1Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center, Tel Hashomer, Israel
2Hadassah-Hebrew University Medical School, Jerusalem, Israel
3Sackler faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
4Smith faculty of Agriculture, food and Environment School of Nutritional Sciences, The Hebrew University of Jerusalem, Rehovot, Israel
 As history repeats itself, recent legalization changes have led cannabis back to our prescription pad. Tracing back to ~4000 BC, the medical merits of cannabis were utilized throughout history. Currently, medical cannabis is rapidly being introduced into the medicinal arsenal, prompting physicians to evaluate its medical and safety properties. Comprising a large part of the population and an even more prominent proportion of health care consumers, elderly patients constitute a substantial target group for treatment with medical cannabis. A recent Israeli study found that of 279 cancer patients receiving medical cannabis 50% were aged 60 or older [1]. Indeed, in light of the unique features of the geriatric population there is a need for a safe drug to address their complaints. Drugs such as antipsychotics or benzodiazepines are being used daily although they pose a high risk for the elderly’s health and well-being. Since medicinal cannabis has shown promise for many conditions that trouble elderly patients, we set out to explore the current therapeutic potential of cannabis in this population.
As history repeats itself, recent legalization changes have led cannabis back to our prescription pad. Tracing back to ~4000 BC, the medical merits of cannabis were utilized throughout history. Currently, medical cannabis is rapidly being introduced into the medicinal arsenal, prompting physicians to evaluate its medical and safety properties. Comprising a large part of the population and an even more prominent proportion of health care consumers, elderly patients constitute a substantial target group for treatment with medical cannabis. A recent Israeli study found that of 279 cancer patients receiving medical cannabis 50% were aged 60 or older [1]. Indeed, in light of the unique features of the geriatric population there is a need for a safe drug to address their complaints. Drugs such as antipsychotics or benzodiazepines are being used daily although they pose a high risk for the elderly’s health and well-being. Since medicinal cannabis has shown promise for many conditions that trouble elderly patients, we set out to explore the current therapeutic potential of cannabis in this population.
• Cannabinoid receptors
To date there are two known cannabinoid receptor subtypes, CB1 and CB2, both classified as Gi/o protein-coupled receptors. Yet the two receptors differ from each other not only in binding site but in distribution as well, with CB1 distribution being more prominent in the central nervous system, whereas CB2 is prominently distributed in the immune system. Although CB1 and CB2 are currently considered the main cannabinoid receptors, evidence suggests that cannabinoid compounds do not bind solely to CB receptors and may interact with other receptors, such as transient receptor potential cation channel subfamily V member 1 channels (TRPV1), peroxisome proliferator-activated receptors (PPARs), and others [3].
The two main endogenous cannabinoid ligands are anandamide (AEA) and 2-arachidonoylglycerol (2-AG). This pair of eCBs are derivatives of arachidonic acid and are synthesized locally in response to demand [3].
• Endocannabinoids synthesis and degradation
AEA is primarily synthesized by the action of N-acyltransferase (NAT) and N-acylphosphatidylethanolamine-specific phospholi- pase D (NAPE-PLD), and degraded predominantly by the activity of fatty acid amide hydrolase (FAAH). 2-AG is synthesized by the action of diacylglycerol lipase (DAGL), and degraded by monoacylglycerol lipase (MAGL). AEA and 2-AG share some oxidation pathways [3].
Different strains of Cannabis sativa encompass more than 545 different compounds, of which more than 100 are classified as phytocannabinoids and are unique to the Cannabis sativa plant. The two most prominent and researched phytocannabinoids are the dynamic duo ∆9tetrahydrocannabinol (THC) and cannabidiol (CBD), each with its own merits and distinctive features. THC, considered the major psychoactive ingredient in Cannabis sativa, is a lipophilic molecule that exerts similar effects to AEA in animal models [2,4]. CBD is a major non- psychoactive constitute of Cannabis sativa and, like THC, is a lipophilic molecule. The pair share a biosynthesis pathway and differ only in the last stage of the process [2].
While cannabinoids can be consumed in their natural form, refined separately or as synthetic analogues, it seems that combined consumption exerts a superior beneficial effect. Surprisingly, and contrary to the “ideal” drug composition paradigm, the Cannabis sativa ensemble of ingredients seems to work synergistically, being more effective than the single ingredient. It appears that, indeed, the whole is greater than the sum of its parts, giving rise to the theory of “the entourage effect” [5].
Parkinson’s disease (PD) is a neurodegenerative disorder mainly of dopaminergic neurons in the substantia nigra. While the etiology of PD is still unclear, the cellular mechanisms that underlie the complex interactions between genetics and envi- ronmental factors are gradually being unraveled. PD is known for its characteristic movement dysfunction and symptoms that commonly include tremor, bradykinesia, rigidity and gait abnormality, collectively known as Parkinsonism. PD may also involve non-motor clinically significant symptoms [6].
Despite the fact that CB1 is absent in the dopaminergic nigrostriatal neuron, the eCB system seems to play a major part in the modulation of dopaminergic transmission in the basal ganglia. Presumably the eCB system modulates GABA and glutamate inputs to the dopaminergic nigrostriatal neuron. Furthermore, evidence of the presence of CB2 and TRPV1 in the nigrostriatal neuron suggests an additional pathway of direct modulation [7]. Additionally, single nucleotide polymorphism within the FAAH gene has been associated with greater risk for PD-related pain [8].
The ability of the eCB to modulate the dopaminergic system holds promise for the future treatment of PD. Preclinical data suggest that selective pharmacological intervention in the eCB signaling pathway may have a beneficial effect in PD, both on motor and non-motor symptoms. An additional benefit may include neuroprotection [6]. Supporting this notion is the open-label observational study conducted by Lotan et al. [10] in 22 PD patients before and 30 minutes after consumption of Cannabis sativa. Following cannabis consumption the patients exhibited a significant improvement of 9.9 points in the mean score in the Unified Parkinson’s Disease Rating Scale (P < 0.001). Furthermore, specific analysis revealed significant amelioration also in rigidity, tremor, bradykinesia, pain and sleeping problems with no significant adverse effect [9]. In another study, conducted as a randomized, double-blind, placebo-controlled, crossover trial, by Sieradzan et al. [11], PD patients reported a significant alleviation of total levodopa-induced dyskinesia following treatment with nabilone (THC analogue). Patients treated with nabilone achieved a 5 point reduction in the Rush Dyskinesia Disability Scale (P = 0.05, n=7) [10]. Notwithstanding both the preclinical data and the results reported by Lotan et al. [10] and Sieradzan et al. [11], other clinical studies aiming to explore the effects of cannabinoids in PD did not demonstrate a significant clinical change. Nonetheless, these studies [12,13] show the relative safety and toleration of cannabinoids in PD patients.
Overall, current preclinical and clinical data suggest a therapeutic potential for PD patients by targeting the eCB system. However, while we acknowledge the beneficial properties of cannabis that are already being utilized in the treatment of PD, it is not without reservation. It is our opinion that the ideal therapeutic effect in these patients is achievable only through specific compounds that target selective parts in the eCB signaling.
Dementia is a clinical mental syndrome mainly affecting older adults, characterized by a progressive pathological impairment of memory, language, orientation, judgment, comprehension and overall cognition that affects the individual’s ability to conduct everyday activities. These symptoms are predominantly accompanied by psychological and behavioral symptoms, i.e., motivation, emotional and social problems, as well as agitation and delusions. Dementia may result from several diseases and assaults, with Alzheimer’s disease (AD) being the leading cause [13]. For the sake of convenience we will mainly address AD and refer to dementia as a syndrome rather than to the individual etiologies.
While the role of the eCB system in dementia has yet to be elucidated, the inherent changes of the eCB system in AD are fairly researched and characterized. These changes affect mainly two sites, the hippocampus and the cerebral cortex, which are also the two prominent sites affected by AD. The aforementioned changes are characterized by: (i) an increase in FAAH enzyme activity and levels in astrocytes associated with neuritic plaque; (ii) elevated 2-AG levels linked to β-amyloid hippocampal degradation, supposedly in an independent manner due to FAAH increase; (iii) CB2 up-regulation in microglial cells adjacent to β-amyloid plaques; and (iv) reduction in the number of CB1-positive neurons [14].
Although preclinical data suggest the potential role of cannabinoids in disease progression and possible prevention, clinical data supporting that notion have yet to be determined. Nonetheless, a substantial amount of clinical data supports the beneficial therapeutic effect of cannabinoids on behavioral symptoms in patients with dementia. In a placebo-controlled crossover-designed study, Volicer et al. [16] were the first to demonstrate an amelioration in behavioral disturbance in AD patients following treatment with dronabinol (THC analogue) (n=15, P value unclear) [15]. Later on, a Cochrane systematic review from 2009 found the Volicer trial to be the only study that met the inclusion criteria. However, due to a lack of quantitative data in the Volicer study, the Cochrane authors concluded that the result could not be adequately validated [16]. In a retrospective systematic chart review, Woodward and co-authors [18] demonstrated a significant reduction in agitation among dementia patients associated with dronabinol intervention (n=40, P < 0.0001) [17]. A similar beneficial effect of dronabinol on noc- turnal agitation was reported in an open-label pilot study (n=6, P < 0.05) by Walther et al. [18]. A new prospective cannabinoid treatment for dementia is illustrated by a recent case report suggesting cannabinoids for the treatment of sexual disinhibition. A 71 year old dementia patient suffering from sexual disinhibition who was non-responsive to conventional psychiatric intervention was treated with nabilone. The outcome was a subsequent reduction in disinhibition in sexual behavior, thus illuminating a new trait that might be beneficial in patients with dementia [19]. Contrary to the results mentioned above, a recent randomized, double-blind, placebo-controlled study failed to achieve significant change in patients’ Neuropsychiatric Inventory (NPI) score following THC treatment. Possibly, the lack of positive results in this study can be attributed to the low THC dosage [20].
Though lacking sufficient data, current data on cannabinoids treatment in both Parkinson’s disease and dementia suggest cannabinoids treatment as beneficial, mainly for motor dysfunction in PD and neuropsychiatric complaints in dementia
CANNABINOIDS AND SLEEP DISTURBANCES IN THE ELDERLY
Changes in sleep patterns and quality of sleep are considered a natural part of aging. Poor sleep quality is often associated with impairment in quality of life, yet its effect on the elderly’s health is much greater. Difficulty maintaining sleep has been found to correspond with a more pronounced cognitive decline in elderly patients with normal cognition [21]. Moreover, in a 12 year follow-up in the general population, short sleepers with poor quality of sleep were found to have a 63% higher risk of cardiovascular disease [22]. Current treatment options include benzodiazepines, non- benzodiazepines and melatonin, which may subject patients to various adverse effects, some endangering their health.
• REM sleep behavior disorder (RBD): CBD appears to be a prospective line of treatment in patients suffering from both Parkinson’s disease and RBD. In a case series of four patients suffering from Parkinson’s disease and RBD, CBD treatment led to a remarkable reduction in frequency of symptoms [24
Studies examining cannabinoids treatment for sleep disturbances and weight loss in the elderly are scarce and insufficient for recommending treatment
CANNABINOIDS AND MALNUTRITION IN THE ELDERLY
Malnutrition and weight loss impose a great risk on the elderly’s health. The pernicious effect of weight loss and malnutrition affects numerous aspects of the elderly’s well-being. The relation between weight loss and mortality emphasizes the significant role of the nutritional state in patients’ prognosis. Moreover, weight loss was also associated with frailty, functional decline, and both severity and rate of disease progression in dementia [25].
Although in popular culture cannabis is known for giving one the “munchies” – a sensation of increased hunger following cannabis consumption – clinical trials exploring cannabis’ beneficial effect on the elderly suffering from cachexia are scarce. In light of the insufficient data, both the Cochrane Review (2009) and the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines on nutrition for patients with dementia (2015) concluded that current data do not support cannabis treatment for cachectic demented patients [16,25]. Nonetheless, preliminary results indicate a potential weight-gaining effect of cannabis. A small scale placebo-controlled crossover study conducted in 15 AD patients showed a significant increase in patients’ body weight and triceps skinfold thickness following treatment with dronabinol (CB1 receptor agonist) [15]. Another small trial examined the effect of dronabinol in patients with involuntary weight loss while residing in long-term care. Twenty-eight patients were followed over a 12 month period while receiving dronabinol for at least 12 weeks. The study’s results diverged, with 53% of patients gaining weight while 39% lost weight [26]. More promising results were accomplished in a recent retrospective study. The charts of 40 patients treated with dronabinol were examined, with mean duration of 16.88 days of dronabinol treatment. Although body weight did not significantly change, a significant change in the amount of food consumed was found. Owing to the limitation in the study’s design, it can be presumed that a longer duration of dronabinol treatment might elicit a change in body weight [17].
Cannabinoids are currently being utilized for a vast range of medical conditions, and although these conditions are not specific to elderly patients a potential efficacy for patients with these diseases can be presumed. Among others, these include chronic pain conditions [27], various cancer morbidities [28,29], epilepsy [30], systemic sclerosis [31], inflammatory bowel disease [32], dysautonomic syndrome [33] and many more [34].
• Adverse effects
The medical literature is abundant with reports of various adverse events, the largest number of adverse events reported to be about 8000, following cannabinoids consumption in their different forms. Among these adverse effects, tachycardia (37%–77%), agitation (16%–41%) and nausea (13%–94%) were the top rated complaints in numerous studies [3]. Since most of these studies were conducted in a general population further elaboration will not be provided.
Cannabinoids present a relatively safe profile of action in elderly patients. Hence, cannabinoid treatment should be considered more readily when other options fail, even in cases of scarce data
• Safety concerns
As with most drugs, cannabinoids lack targeted studies focusing on adverse reactions in the elderly. A recent systematic review set out to explore the safety of cannabinoids in elderly patients. Based on six different studies comprising a total of 260 patients, van den Elsen et al. [35] concluded that sedation/drowsiness was the most frequent complaint among patients. Importantly, no severe adverse effects were reported, apart from two patients with chronic obstructive pulmonary disease (COPD) who developed cardiac arrhythmias and one patient with grand mal seizure who died 2 months later from causes not related to cannabis. Two randomized, double-blind, placebo-controlled, cross-over trials examined the safety of Namisol® (a form of oral THC, Echo Pharmaceuticals, The Netherlands) given to healthy older subjects or demented patients. Among the healthy subjects drowsiness (27% of adverse events) was the most prevalent adverse effect, followed by dry mouth (11%) [36]. Patients suffering from dementia received either Namisol or placebo for a 6 week period (each) separated by a 4 day washout. Namisol treatment resulted in a significant increase in heart rate, VAS (Visual Analogue Scale) internal perception scores, and body sway with eyes closed, while systolic pressure decreased. Contrary to these results, VAS scores for “feeling high” (euphoric) and external perception, body sway with eyes open and diastolic pressure did not differ [37].
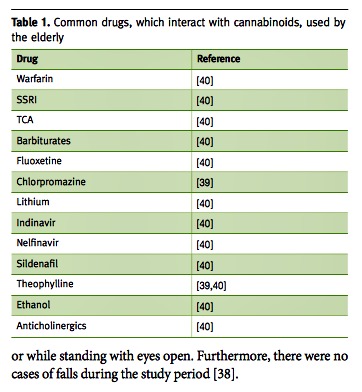
Elderly patients typically consume a large number of prescription drugs. Thus, drug interactions are a major concern for physicians treating elderly patients. Although cannabis is usually regarded as a safe alternative, it should be remembered that no drug is innocent, and the fact that its origin is “natural” does not ensure safety, as seen with digitalis and digoxin. Hence, prescribing medical cannabis should be under advisement with regard to possible interactions. Current data suggest that cannabis or some of its properties are metabolized by CYP3A4, CYP2C9 and CYP2C19, while possibly inducing CYP1A2. These enzymes affect some common drugs used by the elderly such as warfarin and sildenafil. For a more elaborate list see Table 1 [39,40].
In the last decade Cannabis sativa has rebranded itself from a common illicit drug to a new valid medical treatment for various conditions. In light of this new reality, physicians are confronted with a dilemma: the public demand for cannabis versus the lack of medical training regarding cannabis treatment. The dilemma is intensified in geriatric patients, considered to be a unique population due to frailty and over-medication. Thus, knowledge on the safety and therapeutic potential of cannabinoids is crucial when treating an elderly patient. Due to a paucity of research, cannabinoid treatment is predominantly an additive/replacement therapy, regarded as the last resort when conventional therapy fails. As such, in the natural course of Parkinson’s disease, cannabinoids can be utilized as a third-line or supplementary treatment, with evidence indicating their efficacy in reducing tremor, dyskinesia, rigidity and pain, and improving sleep. As in Parkinson’s disease, physicians treating patients with dementia often cannot offer effective treatment for the neuropsychiatric symptoms, and the result is treatment with possible deleterious effects. The use of medical cannabis in dementia appears to be a safe option for behavioral problems although clinical data are still inadequate. Other conditions for which evidence is scarce and preclude recommendations are sleep disturbances and weight loss; additional data are sorely needed. Lastly, the medical literature suggests that cannabinoids have a relatively safe profile for use in geriatrics, with drowsiness being the most common complaint. This safety profile is the core of cannabinoid treatment, making it a possible key player in elderly medical care.
correspondence
33 Rupin Street, Rehovot 7634538, Israel
phone: (972-8) 946-1888, email: itay.katz1@mail.huji.ac.il
references
- Waissengrin B, Urban D, Leshem Y, Garty M, Wolf I. Patterns of use of medical cannabis among Israeli cancer patients: a single institution experience. J Pain Symptom Manage 2015; 49 (2): 223-30.
- Mechoulam R, Hanus L. A historical overview of chemical research on cannabinoids. Chem Phys Lipids 2000; 108 (1-2): 1-13.
- Katz D, Katz I, Porat-Katz BS, Shoenfeld Y. Medical cannabis: another piece in the mosaic of autoimmunity? Clin Pharmacol Ther 2016. In Press
- Katz D, Katz I, Shoenfeld Y. Cannabis and autoimmunity – the neurologic perspective: a brief review. J Neurol Neuromed 2016; 1 (4): 11-15.
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid- terpenoid entourage effects. Br J Pharmacol 2011; 163 (7): 1344-64.
- More SV, Choi D-K. Promising cannabinoid-based therapies for Parkinson’s disease: motor symptoms to neuroprotection. Mol Neurodegener 2015; 10 (1): 17.
- García C, Palomo-Garo C, Gómez-Gálvez Y, Fernández-Ruiz J. Cannabinoid- dopamine interactions in the physiology and physiopathology of the basal ganglia. Br J Pharmacol 2016; 173 (13): 2069-79.
- Greenbaum L, Tegeder I, Barhum Y, Melamed E, Roditi Y, Djaldetti R. Contribution of genetic variants to pain susceptibility in Parkinson disease. Eur J Pain 2012; 16 (9): 1243-50.
- Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical marijuana) treatment for motor and non–motor symptoms of parkinson disease. Clin Neuropharmacol 2014; 37 (2): 41-4.
- Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology 2001; 57 (11): 2108-11.
- Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology 2004; 63 (7): 1245-50.
- Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 2014; 28 (11): 1088-98.
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dement 2013; 9 (1): 63-75.e2.
- Fernández-RuizJ,RomeroJ,RamosJA.EndocannabinoidsandNeurodegenerative Disorders: Parkinson’s Disease, Huntington’s Chorea, Alzheimer’s Disease, and Others. Springer International Publishing, 2015: 233-59.
- Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 1997; 12 (9): 913-19.
17. Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry 2014; 22 (4): 415-19.
18. Walther S, Mahlberg R, Eichmann U, Kunz D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl) 2006; 185 (4): 524-8.
19. ZajacDM,SikkemaSR,ChandrasenaR.Nabiloneforthetreatmentofdementia- associated sexual disinhibition. Prim Care Compan CNS Disord 2015; 17 (1).
20. vandenElsenGAH,AhmedAIA,VerkesR-J,FeuthT,vanderMarckMA,Olde Rikkert MGM. Tetrahydrocannabinol in behavioral disturbances in dementia: a crossover randomized controlled trial. Am J Geriatr Psychiatry 2015; 23 (12): 1214-24.
21. Johar H, Kawan R, Emeny RT, Ladwig K-H. Impaired sleep predicts cognitive decline in old people: findings from the prospective KORA age study. Sleep 2016; 39 (1): 217-26.
22. Hoevenaar-Blom MP, Spijkerman AMW, Kromhout D, van den Berg JF, Verschuren WMM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep 2011; 34 (11): 1487-92.
23. GatesPJ,AlbertellaL,CopelandJ.Theeffectsofcannabinoidadministrationon sleep: a systematic review of human studies. Sleep Med Rev 2014; 18 (6): 477-87.
24. Chagas MHN, Eckeli AL, Zuardi AW, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther 2014; 39 (5): 564-6.
25. VolkertD,ChourdakisM,Faxen-IrvingG,etal.ESPENguidelinesonnutrition in dementia. Clin Nutr 2015; 34 (6): 1052-73.
26. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant? – a pilot study. J Nutr Health Aging 2007; 11 (2): 195-8.
27. LynchME,CampbellF.Cannabinoidsfortreatmentofchronicnon-cancerpain; a systematic review of randomized trials. Br J Clin Pharmacol 2011; 72 (5): 735-44.
28. Turgeman I, Bar-Sela G. Cannabis use in palliative oncology: a review of evidence for popular indications. IMAJ 2017; 19: 85-8.
29. GolanH,FisherT,TorenA.Theroleofcannabinoidsinthetreatmentofchildren with cancer. IMAJ 2017; 19: 89-94.
30. Hausman-Kedem M, Kramer U. Efficacy of medical cannabis for treating refractory epilepsy in children and adolescents, with emphasis on the Israeli experience. IMAJ 2017; 19: 76-8.
31. Garcia-Gonzalez E, Galeazzi M, Selvi E. Can cannabinoids modulate fibrotic progression in systemic sclerosis? IMAJ 2016; 18 (3-4): 156-8.
32. Naftali T, Konikoff FM. Cannabis in inflammatory bowel diseases: from anecdotal use to medicalization? IMAJ 2017; 19: 95-7.
33. Palmieri B, Laurino C, Vadala M. Short-term efficacy of cannabidiol-enriched hemp oil in girls with dysautonomic syndrome after human papillomavirus vaccination. IMAJ 2017; 19: 79-84.
34. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use. JAMA 2015; 313 (24): 2456.
35. Van den Elsen GAH, Ahmed AIA, Lammers M, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev 2014; 14 (1): 56-64.
36. Ahmed AIA, van den Elsen GAH, Colbers A, et al. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. Eur Neuropsychopharmacol 2014; 24 (9): 1475-82.
37. Ahmed AIA, van den Elsen GAH, Colbers A, et al. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl) 2015; 232 (14): 2587-95.
38. van den Elsen GA, Tobben L, Ahmed AI, et al. Effects of tetrahydrocannabinol on balance and gait in patients with dementia: A randomised controlled crossover trial. J Psychopharmacol 2016; 0269881116665357
39. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev 2014; 46 (1): 86-95.
40. Lindsey WT, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse 2012; 38 (4): 334-43.