Biosci (Elite Ed). 2015;7:168–92. 10.2741/E726. [DOI] [PubMed]
- [142].Perez XA, Bordia T, Quik M. The striatal cholinergic system in L-dopa-induced dyskinesias. J Neural Transm (Vienna). 2018;125:1251–62. 10.1007/s00702-018-1845-9. [DOI] [PMC free article] [PubMed]
- [143].Wang Y, Zhang G-J, Sun Y-N, Yao L, Wang H-S, Du C-X, et al. Identification of metabolite biomarkers for L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease by metabolomic technology. Behav Brain Res. 2018;347:175–83. 10.1016/j.bbr.2018.03.020. [DOI] [PubMed]
- [144].Rojo-Bustamante E, Abellanas MA, Clavero P, Thiolat M-L, Li Q, Luquin MR, et al. The expression of cannabinoid type 1 receptor and 2-arachidonoyl glycerol synthesizing/degrading enzymes is altered in basal ganglia during the active phase of levodopa-induced dyskinesia. Neurobiol Dis. 2018;118:64–75. 10.1016/j.nbd.2018.06.019. [DOI] [PubMed]
- [145].Fox SH, Henry B, Hill M, Crossman A, Brotchie J. Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord. 2002;17:1180–7. 10.1002/mds.10289. [DOI] [PubMed]
- [146].Gilgun-Sherki Y, Melamed E, Mechoulam R, Offen D. The CB1 cannabinoid receptor agonist, HU-210, reduces levodopa-induced rotations in 6-hydroxydopamine-lesioned rats. Pharmacol Toxicol. 2003;93:66–70. 10.1034/j.1600-0773.2003.930202.x. [DOI] [PubMed]
- [147].Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18:1607–14. 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed]
- [148].Gutiérrez-Valdez AL, García-Ruiz R, Anaya-Martínez V, Torres-Esquivel C, Espinosa-Villanueva J, Reynoso-Erazo L, et al. The combination of oral L-DOPA/rimonabant for effective dyskinesia treatment and cytological preservation in a rat model of Parkinson’s disease and L-DOPA-induced dyskinesia. Behav Pharmacol. 2013;24:640–52. 10.1097/FBP.0000000000000004. [DOI] [PubMed]
- [149].Cao X, Liang L, Hadcock JR, Iredale PA, Griffith DA, Menniti FS, et al. Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther. 2007;323:318–26. 10.1124/jpet.107.125666. [DOI] [PubMed]
- [150].Brotchie JM. CB1 cannabinoid receptor signalling in Parkinson’s disease. Curr Opin Pharmacol. 2003;3:54–61. 10.1016/s1471-4892(02)00011-5. [DOI] [PubMed]
- [151].Pérez-Rial S, García-Gutiérrez MS, Molina JA, Pérez-Nievas BG, Ledent C, Leiva C, et al. Increased vulnerability to 6-hydroxydopamine lesion and reduced development of dyskinesias in mice lacking CB1 cannabinoid receptors. Neurobiol Aging. 2011;32:631–45. 10.1016/j.neurobiolaging.2009.03.017. [DOI] [PubMed]
- [152].Fernandez-Espejo E, Caraballo I, de Fonseca FR, El Banoua F, Ferrer B, Flores JA, et al. Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with very severe nigral lesion in experimental parkinsonism. Neurobiol Dis. 2005;18:591–601. 10.1016/j.nbd.2004.10.015. [DOI] [PubMed]
- [153].García-Arencibia M, Ferraro L, Tanganelli S, Fernández-Ruiz J. Enhanced striatal glutamate release after the administration of rimonabant to 6-hydroxydopamine-lesioned rats. Neurosci Lett. 2008;438:10–3. 10.1016/j.neulet.2008.04.041. [DOI] [PubMed]
- [154].Han Q-W, Yuan Y-H, Chen N-H. The therapeutic role of cannabinoid receptors and its agonists or antagonists in Parkinson’s disease. Prog Neuro-psychopharmacol Biol Psychiatry. 2020;96:109745. 10.1016/j.pnpbp.2019.109745. [DOI] [PubMed]
- [155].dos Santos Pereira M, Abreu GHD, Rocca J, Hamadat S, Raisman-Vozari R, Michel PP, et al. Contributive role of TNF-α to L-DOPA-induced dyskinesia in a unilateral 6-OHDA lesion model of Parkinson’s disease. Front Pharmacol. 2021;11:617085. 10.3389/fphar.2020.617085. [DOI] [PMC free article] [PubMed]
- [156].Fakhfouri G, Ahmadiani A, Rahimian R, Grolla AA, Moradi F, Haeri A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway. Neuropharmacology. 2012;63:653–66. 10.1016/j.neuropharm.2012.05.013. [DOI] [PubMed]
- [157].Payandemehr B, Ebrahimi A, Gholizadeh R, Rahimian R, Varastehmoradi B, Gooshe M, et al. Involvement of PPAR receptors in the anticonvulsant effects of a cannabinoid agonist, WIN 55,212-2. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:140–5. 10.1016/j.pnpbp.2014.11.005. [DOI] [PubMed]
- [158].Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–15. 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed]
- [159].Szallasi A, Di Marzo V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000;23:491–7. 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed]
- [160].Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F, et al. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol. 2004;27:108–10. 10.1097/00002826-200405000-00003. [DOI] [PubMed]
- [161].Sieradzan KA, Fox SH, Hill M, Dick JPR, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: A pilot study. Neurology. 2001;57:2108–11. 10.1212/WNL.57.11.2108. [DOI] [PubMed]
- [162].Venderová K, Růžička E, Voříšek V, Višňovský P. Survey on cannabis use in Parkinson’s disease: Subjective improvement of motor symptoms. Mov Disord. 2004;19:1102–6. 10.1002/mds.20111. [DOI] [PubMed]
- [163].Consroe P, Sandyk R, Snider SR. Open label evaluation of cannabidiol in dystonic movement disorders. Int J Neurosci. 1986;30:277–82. 10.3109/00207458608985678. [DOI] [PubMed]
- [164].Frankel JP, Hughes A, Lees AJ, Stern GM. Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry. 1990;53:436. 10.1136/jnnp.53.5.436. [DOI] [PMC free article] [PubMed]
- [165].Zuardi A, Crippa J, Hallak J, Pinto J, Chagas M, Rodrigues G, et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol. 2009;23:979–83. 10.1177/0269881108096519. [DOI] [PubMed]
- [166].Finseth TA, Hedeman JL, Brown RP, Johnson KI, Binder MS, Kluger BM. Self-reported efficacy of cannabis and other complementary medicine modalities by Parkinson’s disease patients in colorado. Evidence-Based Complementary Altern Med. 2015;2015:1–6. 10.1155/2015/874849. [DOI] [PMC free article] [PubMed]
- [167].Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (Medical Marijuana) treatment for motor and non–motor symptoms of Parkinson disease: An open-label observational study. Clin Neuropharmacol. 2014;37:41–4. 10.1097/WNF.0000000000000016. [DOI] [PubMed]
- [168].Shohet A, Khlebtovsky A, Roizen N, Roditi Y, Djaldetti R. Effect of medical cannabis on thermal quantitative measurements of pain in patients with Parkinson’s disease. Eur J Pain. 2017;21:486–93. 10.1002/ejp.942. [DOI] [PubMed]
- [169].Yust-Katz S, Hershkovitz R, Gurevich T, Djaldetti R. Pain in extrapyramidal neurodegenerative diseases. Clin J Pain. 2017;33:635–9. 10.1097/AJP.0000000000000437. [DOI] [PubMed]
- [170].Balash Y, Bar-Lev Schleider L, Korczyn AD, Shabtai H, Knaani J, Rosenberg A, et al. Medical cannabis in Parkinson disease: Real-life patientsʼ experience. Clin Neuropharmacol. 2017;40:268–72. 10.1097/WNF.0000000000000246. [DOI] [PubMed]
- [171].Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, et al. Cannabis use in people with Parkinson’s disease and Multiple Sclerosis: A web-based investigation. Complementary Ther Med. 2017;33:99–104. 10.1016/j.ctim.2017.07.002. [DOI] [PubMed]