1Pediatric Hematology-Oncology Research Laboratory, Cancer Research Center, Sheba Medical Center, Tel Hashomer, Israel 2Department of Pediatric Hematology-Oncology, Safra Children’s Hospital, Sheba Medical Center, Tel Hashomer, Israel 3Sackler faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- phytocannabinoids, which are natural compounds present in the Cannabis sativa plant [1]
- endogenous cannabinoids, also known as endocannabinoids
- the synthetic analogs of both groups, called synthetic cannabinoids [2].The well studied cannabinoid is Δ9-tetrahydrocannabinol (THC), which is also the most active constituent of the plant. In addition to THC, cannabis has high concentrations of cannabidiol (CBD), a non-psychotropic constituent of the plant [3]. The two most studied compounds of the endocannabinoid system are anandamide (AEA) and 2-arachidonoylglycerol (2-AG). Commercially available synthetic cannabinoids and THC analogs include Sativex®( GW Pharmaceuticals, UK), Dronabinol® (AbbVie Inc., USA) and Nabilone® (Meda Pharmaceuticals Inc, Sweden), which are approved for the treatment of cancer related side effects [4].Cannabinoid activity is regulated by the endocannabinoid system (ECS), which comprises cannabinoid receptors, transporters, and enzymes involved in cannabinoid synthesis and breakdown. So far, two major cannabinoid-specific receptors – CB1 and CB2 – have been cloned and characterized from mammalian tissues [2]. These receptors are present throughout the body and regulate a variety of physiological functions, including neuronal development and energy metabolism. The distribution of CB1 and CB2 accounts for many of the observed effects associated with cannabis use. CB1 receptors appear to be ubiquitously located throughout the body, with the highest concentration of receptors found in the central nervous system [5]. CB2 receptor expression occurs mainly in the immune system, the highest expression in B lymphocytes which are involved in immune suppression and cell migration induction [2]. Both CB1 and CB2 belong to the large family of G protein- coupled receptors. Other receptors have been proposed to act as endocannabinoid receptors, including the transient receptor potential cation channel subfamily V member 1 (TRPV1) and certain orphan G protein-coupled receptors – GPR55, GPR119 and GPR18 [6].Cannabis has been used in folk medicine to alleviate pain, depression, amenorrhea, inflammation and numerous other medical conditions. In cancer patients specifically, cannabinoids are well known to exert palliative effects; their best-established use is the inhibition of chemotherapy-induced nausea and vomiting, but they are applied also to alleviate pain, stimulate appetite, and attenuate wasting [7]. More recently, cannabinoids have gained special attention for their role in cancer cell proliferation and death. A huge number of cannabinoids has been elucidated, and significant research has been undertaken to evaluate the therapeutic utility of these compounds in the treatment of cancer.
 The current state of cannabis use for both medical and recreational purposes is highly debated. While the legality of its use varies from country to country, possession of cannabis is illegal in most countries. In the United States, it is a controlled substance and is classified as a drug with a high potential for abuse and no currently accepted medical use. Despite this classification, many states in the United States have moved to decriminalize or legalize marijuana for medical or recreational use [8]. In addition, Canada, New Zealand and several countries in Europe approved a few synthetic cannabinoid analogs for spasticity associated with multiple sclerosis, as an adjunct analgesic for patients with advanced cancer, and for prevention of chemotherapy-induced nausea and vomiting. In Israel, cannabis for recreational purposes is illegal but it is approved for cancer patients and for patients with pain-related illnesses such as Parkinson’s, multiple sclerosis, Crohn’s disease, other chronic pain and post-traumatic stress disorder.
The current state of cannabis use for both medical and recreational purposes is highly debated. While the legality of its use varies from country to country, possession of cannabis is illegal in most countries. In the United States, it is a controlled substance and is classified as a drug with a high potential for abuse and no currently accepted medical use. Despite this classification, many states in the United States have moved to decriminalize or legalize marijuana for medical or recreational use [8]. In addition, Canada, New Zealand and several countries in Europe approved a few synthetic cannabinoid analogs for spasticity associated with multiple sclerosis, as an adjunct analgesic for patients with advanced cancer, and for prevention of chemotherapy-induced nausea and vomiting. In Israel, cannabis for recreational purposes is illegal but it is approved for cancer patients and for patients with pain-related illnesses such as Parkinson’s, multiple sclerosis, Crohn’s disease, other chronic pain and post-traumatic stress disorder.In contrast, physicians caring for cancer patients in the U.S. recommend medicinal cannabis for symptom management. A survey conducted in 2013 among 1446 physicians on their attitudes regarding the legalization of medical marijuana use found that 76% approved using it for a medical purpose. Most physicians in this study cited their “responsibility as caregivers to alleviate suffering” as their reason for support [8].
Regarding the use of cannabis in children and adolescents the dilemma and concern are even greater since there are no published studies on the use of medicinal marijuana or phar- maceutical cannabinoids in pediatric populations. Growing evidence suggests a differential effect of cannabis exposure on the human brain based on the age of exposure. In a recently published statement [10], the American Academy of Pediatrics (AAP) did not approve cannabis and cannabinoid use in children due to concerns about brain development and long-term cognitive effects. In the statement, the AAP warns that marijuana can affect memory and concentration and interfere with learning, motor control, coordination, judgment, reaction time, tracking ability, and enhanced vulnerability to addiction and psychiatric disorders in later life. Despite this, the AAP’s position does allow exceptions. It acknowledges that “marijuana may currently be an option for cannabinoid administration for children with life-limiting or severely debilitating conditions and for whom current therapies are inadequate.”
ANTI-CANCER EFFICACY OF CANNABINOIDS: PRECLINICAL EVIDENCE
The ability of cannabinoids to reduce tumor growth was reported for the first time by Munson et al. in 1975 [11]. They showed by in vitro and in vivo experiments that several phytocannabinoids, including THC, decreased Lewis lung adenocarcinoma proliferation in a dose-dependent manner. Nevertheless, it was not until the 2000s that the interest in these compounds as anti-cancer agents was renewed, predominantly due to the work of Guzman [12] in gliomas, and the demonstration of cannabinoids’ anti-cancer effects on various types of tumors [6]. The anti-tumorigenic effect of the endo- and phytocannabinoids was demonstrated in several in vitro and in vivo models of a wide variety of adult tumors including glioma, prostate, breast, leukemia, lymphoma, pancreas, melanoma, thyroid, colorectal and hepatocellular carcinoma tumors [13] [Table 1]. Significant levels of the cannabinoid receptors – CB1 and CB2 – were found to be highly expressed in these tumors especially in comparison with normal tissues [14,15], and were highly correlated to tumor progression, tumor aggressiveness and disease outcome [14].
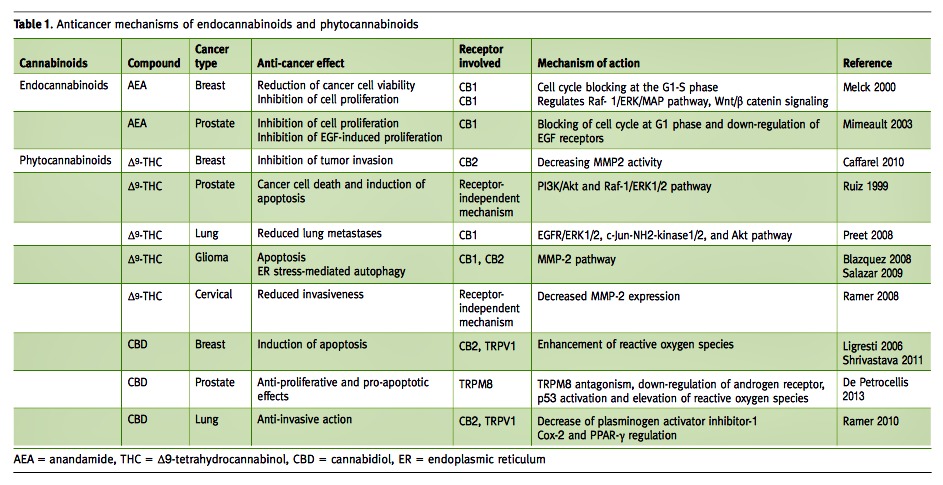
Our group studied the effects of administration of THC to glioblastoma multiforme (GBM) cell lines and showed that administration of THC results in a significant decrease in cell viability [Figure 1A] via a mechanism that appears to elicit G1 arrest [Figure 1B]. Western blot analyses revealed the THC-altered cellular content of proteins that regulate cell progression through the cell cycle. The cell content of E2F1 and cyclin A, two proteins that promote cell cycle progression, were suppressed whereas the level of p16INK4A, a cell cycle inhibitor, was up-regulated [Figure 1C]. The decrease in E2F1 levels resulted from proteasome-mediated degradation and was prevented by proteasome inhibitors [Figure 1D+E] [21].
ANTI-CANCER EFFICACY OF CANNABINOIDS: CLINICAL EVIDENCE
Clinical trials conducted on medicinal cannabis are limited. However, the emergence of preclinical studies demonstrating the anti-tumor effects of cannabinoids are growing in number and have formed the basis of limited clinical studies that are beginning to shed light on the translational value of the preclinical work. There has been only one clinical trial examining the effects of THC on cancer [12]. In this first phase I human study Guzmán et al. [23] studied intracranial administration of THC to nine patients with recurrent GBM. Treatment with THC decreased tumor growth and tumor progression, as assessed by magnetic resonance imaging and biomarker expression, in at least two of the nine patients studied (Clinical trial ID# NCT01812603). Importantly, intracranial administration of THC was found to be a safe and tolerable approach with no apparent psychoactive effects. However, the study is limited by the small sample size, lack of a control group, and the study design’s inability to comment on the effects of THC on survival time [23].
There are no published clinical studies describing the use of cannabinoids in pediatric patients. There are, in fact, only a few anecdotal case reports that demonstrate regression of tumor during cannabinoid treatment that was given with palliative intent to terminally ill children [24,25].
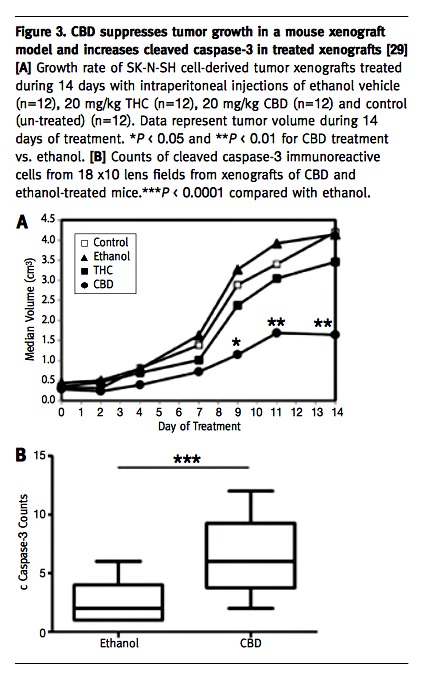
Basic research exploring the putative anti-tumorigenic properties of cannabinoids in pediatric tumors is still limited, and the molecular mechanisms underlying the anti-tumorigenic effect are poorly understood. The anti-tumorigenic activity of cannabinoids was studied so far in only two pediatric tumors: alveolar rhabdomyosarcoma [26] and osteosarcoma [27]. We therefore investigated the role of cannabinoids in another aggressive pediatric tumor, neuroblastoma (NBL), which is the most frequent extracranial solid tumor of childhood and which still carries a very poor prognosis despite a multimodal and intensive therapy [28]. The results obtained in our in vitro studies showed that THC and in particular CBD reduced the viability and invasiveness of NBL cells. The effect of CBD seemed to be mediated by apoptotic cell death, as demonstrated by morphology changes, annexin V assay, and increased expression of cleaved caspase 3 [Figure 2A-D]. Based on that first set of results, we studied the effect of CBD and THC on xenograft tumors generated in NOD/SCID mice from the NBL cell line SK-N-SH that already demonstrated the greatest sensitivity to the effect of those molecules in vitro. In accordance with the findings from the in vitro experiments, THC and CBD both reduced the xenograft growth rate, with CBD showing a superior effect [29]. The results obtained in our study indicate that of the two cannabinoids tested CBD was more effective on the NBL cell line and on xenografts in comparison to THC. As a potential therapeutic agent, CBD could have many advantages compared with psychoactive THC [30] because most – if not all – of the psychoactive effects of cannabinoids are produced by activation of the central CB1 receptors. CBD, which has been shown to act independently of CB1, is devoid of psychoactive effect [31] and can serve as a more suitable treatment, especially in children. Additionally, CBD shares the palliative properties and low toxicity profile described for other cannabinoids, has none of the strong side effects associated with chemotherapeutic agents, and might have synergistic activity with well established anti-neoplastic substances.
SUMMARY
Overall, the cannabinoids, and specifically the non-psychoactive CBD, may show future promise in the treatment of cancer, but there are still many significant hurdles to be overcome. Questions surrounding the effects of chronic cannabinoid use continue to increase especially with regard to its use in pediatric patients. Currently, much of the data are based on animal data and small trials and are outdated. Moreover, the results from studies lack sufficient depth of understanding and do not allow the acceptance and use of cannabinoid outside a clinical study.
Finally, while most eyes have been set on the presidential race in the U.S. during the 2016 election, additional states vote to legalize the use of marijuana. This decision reflects an increasing change and a shift in attitudes to cannabis use and reinforces the need to further conduct more robust preclinical and clinical studies that will increase our knowledge on the effectiveness and consequences of the use of these compounds in general and in children in particular.
dr. a. toren
Dept. of Pediatric Hematology-Oncology, Safra Children’s Hospital, Sheba Medical Center, Tel Hashomer 52621, Israel
Phone: (972-3) 530-3037
Fax: (972-3) 530-3031
email: amost@post.tau.ac.il
references
1. Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Lett 2009; 285 (1): 6-12.
2. Fraguas-Sanchez AI, Fernandez-Carballido A, Torres-Suarez AI. Phyto-, endo- and synthetic cannabinoids: promising chemotherapeutic agents in the treatment of breast and prostate carcinomas. Exp Opin Invest Drugs 2016; 25 (11): 1311-23.
3. Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol – recent advances. Chem Biodivers 2007; 4 (8): 1678-92.
4. Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol 2009; 156 (3): 397-411.
5. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol 2013; 64: 21-47.
6. Velasco G, Sanchez C, Guzman M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer 2012; 12 (6): 436-44.
7. Mechoulam R, Hanu L. The cannabinoids: an overview. Therapeutic implications in vomiting and nausea after cancer chemotherapy, in appetite promotion, in***
- Wilkie G, Sakr B, Rizack T. Medical marijuana use in oncology: a review. JAMAOncol 2016; 2 (5): 670-4.
- Koppel BS, Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014; 82 (17): 1556-63.
- Ammerman S, Ryan S, Adelman WP. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics 2015; 135 (3): e769-85.
- MunsonAE,HarrisLS,FriedmanMA,DeweyWL,CarchmanRA.Antineoplastic activity of cannabinoids. J Natl Inst 1975; 55 (3): 597-602.
- Guzman M. Cannabinoids: potential anticancer agents. Nature Rev Cancer 2003; 3 (10): 745-55.
- Romano B, Borrelli F, Pagano E, Cascio MG, Pertwee RG, Izzo AA. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2014; 21 (5): 631-9.
- Qamri Z, Preet A, Nasser MW, et al. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther 2009; 8 (11): 3117-29.
- Sarfaraz S, Afaq F, Adhami VM, Mukhtar H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res 2005; 65 (5): 1635-41.
- Blazquez C, Casanova ML, Planas A, et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J 2003; 17 (3): 529-31.
- CridgeBJ,RosengrenRJ.Criticalappraisalofthepotentialuseofcannabinoids in cancer management. Cancer Manag Res 2013; 5: 301-13.
- Ramer R, Bublitz K, Freimuth N, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J 2012; 26 (4): 1535-48.
- Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol 2005; 144 (8): 1032-6.
21. Galanti G, Fisher T, Kventsel I, et al. Delta 9-tetrahydrocannabinol inhibits cell cycle progression by downregulation of E2F1 in human glioblastoma multiforme cells. Acta Oncol 2008; 47 (6): 1062-70.
22. McKallip RJ, Nagarkatti M, Nagarkatti PS. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol 2005; 174 (6): 3281-9.
23. Guzman M, Duarte MJ, Blazquez C, et al. A pilot clinical study of Delta9- tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer 2006; 95 (2): 197-203.
24. Foroughi M, Hendson G, Sargent MA, Steinbok P. Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas – possible role of Cannabis inhalation. Childs Nerv Syst 2011; 27 (4): 671-9.
25. Singh Y, Bali C. Cannabis extract treatment for terminal acute lymphoblastic leukemia with a Philadelphia chromosome mutation. Case Rep Oncol 2013; 6 (3): 585-92.
26. Oesch S, Walter D, Wachtel M, et al. Cannabinoid receptor 1 is a potential drug target for treatment of translocation-positive rhabdomyosarcoma. Mol Cancer Ther 2009; 8 (7): 1838-45.
27. Notaro A, Sabella S, Pellerito O, et al. Involvement of PAR-4 in cannabinoid- dependent sensitization of osteosarcoma cells to TRAIL-induced apoptosis. Int J Biol Sci 2014; 10 (5): 466-78.
28. Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am 2015; 62 (1): 225-56.
29. Fisher T, Golan H, Schiby G, et al. In vitro and in vivo efficacy of non- psychoactive cannabidiol in neuroblastoma. Curr Oncol 2016; 23 (2): S15-22.
30. Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol 2013; 75 (2): 303-12.
31. Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ. The role of CB1 receptors in psychostimulant addiction. Addict Biol 2008; 13 (2): 225-38.
“Cowardice asks the question, ‘Is it safe?’ expediency asks the question, “Is it politic?” vanity asks the question, “Is it popular?” but, conscience asks the question, “Is it right?” And there comes a time when one must take a position that is neither safe, nor politic, nor popular but one must take it because one’s conscience tells one that it is right”
Martin Luther King, Jr (1929-1968), American Baptist minister and leader in the Civil Rights Movement. He helped organize the 1963 March on Washington, where he delivered his famous “I Have a Dream” speech. In 1964 King received the Nobel Peace Prize. following his assassination in Memphis, Tennessee in 1968, riots broke out in many U.S. cities. King was posthumously awarded the Presidential Medal of freedom and the Congressional Gold Medal. Martin Luther King Jr. Day was established as a holiday in numerous cities and states and as a U.S. federal holiday in 1986. Hundreds of streets in the U.S. have been renamed in his honor. The Martin Luther King Jr. Memorial on the National Mall in Washington, D.C., was dedicated in 2011


