Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Sep 11;6:36. doi: 10.1186/s42238-024-00245-9
Abstract
Background
The psychosocial impact of medical marijuana use is not yet known. This study evaluated short-term changes in health-related quality of life (HRQoL) over the first three months of medical marijuana use.
Methods
This prospective, observational, longitudinal study followed adults newly recommended for medical marijuana by a physician for any of the more than 20 qualifying medical conditions in Pennsylvania. Participants (N = 438) provided their clinical status and demographic information, and completed semi-structured interviews prior to medical marijuana initiation (baseline) and at three months. HRQoL was assessed by the Short Form-36 (SF-36). Paired-samples t-tests evaluated changes in HRQoL over time.
Results
Participants (M age = 46.4 years [15.6]; 66.4% female) were mostly commonly referred for medical marijuana to treat anxiety disorders (61.9%) or severe chronic or intractable pain (53.6%). Participants reported rapid and significant improvements in all of the domains of HRQoL from baseline to three months after initiating medical marijuana use (physical functioning, role limitations due to physical health problems, emotional well-being, role limitations due to emotional problems, bodily pain, social functioning, energy/fatigue and general health, P < .001 for all). Age was negatively predictive of level of improvement over time for the physical functioning (P < .0001), role limitations due to physical health problems (P < .001), and pain (P < .0001) domains after controlling for baseline, with older participants displaying less improvement than younger participants.
Conclusions
Gains were observed in all HRQoL domains assessed after three months of medical marijuana use. In several domains, age was a significant predictor of degree of improvement.
Despite the increasing utilization of marijuana for medical purposes [1, 2], the effects of medical marijuana use on the health-related quality of life (HRQoL) of individuals who begin using cannabis-based products for medical reasons are largely unknown. According to the Centers for Disease Control and Prevention [3], HRQoL is a multidimensional construct that reflects “physical wellbeing, social and emotional wellbeing, and the impact of social determinants of health” (p.1). While the assessment of HRQoL provides valuable information regarding the wellbeing of individuals, including those living with chronic medical conditions, studies suggest HRQoL has prognostic value in regards to clinical outcomes such as mortality risk in older adults and in individuals with cancer diagnoses [4, 5].
Ongoing monitoring of HRQoL in individuals using medical marijuana to treat various mental and physical health conditions has the potential to capture changes in both the burden of the referring condition and the impact of medical marijuana on the wellbeing and functioning of users as treatment progresses. Several studies have demonstrated HRQoL scores in individuals initiating medical marijuana treatment to be comparable, or in some domains lower, than those observed in other studies of individuals with chronic health conditions [6, 7]. Furthermore, a number of studies provide preliminary evidence that medical marijuana treatment may enhance patients’ HRQoL [8–10]. For example, a prospective three-month study of medical marijuana users in the UK reported improvements in the pain/discomfort and anxiety/depression domains of HRQoL [9] and a retrospective case series of medical marijuana users in Australia reported similar findings in the domains of pain and mental health [11]. In Florida, middle aged and older patients using medical marijuana for pain reported higher HRQoL three months after baseline [10]. However, a review of studies evaluating HRQoL and medical marijuana found the existing literature to be inconclusive for conditions other than pain and called for more rigorous study designs and longer study durations in future investigations [12–15]. Studies employing prospective designs and larger samples, utilizing well-established QoL measures, and recruiting patients when they first begin medical marijuana use, are needed to clarify the relationship between HRQoL and medical marijuana use.
The current prospective, longitudinal study evaluated changes in eight domains of HRQoL over the first three months of medical marijuana treatment in a large sample of adults initiating medical marijuana treatment for the first time in Pennsylvania (PA) for any of the more than 20 approved health conditions, which include mental health disorders (e.g., anxiety disorders, posttraumatic stress disorder) and physical health disorders (e.g., severe chronic or intractable pain, neurodegenerative diseases, inflammatory bowel disease, intractable seizures). This study included only new medical marijuana users to most effectively capture changes in HRQoL with the initiation of medical marijuana use, and utilized a well-validated measure of HRQoL, the Short Form-36 (SF-36) [16, 17]. We hypothesized that adults would report significant improvements in all aspects of HRQoL from baseline to three months after initiating medical marijuana use. In addition, we examined potential predictors of change in HRQoL during this same time period.
Methods
Individuals aged 18 years or older were eligible to participate in the study if they had obtained a recommendation from a PA physician for medical marijuana to treat any qualifying medical condition, had a valid state-issued medical marijuana card, and were naïve to medical marijuana. Individuals were excluded from participation if they were unable to provide informed consent, reported active heavy recreational marijuana use (use on most days for the past month), or were not English speaking. Recruitment occurred at four dispensaries in the greater Harrisburg and Pittsburgh, PA areas.
Potentially eligible individuals were introduced to onsite study research staff by the dispensary pharmacists after they presented for their initial medical marijuana consultation. New medical marijuana patients at the dispensary sites where recruitment took place meet with a pharmacist prior to their initial medical marijuana purchase. Interested individuals completed informed consent, granted research staff access their dispensary medical record, and completed the structured baseline interview including the HRQoL measure within one week of consent. Participants consented to be followed over the first year of medical marijuana use and to return for study visits at 3, 6, 9 and 12-months post-enrollment. Follow-up study assessments were completed either in person during dispensary visits to purchase products or via phone. Participants’ medical marijuana purchases from dispensaries were collected over the study year. In general, the baseline appointment, including the informed consent process, took approximately 60–90 min to complete. Participants were remunerated $25 USD for each study visit completed. Baseline HRQoL data were previously reported [7].
Study data were collected and managed using REDCap (Research Electronic Data Capture) hosted at the lead institution. REDCap is a secure, web-based software platform designed to support data capture for research studies.[18] The Philadelphia College of Osteopathic Medicine Institutional Review Board (#H17-060) approved the research protocol and provided ongoing ethical oversight of the study. The dataset analyzed for the current study is available in the DigitalCommons repository, https://digitalcommons.pcom.edu/.
Measures
Demographic and Medical Information
Participants reported their age, identified gender, marital status, race, ethnicity, completed education level, socioeconomic status (e.g., household income), current medical diagnoses, and the medical reason(s) for their medical marijuana recommendation. Current (past 90 days) and lifetime use of recreational marijuana was collected using the Addiction Severity Index (ASI).[19].
Health-Related Quality of Life (HRQoL)
The Short-Form—36 (SF-36) is a 36-item structured clinical assessment evaluating physical and mental health functioning, with most questions referring to the past four weeks.[16, 17] The SF-36 measures several areas of HRQoL: physical functioning, role limitations due to physical health problems, emotional well-being, role limitations due to emotional problems, social functioning, energy/fatigue, bodily pain, and general health. Scores for each scale range from 0 (poorest) to 100 (highest). The SF-36 was administered at baseline (prior to the start of medical marijuana use) and again three months after starting medical marijuana (± 2 weeks). Lower scores are indicative of poorer functioning.
Statistical approach
Descriptive statistics (mean, standard deviations and frequencies) were generated to characterize the overall sample at study entry. A series of paired t-tests were performed to evaluate the extent to which HRQoL subscale scores differed significantly from baseline to month three for each domain. Finally, a series of linear regression analyses were used to examine the extent to which participant-level characteristics at baseline (i.e., age, race, gender identity [= female in analyses], chronic pain as a referring condition, anxiety as a referring condition) were associated with the degree of change observed at month three for each subscale of the SF—36 (i.e., month three scale score – baseline scale score) after controlling for the corresponding baseline score. For each subscale, t-test and regression analyses included all individuals with non-missing data at baseline and the three-month follow-up assessments. Effect sizes (Cohen’s d) were calculated to estimate clinically significant change in each HRQoL domain. Significance levels were set at P < 0.05.
Results
From September 2020 – June 2023, 1,314 new medical marijuana patients were approached regarding the study and 452 (34.4%) enrolled and completed the baseline assessment. Of the 452 enrolled, 14 participants withdrew prior to the three-month assessment (N = 438). Most individuals who declined participation reported time constraints and a desire to initiate medical marijuana treatment immediately (vs. after completion of the baseline assessment) as the primary reason they did not enroll in the study. In total, 399 participants completed the three-month follow-up assessment (91% follow-up rate) and were included in the longitudinal HRQoL analyses. At the time of these analyses, the study was ongoing and data collection continued for one-year post-enrollment.
Participant characteristics
Participants (N = 438) were a mean age of 46.4 years (SD = 15.6) and predominantly identified as White (94.5%) and female (66.4%). The majority of participants were referred for medical marijuana for the treatment of chronic intractable pain (53.6%) and/or an anxiety disorder (61.9%). Sample characteristics are presented in Table 1.
Table 1.
Sample characteristics of first-time medical marijuana users in Pennsylvania (N = 438)
| Mean (SD) | n (%) | |
|---|---|---|
| Age (years) | 46.4 (15.6) | |
| Gender | ||
| Male | 142 (32.6) | |
| Female | 289 (66.4) | |
| Transgender, Non-binary, Not Specified | 7 (1.0) | |
| Race | ||
| White | 414 (94.5) | |
| Black | 16 (3.7) | |
| Other | 8 (1.8) | |
| Latino/a/x Ethnicity | 12 (2.7) | |
| Marital Status | ||
| Married or Domestic Partnership | 244 (55.7) | |
| Single (Never Married) | 108 (24.6) | |
| Separated or Divorced | 69 (15.8) | |
| Widowed or Unknown | 17 (3.9) | |
| Highest Degree Completed | ||
| High School Diploma or GED | 101 (23.1) | |
| Associate’s Degree or Trade/Technical | 70 (16.0) | |
| Bachelor’s Degree | 106 (24.3) | |
| Graduate Degree | 59 (13.5) | |
| Other | 102 (23.3) | |
| Most Common Referring Conditions for Medical Marijuana* | ||
| Chronic or intractable pain | 235 (53.7) | |
| Anxiety disorder | 271 (61.9) | |
| Post-Traumatic Stress Disorder | 50 (11.4) | |
| Neuropathies | 27 (6.2) | |
| Cancer | 25 (5.7) | |
| Irritable Bowel Syndrome | 17 (3.9) | |
| Multiple Sclerosis | 11 (2.5) | |
| Glaucoma | 6 (1.4) | |
| Recreational Marijuana Use | ||
| Any use, past 90 days (yes) | 107 (25.4) | |
| Any use, lifetime (yes) | 222 (52.7) | |
| Medical Marijuana Product Type Purchased Over 3 Months** | ||
| Oral | 237 (56.0) | |
| Vape/Inhaled | 256 (60.5) | |
| Concentrate/Extract | 77 (18.2) | |
| Topical | 111 (26.2) |
Changes in HRQoL scale scores from baseline to month 3
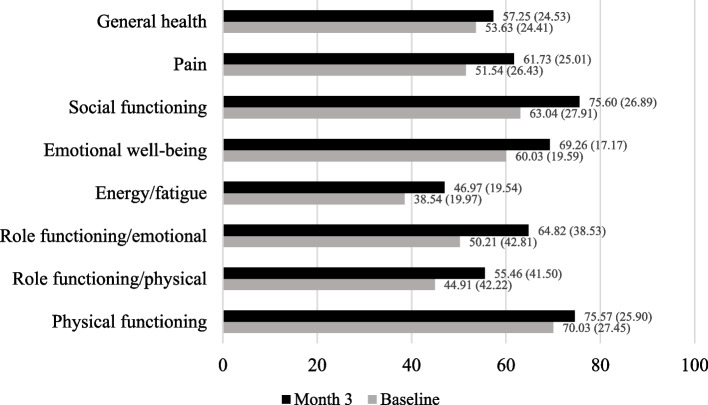
Participants reported significant improvements across all domains of HRQoL after the first three months of medical marijuana use (see Fig. 1, P‘s < 0.001 for all domains). Specifically, participants endorsed gains from baseline to month three in physical functioning (t[395] = -5.23, + 6.5%, d = 0.26), role limitations due to physical health problems (t[397] = -5.59, + 23.5%, d = 0.28), role limitations due to emotional problems (t[396] = -7.47, + 29.1%, d = 0.37), energy/fatigue (t[397] = -9.31, 18.8%, d = 0.47), emotional well-being (t[397] = -11.25, + 15.4%, d = 0.56), social functioning (t[396] = -9.08, + 25.9%, d = 0.46), bodily pain (t[396] = -9.54, + 19.8%, d = 0.48), and general health (t[394] = -4.81, + 6.7%, d = 0.24).
Fig. 1.
Predictors of HRQoL change at month three
Results from the series of regression analyses predicting the three-month change score are presented in Table 2. Baseline score was a significant predictor of degree of change across each of the SF-36 domains (all P’s < 0.0001); participants with higher baseline HRQoL scores displayed lower levels of change than those with lower baseline HRQoL scores within each domain. In addition, age significantly predicted the observed degree of change for the physical functioning (t(Bruce et al. [7] June [7]) = -4.70, P < 0.001), role limitations due to physical health problems, (t(Bruce et al. [7] June [7]) = -3.71, P < 0.001), and bodily pain (t(Bruce et al. [7] June [7]) = -4.46, P < 0.0001) domains. Specifically, age was negatively associated with the degree of observed change with older participants displaying less improvement from baseline to the three-month follow-up than younger participants. Gender, anxiety as a referring condition, and chronic pain as a referring condition did not reach statistical significance in any of the models.
Table 2.
Regression analyses predicting change score at month three for each SF-36 domain
| Domain | Variable | Beta | SE | t | R2 for model |
|---|---|---|---|---|---|
| Physical functioning
(n = 393) |
(Intercept)**** | 41.66 | 4.68 | 8.90 | 0.22 |
| Baseline score**** | -0.33 | 0.03 | -10.03 | ||
| Female gender | -1.00 | 1.66 | -0.60 | ||
| Age**** | -0.27 | 0.06 | -4.70 | ||
| Anxiety | 0.30 | 1.85 | 0.16 | ||
| Chronic pain | -1.63 | 1.81 | -0.90 | ||
| Role limitations due to physical health
(n = 395) |
(Intercept)**** | 58.89 | 7.60 | 7.75 | 0.26 |
| Baseline score**** | -0.49 | 0.04 | -11.29 | ||
| Female gender | -6.71 | 3.53 | -1.90 | ||
| Age*** | -0.43 | 0.12 | -3.71 | ||
| Anxiety | 1.45 | 3.92 | 0.37 | ||
| Chronic pain | -5.32 | 3.82 | -1.40 | ||
| Role limitations due to emotional problems
(n = 394) |
(Intercept)**** | 38.65 | 7.27 | 5.31 | 0.30 |
| Baseline score**** | -0.50 | 0.04 | -11.92 | ||
| Female gender | 1.09 | 3.53 | 0.31 | ||
| Age | 0.11 | 0.12 | 0.94 | ||
| Anxiety | -2.65 | 3.94 | -0.67 | ||
| Chronic pain | -5.41 | 3.72 | -1.46 | ||
| Energy/fatigue
(n = 395) |
(Intercept)**** | 27.90 | 3.80 | 7.34 | 0.23 |
| Baseline score**** | -0.44 | 0.04 | -10.61 | ||
| Female gender | -2.27 | 1.73 | -1.31 | ||
| Age | -0.02 | 0.06 | -0.42 | ||
| Anxiety | 0.79 | 1.88 | 0.42 | ||
| Chronic pain | -1.01 | 1.81 | -0.56 | ||
| Emotional well-being
(n = 395) |
(Intercept)**** | 37.06 | 3.72 | 9.97 | 0.31 |
| Baseline score**** | -0.46 | 0.04 | -12.18 | ||
| Female gender | 0.98 | 1.48 | 0.66 | ||
| Age | -0.01 | 0.05 | -0.16 | ||
| Anxiety | -0.42 | 1.65 | -0.26 | ||
| Chronic pain | -0.10 | 1.55 | -0.06 | ||
| Social functioning
(n = 394) |
(Intercept)**** | 50.18 | 5.77 | 8.69 | 0.29 |
| Baseline score**** | -0.53 | 0.04 | -12.19 | ||
| Female gender | -3.65 | 2.53 | -1.44 | ||
| Age | -0.05 | 0.08 | -0.56 | ||
| Anxiety | 1.98 | 2.77 | 0.71 | ||
| Chronic pain | -2.08 | 2.67 | -0.78 | ||
| Pain
(n = 394) |
(Intercept)**** | 45.39 | 4.99 | 9.10 | 0.27 |
| Baseline score**** | -0.44 | 0.04 | -10.69 | ||
| Female gender | 0.79 | 2.00 | 0.40 | ||
| Age**** | -0.30 | 0.07 | -4.46 | ||
| Anxiety | 1.42 | 2.18 | 0.65 | ||
| Chronic pain | -0.70 | 2.25 | -0.31 | ||
| General health
(n = 392) |
(Intercept)**** | 15.09 | 3.43 | 4.40 | 0.12 |
| Baseline score**** | -0.20 | 0.03 | -6.82 | ||
| Female gender | 1.64 | 1.51 | 1.08 | ||
| Age | -0.02 | 0.05 | -0.34 | ||
| Anxiety | 0.79673 | 1.66 | 0.48 | ||
| Chronic pain | -2.44937 | 1.61 | -1.52 |
Note: *p < .05, **p < .01, ***p < .001, ****p < .0001
Discussion
As hypothesized, new medical marijuana users experienced improvements across all domains of HRQoL over the first three months of medical marijuana use for any of the more than 20 qualifying medical conditions for use in PA. Notably, participants endorsed greater than 20% increases in ratings of their role limitations due to physical health problems and emotional problems, and in social functioning after three months of medical marijuana use. The physical health and emotional limitations subscales of the SF-36 capture time spent, amount accomplished, or difficulties encountered when performing work or other activities due to physical health or emotional problems over the past four weeks, while the social functioning subscale assesses the extent to which physical or emotional problems have interfered with normal social activities.[16] We believe that these HRQoL gains represent clinically meaningful change in our participants. According to Wyrwich and colleagues, “clinically significant change in QoL is a difference score that is large enough to have an implication for the patient’s treatment or care” (p. 286).[20] While our study did not ask participants to define this threshold, the moderate effect sizes found in several domains including energy/fatigue, emotional well-being, social functioning, and bodily pain suggest that meaningful change occurred. Further, HRQoL directly relates to clinical outcomes. In individuals with chronic pain conditions, greater severity of pain is associated with greater impairments in HRQoL [21]; however, HRQoL improves as pain levels decline with treatment.[22].
Notably, the changes in HRQoL seen in our study were comparable to HRQoL gains in others evaluating different treatment modalities for the two most common referring conditions, anxiety and chronic pain. A study of HRQoL after 13 weeks of oral analgesic use for chronic knee pain found improvements in bodily pain scores on the SF-36 that were similar to the changes in bodily pain scores in our study after 12 weeks of medical marijuana use.[23] HRQoL has also been evaluated in individuals with generalized anxiety disorder (GAD). A double-blind, placebo-controlled study evaluating HRQoL following treatment with the prescription medication vortioxetine for GAD also found similar gains in social functioning scores after eight weeks as we found after 12 weeks of medical marijuana use.[24] Future studies that employ randomized controlled designs are needed to better understand the effectiveness of medical marijuana on HRQoL compared to other treatments for various conditions.
How the use of medical marijuana may relate to the observed gains in HRQoL, particularly in the domains that increased most, requires further study. For example, levels of energy increased in medical marijuana users as reported in the energy/fatigue subscale; future studies may benefit from examining potential mediators of this effect such as sleep quality and duration. It is also plausible that levels of bodily pain could mediate the gains observed at three months in physical and social functioning, especially given the high number of participants referred for medical marijuana to treat chronic pain. Additionally, the observed social functioning gains may be particularly relevant to individuals referred for medical marijuana for the treatment of anxiety disorders, as the symptoms associated with many anxiety disorders can substantially interfere with interpersonal functioning. The HRQoL gains in our study are similar to those reported in a study of patients with chronic pain [10] and in a smaller study of UK patients over three months of medical marijuana use.[9] Notably, referring condition (i.e., anxiety, chronic pain) was not related to the degree of improvement in HRQoL for any of the domains examined. Older participants in our study, however, reported less robust gains in their physical functioning and pain than younger participants, which may reflect the changes in functioning that normally coincide with older age, or may indicate a possible limitation of medical marijuana use in older adults. Additional studies are needed to clarify this finding.
This study has multiple strengths. To our knowledge, this study is one of the largest longitudinal studies of quality of life in individuals using medical marijuana in the US. This study utilized a well-established and widely used measure of HRQoL in both research and clinical domains, and had a high follow-up rates at month three (91%). The study also had several limitations. Our sample identified as predominantly White and female, limiting the generalizability of our findings to other groups. The reasons why this particular demographic is overrepresented are unclear, but may relate to the higher prevalence of anxiety disorders [25] and chronic pain [26] in women (the two most common referring conditions in this study), the greater likelihood of women to utilize complementary and alternative medicine compared to men, or fears of potentially negative outcomes in racial and ethnic minorities despite the legalization of cannabis use for medicinal purposes.[27] The observational study design did not allow for the attribution of causality in regards to the observed changes in HRQoL and the initiation of medical marijuana use; the current Schedule I designation of products containing tetrahydrocannabinol (THC) in the US limits the utilization of more rigorous study designs.
Individuals seeking medical marijuana for the first time may be experiencing particularly acute, intense or refractory medical conditions that more adversely impact their HRQoL compared to those seeking more evidence-based behavioral or prescription treatment options. It is also important to note that while individuals who enrolled in this study were naïve to medical marijuana use, 25% reported recent recreational use. The rate of recent recreational marijuana use was higher than rates of marijuana use reported in the general U.S. population with medical conditions [28] and could therefore limit the generalizability of our findings. Further, the high number of participants reporting previous experience with marijuana allows for the potential for both expectancy biases and placebo effects surrounding the use of marijuana for medical purposes. Beyond the reasons for declining study enrollment, no additional information was collected regarding the individuals who declined to participate in this study. It is possible that these individuals were different than enrolled participants in meaningful ways and future studies may benefit from obtaining descriptive information on those who decline to participate. While the majority of participants who did not complete three-month assessments were lost to follow-up (> 80%) and therefore the reasons for drop out remain unknown, several participants reported their reasons for dropping to be low remuneration and a high burden of time. Further, the present analyses did not evaluate potentially adverse psychosocial outcomes of medical marijuana use, though Arkell and colleagues found use to be associated with a low risk of serious adverse events.[11] Future studies could evaluate the adverse or unintended consequences of use or the reasons for initiating use, and may benefit from following participants for longer durations. The present study represents interim analyses and future analyses of this ongoing study will help to determine whether the observed gains in HRQoL at three months are sustained over the first year of medical marijuana use.
In conclusion, the use of medical marijuana for three months was associated with improvements in physical, social, emotional and pain-related HRQoL. Ongoing surveillance of HRQoL in individuals with physical and mental health conditions can help to treat the “whole person” and to capture any collateral impact of selected therapeutic approaches as treatment initiates and progresses. Results from this study can help patients, their caregivers, and their providers to make more informed and evidence-based decisions on whether to incorporate medical marijuana into their treatment regimens.
Acknowledgements
The authors would like to acknowledge the late David S. Festinger, PhD, who was instrumental in the conceptualization of the study and development of study procedures, Organic Remedies for funding this work, and all of the dispensary patients, pharmacists and staff that made this research possible.
Abbreviation
- HRQoL
-
Health-related quality of life
Authors’ contributions
MRL designed the study, conducted the literature review, oversaw study administration, and wrote the first manuscript draft. MRL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. TRM recruited participants, collected data, designed the database, and edited the manuscript draft. MMS recruited participants, collected data, designed the database, and edited the manuscript draft. KLD designed the study, collaborated on study administration, conducted all analyses, and edited the manuscript draft.
Funding
This study was funded by Organic Remedies, Inc. The funder had no role in the study design, or in the analysis or interpretation of data.
Availability of data and materials
Study data and a data dictionary are available at https://digitalcommons.pcom.edu/.
Declarations
Ethics approval and consent to participate
The Philadelphia College of Osteopathic Medicine Institutional Review Board (#H17-060) approved the research protocol and provided ongoing ethical oversight of the study. All participants provided informed consent.
Consent for publication
N/A.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abelev S, Warne LN, Benson M, Hardy M, Nayee S, Barlow J. Medicinal Cannabis for the Treatment of Chronic Refractory Pain: An Investigation of the Adverse Event Profile and Health-Related Quality of Life Impact of an Oral Formulation. Med Cannabis Cannabinoids. 2022;5(1):20–31. 10.1159/000521492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal SK, Carter GT, Sullivan MD, Zumbrunnen C, Morrill R, Mayer JD. Prospectively surveying health-related quality of life and symptom relief in a lot-based sample of medical cannabis-using patients in urban Washington State reveals managed chronic illness and debility. Am J Hosp Palliat Care. 2013;30(6):523–31. 10.1177/1049909112454215 [DOI] [PubMed] [Google Scholar]
- 3.Arkell TR, Downey LA, Hayley AC, Roth S. Assessment of Medical Cannabis and Health-Related Quality of Life. JAMA Netw Open. 2023;6(5): e2312522. 10.1001/jamanetworkopen.2023.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett RM, Schein J, Kosinski MR, Hewitt DJ, Jordan DM, Rosenthal NR. Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis Care & Research: Official Journal of the American College of Rheumatology. 2005;53(4):519–27. 10.1002/art.21319 [DOI] [PubMed] [Google Scholar]
- 5.Brown DS, Thompson WW, Zack MM, Arnold SE, Barile JP. Associations between health-related quality of life and mortality in older adults. Prev Sci. 2015;16:21–30. 10.1007/s11121-013-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce D, Foster E, Shattell M. Perceived efficacy of medical cannabis in the treatment of co-occurring health-related quality of life symptoms. Behav Med. 2021;47(2):170–4. 10.1080/08964289.2019.1683712 [DOI] [PubMed] [Google Scholar]
- 7.Bruce D, Grove TJ, Foster E, Shattell M. Gender Differences in Medical Cannabis Use: Symptoms Treated, Physician Support for Use, and Prescription Medication Discontinuation. J Womens Health (larchmt). 2021;30(6):857–63. 10.1089/jwh.2020.8437 [DOI] [PubMed] [Google Scholar]
- 8.Buonomano LS, Mitnick MM, McCalmont TR, Syracuse P, Dugosh KL, Festinger DS, et al. Clinical Characteristics and Quality of Life in Adults Initiating Medical Marijuana Treatment. Med Cannabis Cannabinoids. 2022;5(1):95–101. 10.1159/000524831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. HRQOL Concepts. 2018; . Accessed May 17, 2023.
- 10.Dai H, Richter KP. A national survey of marijuana use among US adults with medical conditions, 2016–2017. JAMA Netw Open. 2019;2(9): e1911936. 10.1001/jamanetworkopen.2019.11936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erridge S, Salazar O, Kawka M, Holvey C, Coomber R, Usmani A, et al. An initial analysis of the UK Medical Cannabis Registry: Outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. 2021;41(3):362–70. 10.1002/npr2.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain. Clin J Pain. 2016;32(12):1036–43. 10.1097/AJP.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ 1993 October 01;2(3):217–227. [DOI] [PubMed]
- 15.Mahableshwarkar AR, Jacobsen PL, Serenko M, Chen Y. A randomized, double‐blind, fixed‐dose study comparing the efficacy and tolerability of vortioxetine 2.5 and 10 mg in acute treatment of adults with generalized anxiety disorder. Hum Psychopharmacol Clin Exp 2014;29(1):64–72. [DOI] [PubMed]
- 16.Martins SS, Segura LE, Levy NS, Mauro PM, Mauro CM, Philbin MM, et al. Racial and ethnic differences in cannabis use following legalization in US states with medical cannabis laws. JAMA Netw Open. 2021;4(9): e2127002. 10.1001/jamanetworkopen.2021.27002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45(8):1027–35. 10.1016/j.jpsychires.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 1992;9(3):199–213. [DOI] [PubMed]
- 19.Osborne NR, Davis KD. Sex and gender differences in pain. International Review of Neurobiology: Elsevier; 2022. p. 277–307. [DOI] [PubMed]
- 20.Papou A, Hussain S, McWilliams D, Zhang W, Doherty M. Responsiveness of SF-36 Health Survey and Patient Generated Index in people with chronic knee pain commenced on oral analgesia: analysis of data from a randomised controlled clinical trial. Qual Life Res. 2017;26:761–6. 10.1007/s11136-016-1484-2 [DOI] [PubMed] [Google Scholar]
- 21.Peterson AM, Le C, Dautrich T. Measuring the Change in Health-Related Quality of Life in Patients Using Marijuana for Pain Relief. Med Cannabis Cannabinoids. 2021;4(2):114–20. 10.1159/000517857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safakish R, Ko G, Salimpour V, Hendin B, Sohanpal I, Loheswaran G, et al. Medical cannabis for the management of pain and quality of life in chronic pain patients: a prospective observational study. Pain Med. 2020;21(11):3073–86. 10.1093/pm/pnaa163 [DOI] [PubMed] [Google Scholar]
- 23.Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surg Oncol Clin. 2018;27(4):675–84. 10.1016/j.soc.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svensson O, Malmenäs M, Fajutrao L, Roos EM, Lohmander LS. Greater reduction of knee than hip pain in osteoarthritis treated with naproxen, as evaluated by WOMAC and SF-36. Ann Rheum Dis. 2006;65(6):781–4. 10.1136/ard.2005.040519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Jean Jacques J, Li Z, Sibille KT, Cook RL. Health Outcomes among Adults Initiating Medical Cannabis for Chronic Pain: A 3-month Prospective Study Incorporating Ecological Momentary Assessment (EMA). Cannabis. 2021October 01;4(2):69–83. 10.26828/cannabis/2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992 June 01;30(6):473–483. [PubMed]
- 27.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2030 June 23;313(24):2456–2473. [DOI] [PubMed]
- 28.Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL, Symonds T. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14:285–95. 10.1007/s11136-004-0705-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study data and a data dictionary are available at https://digitalcommons.pcom.edu/.