Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Oct 11;7(1):171–182. doi: 10.1159/000540353
Abstract
Introduction
Cannabidiol (CBD) has sparked considerable interest because of its wide range of pharmacological uses and the fact that it does not induce psychoactive effects. CBD formulation development presents significant challenges due to its limited water solubility and susceptibility to first-pass metabolism, both of which restrict its overall bioavailability. The current research aimed to use hot-melt extrusion (HME) technology to develop mucoadhesive buccal films to improve CBD solubility and reduce first-pass metabolism.
Methods
Five formulations containing 10% w/w CBD were extruded using a counter-rotating twin-screw extruder (Haake Minilab II, Thermo Fisher Scientific). Different characterization studies were conducted on the developed formulations.
Results
Differential scanning calorimetry (DSC) revealed that the CBD endothermic peak disappeared in some of the developed films, indicating that CBD was converted from crystalline to amorphous form. A bio-adhesion study showed that the formulations containing Carbopol® (BF2, BF3, BF4, and BF5) had higher adhesiveness properties. In vitro release and solubility studies showed an increase in CBD release and water solubility in the developed formulations when compared to pure CBD. Stability studies revealed that CBD content and release in the lead formulation (BF2) was stable over 15 months.
Conclusion
The current study demonstrates that HME was successfully used as an approach to develop CBD mucoadhesive buccal films and CBD solubility was enhanced.
Introduction
Natural products and their structural analogs have consistently been vital contributors to the field of pharmacotherapy. In contrast to conventional synthetic molecules, natural products exhibit distinctive attributes that offer both advantages and challenges to the drug discovery process [1]. As an example, cannabidiol (CBD) is a crystalline compound isolated from a high CBD chemovar of Cannabis sativa plant. It has been reported to possess various pharmacological activities such as antipsychotic, anti-inflammatory, anti-seizure, and anxiolytic effects [2]. Its antitumor activity has also been reported in breast carcinoma cases as it slows the proliferation of tumor cells [3]. Unlike Δ9-tetrahydrocannabinol (Δ9 -THC) which is another pharmacologically active component isolated from cannabis, CBD has no psycho-activity, which can be attributed to its low binding affinity to CB1 endocannabinoid receptor. Moreover, it behaves as CB2 receptor inverse agonist [4]. On the other hand, CBD formulation development faces some challenges due to its high lipophilicity (log P of 6.3) [5, 6], low water solubility (0.1 µg/mL), and excessive first-pass metabolism, consequently leading to a low oral bioavailability estimated at 6% [7]. Additionally, many studies have revealed that CBD is susceptible to degradation by light, temperature, and oxidation, which poses significant challenges in its formulation development [8, 9]. Currently, Epidiolex® (sesame oil-based solution, 100 mg CBD/mL) is the only commercially available preparation containing CBD that has been approved by the US-FDA for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndromes [10, 11]. Consequently, it is essential to focus on the development of different CBD formulations with higher solubility and oral bioavailability.
Oral drug delivery is the most common and preferred route of administration whenever feasible due to its ease of administration and patient compliance [12]. Despite these advantages, many drugs face challenges in being delivered effectively through the conventional oral route due to different reasons. The most popular challenges are the first-pass metabolism (pre-systemic clearance in the liver) and the low aqueous solubility which lead to a decrease in oral bioavailability [13]. Parenteral drug delivery serves as an alternative route for administering drugs with limited oral bioavailability; however, it is not the preferred choice for patients. Hence, there arises a demand for alternative means of administering such drugs. The use of a buccal drug delivery system presents a promising alternative to traditional oral administration, particularly for drugs affected by first-pass metabolism [14, 15]. This system facilitates rapid drug transport to the systemic circulation and bypasses the first-pass hepatic metabolism, ultimately achieving higher bioavailability [16]. The term buccal refers to the lining of the cheek, which represents one-third of the total oral mucosal surface [16]. The buccal mucosa features smooth muscle and exhibits relative permeability, benefiting from a rich blood supply. It also serves as a passive absorption for drugs, and the presence of saliva provides a higher amount of water for drug dissolution compared to the rectal or transdermal routes [17]. In the development of buccal formulations, mucoadhesiveness is an important property that enhances the attachment of the dosage form to the mucous membrane and extends the contact time between the drug and the membrane [18]. To achieve proper drug release and mucoadhesive properties in buccal formulation development, the selection of polymer blends for film formulation is essential [19]. Previous studies have demonstrated the therapeutic efficacy of various active pharmaceutical ingredients, such as propranolol, salbutamol, and progesterone, when formulated as mucoadhesive buccal formulations, surpassing the effectiveness of conventional oral dosage forms [20–22]. Film preparations are typically produced using the solvent-casting method. This method involves dissolving polymers and excipients, such as plasticizers, in a suitable solvent to create a clear, viscous solution or gel [23]. The solution is then allowed to undergo solvent evaporation to form a uniform and flexible film. Additionally, solvent-cast films may require the use of organic solvents for water-insoluble polymers, which can be hazardous and may leave behind residual solvents after drying. The selection of an appropriate solvent can also be challenging, and the associated waste disposal creates significant environmental concerns [24, 25]. Thus, there is a need to find an alternative method for the preparation of buccal films, and hot-melt extrusion (HME) stand out as one of the alternative techniques that has been utilized in the development of different buccal films [26].
HME has been used in pharmaceutical applications since the mid-nineteenth century and was first developed for manufacturing lead pipes in the late 18th century [27]. HME involves melting and mixing drug molecules with hydrophilic polymeric carriers at temperatures above their glass transition temperatures or melting temperatures [28]. This continuous, solvent-free process enhances the solubility of poorly water-soluble drugs by transforming them from a crystalline state to an amorphous state [29]. HME can also be used to develop modified-release systems and mask bitter tastes [30]. It is suitable for developing various dosage forms such as tablets, capsules, films, and implants for oral, transdermal, and trans-mucosal routes of drug delivery [31]. HME technology has gained significant attention, especially after the US-FDA recommended the use of continuous manufacturing processes [32]. On the other hand, HME faces some challenges as a thermal process, where the drug and polymer thermal stability should be considered; therefore, it is not suitable for heat-sensitive pharmaceutical active ingredients [29, 33].
The present study aimed to explore the utilization of HME technology for the preparation of CBD mucoadhesive buccal films, to enhance CBD water solubility and oral bioavailability. It is essential to identify suitable key ingredients in order to formulate CBD mucoadhesive films by HME. The selection of an appropriate polymer holds importance in HME technology, wherein a variety of polymers, both biodegradable and non-biodegradable, has been employed [34]. In this study, polyethylene oxide-N80 (PEO-N80) and hydroxypropyl cellulose (klucel™ EF) were chosen as polymeric carriers for CBD. All the prepared films were evaluated for various characteristics.
Materials and Methods
Materials
CBD was isolated from CBD cannabis chemovar grown at the University of Mississippi with a purity of 99.8% as determined by HPLC. PEO-N80 was gifted from Colorcon (Harleysville, PA, USA). Copolymers of polyacrylic acid (Carbopol® 980 and 934) were gifted by Lubrizol (Wickliffe, OH, USA). D-α tocopherol (vitamin E) polyethylene glycol succinate (Kolliphor® TPGS) was obtained as a gift from BASF corporation (Florham Park, NJ, USA), and hydroxypropyl cellulose (Klucel™ EF) was also received as a gift from Ashland specialty ingredients (Wilimington, DE, USA). All other chemicals were of HPLC analytical grade and were purchased from Fisher Scientific (Fair Lawn, NJ, USA).
Methods
HME Process
Five formulations (BF1, BF2, BF3, BF4, and BF5) were developed using HME. The compositions of these formulations are shown in Table 1. PEO-N80 (molecular weight 200,000, viscosity 65-115 cp) and klucel™ EF (molecular weight 80,000, viscosity 200-600 cp) were used as the polymeric carriers for the CBD. Kolliphor® TPGS served as a stabilizer to prevent CBD from oxidizing. Additionally, Carbopol® was used as a mucoadhesive substance. Batches of 10 g physical mixtures (PMs) were prepared as follows: TPGS was melted and mixed with the other excipients, then 10% w/w CBD was added and geometrically mixed. The degradation temperature of CBD was determined using a thermogravimetric analyzer (TGA) model TA TGA55 before the extrusion process. The resulting PMs were fed into HAAKE Minilab II (Thermo Fisher Scientific, Waltham, MA, USA), at 110°C, and 50 rpm. A 4.5 mm × 1 mm film die was used to shape the CBD films. All the developed films went under different characterization studies.
Table 1.
CBD films formulations composition
| Name | PEO-N80 (% w/w) | KlucelTM EF (% w/w) | Kolliphor® TPGS (% w/w) | Carbopol® 980 (% w/w) | Carbopol® 934 (% w/w) | CBD (% w/w) |
|---|---|---|---|---|---|---|
| BF 1 | 85 | 0 | 5 | 0 | 0 | 10 |
| BF2 | 75 | 0 | 5 | 10 | 0 | 10 |
| BF3 | 75 | 0 | 5 | 0 | 10 | 10 |
| BF4 | 0 | 75 | 5 | 10 | 0 | 10 |
| BF5 | 37.5 | 37.5 | 5 | 10 | 0 | 10 |
PEO-N80, polyethylene oxide-N80; KlucelTM EF, hydroxypropyl cellulose; Kolliphor® TPGS, vitamin E polyethylene glycol succinate; CBD, cannabidiol.
Analytical Method and Chromatographic Conditions
The HPLC analytical method used to quantify CBD was based on a previously published method [35]. The chromatographic system employed was a Waters HPLC-UV system (Waters Corporation, Milford, MA, USA) with a Phenomenex Luna column [5 µm C18 (2) 100 Å, LC, 150 × 4.6 mm]. The mobile phase was a mixture of 23% v/v water and 77% v/v acetonitrile with 0.1% v/v formic acid, at a flow rate of 1 mL/min. CBD detection occurred at 220 nm with an injection volume of 10 µL. A five-point calibration curve was constructed with concentrations ranging from 5 to 100 µg/mL. The run time for the method was 10 min, with a CBD retention time at 7.5 min. Regression analysis of the data points produced a calibration curve with an R2 value >0.999.
Drug Content and Content Uniformity Test
The drug content of the developed CBD films was evaluated using the abovementioned HPLC analytical method. Samples (20–30 mg) were diluted 200 times with acetonitrile for CBD extraction and then analyzed for CBD content. CBD content uniformity in the prepared films was detected by taking random samples from different film segments. All experiments were conducted in triplicates.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC; TA DSC 25) was used to investigate the thermal characterization of CBD, polymeric carriers, and determine the physical state of CBD in the PMs of BF2 and BF4 (PM2 and PM4) and the developed HME films (BF1, BF2, BF3, BF4, and BF5). Samples were weighed in Tzero pans (3–5 mg), the pans were covered with Tzero lids and hermetically sealed. The test included one heating cycle for each sample with a temperature elevated from 25°C to 200°C at a heating rate of 10°C/min under a flow of ultrahigh purity nitrogen gas.
In vitro Adhesion Test
Texture Analyzer TA. XT2i (Texture Technologies, Hamilton, MA, USA) was used to study the adhesiveness properties of the developed CBD buccal films. Random Samples from each film (n = 5) were cut and hydrated with prepared simulated saliva fluid. The film was fixed on a die (TA-303 Indexable Adhesive Test Rig) using a double-faced tap. The rig was positioned on the lower base of the TA apparatus and a circular steel probe (TA-57R) with a diameter of 7 mm approached the film at test speed 0.1 mm/s, applied force 5.000 N and a contact time of 60 s. The probe attached to the film then separated at a speed 0.1 mm/s. The peak force, adhesiveness, and work of adhesion were determined and recorded for each film.
In vitro Drug Release Studies
An in vitro drug release study was carried out using a multi-position stirring hot plate (IKA® RT10). The in vitro release testing was conducted under non-sink conditions to differentiate between the pure drug and the developed formulations. Three films from each formulation, each weighing 100 mg and containing 10 mg of CBD, along with three samples of pure CBD, each weighing 10 mg, were tested to ensure all samples had the same CBD dose. The dissolution medium consisted of 100 mL of simulated saliva (pH 6.8) with 0.1% w/v sodium lauryl sulfate (SLS) at 37 ± 0.5°C and stirred at 250 rpm. Samples of 1.5 mL were withdrawn at intervals of 15, 30, 60, 90, 120, and 180 min; additionally, the release test for BF4 was extended to 240 min. An equal volume of fresh medium added to replace the withdrawn aliquots. The samples were then centrifuged at 16,200 g for 10 min, and the supernatants were analyzed using the specified HPLC method to quantify the percentage of CBD released. All experiments performed in triplicate.
Swelling Index
To conduct the swelling test, the initial weight of each film was measured and recorded (W1). Then the film was placed in a petri dish containing 10 mL of phosphate buffer (pH 6.8). The film was removed carefully from the petri dish at time points, 10 and 30 min. The excess solvent was dried using a paper towel. The weight of the swelled film was recorded (W2).
The swelling index was calculated by using the following formula:
| %=(2−1)1×100 |
where, SI = swelling index, W1 = original weight of film, W2 = weight of swelled film.
Solubility Studies
Solubility studies were performed in both distilled water and dissolution medium (pH 6.8 with 0.1% w/v SLS). In distilled water, the solubility of CBD in the developed films (BF1, BF2, and BF3) was compared to pure CBD to assess the enhancement of CBD aqueous solubility in the prepared buccal films. In the dissolution, medium solubility tests included pure CBD, BF1, BF2, BF3, and their PMs (PM1, PM2, and PM3). Excess amounts of CBD film, PMs (PM), or pure CBD (n = 3) were added to 5 mL of either distilled water or pH 6.8 + 0.1% SLS in 20-mL scintillation vials. The samples were shaken on a horizontal shaker (VWR microplate shaker) at room temperature for 48 h. Samples were withdrawn at 48 h, diluted as necessary, and centrifuged at 16,200 g for 20 min. The supernatants were analyzed using HPLC to evaluate the CBD concentration.
Fourier Transform Infrared Spectroscopy (FTIR)
CBD, PEO-N80, Carbopol® and the developed film (BF1, BF2, and BF3) were subjected to Fourier transform infrared spectroscopy (FTIR) spectral analysis using an Agilent Cary 660 FTIR spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). This FTIR test aimed to explore any potential interactions between CBD and other excipients within the formulation. The scanning range was set between 600 and 4,000 cm−1.
Stability Evaluation at Room Temperature
The stability study was conducted for the film formulations, BF1, BF2, and BF3. The Films were stored at room temperature for 15 months, and the CBD content was evaluated using HPLC. Additionally, BF2 as the lead formulation was evaluated for the drug release within the period of the stability study to detect any significant change in CBD release at room temperature storage condition.
Statistical Analysis
The statistical analysis was performed using JASP software (JASP-0.18.1.0). Paired sample T tests were performed on the release study results and the solubility study results of each formulation to compare between each formulation and pure CBD. A p value below 0.05 was considered statistically significant.
Results and Discussion
Formulation Composition and HME Process
The composition of the developed formulations is presented in Table 1. PEO-N80 and klucel™ EF were used as polymeric carriers and film formers. The adhesiveness properties are crucial for the buccal formulations and various components were reported as mucoadhesive substances [36]. In this study, Carbopol®, a copolymer of polyacrylic acid, was used for its mucoadhesive properties. Given CBD’s susceptibility to degradation from light and temperature, a stabilizer (antioxidant) was necessary. Kolliphor® TPGS was incorporated to maintain the drug’s stability in the formulated product, as it is known to act as an antioxidant stabilizer in drug delivery systems [37].
To optimize the extrusion process parameters (temperature and screw speed), different placebos were extruded and visually evaluated. The melting point of PEO-N80 (69°C) is close to that of CBD (66°C), while the degradation temperature of CBD, evaluated using TGA, was found to be at 230°C. Therefore, the initial extrusion temperature was set and tested at 90°C and 50 rpm. However, the prepared films were rough, brittle, and contained many air bubbles, indicating that 90°C was not optimal. The temperature was then adjusted to 110°C and 50 rpm, allowing for proper melting and dispersion of CBD within the polymeric matrix, and five formulations (BF1, BF2, BF3, BF4, and BF5) were prepared using these optimized conditions.
Throughout the entire process, the torque remained low and stable (15–20 Ncm) during the preparation of both placebos and CBD-loaded formulations, indicating a smooth and efficient process. All the developed films were white to yellowish white, with a uniform shape, smooth surface texture, and good elasticity.
Drug Content
The drug content results (Table 2) showed that CBD content in the developed buccal films was in the range (96.9–100.3), with relative standard deviation ≤5% for the tested samples. The samples were taken from different segments (N = 3), and the results indicated that CBD in all developed films was uniformly distributed.
Table 2.
Drug content for the developed CBD films ± SD (n = 3)
| Formulation name | CBD (%) |
|---|---|
| BF1 | 99.28±2.40 |
| BF2 | 100.22±0.26 |
| BF3 | 100.31±1.39 |
| BF4 | 98.60±1.18 |
| BF5 | 96.90±3.62 |
Differential Scanning Calorimetry
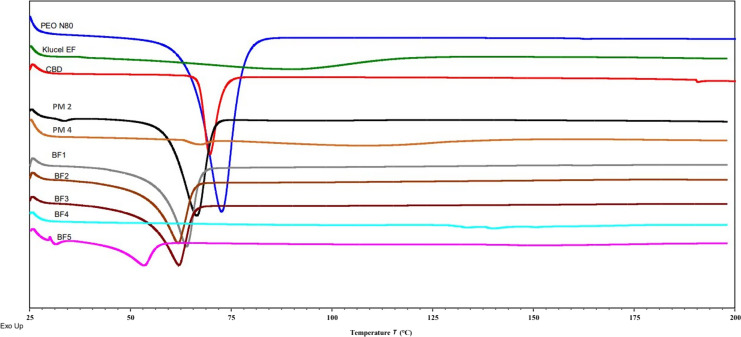
DSC study was performed to detect the thermal behavior of pure CBD, PEO-N80, the PMs of BF2 (PM2) and BF4 (PM4), and the prepared films (BF1, BF2, BF3, BF4, and BF5). The thermograms illustrated in Figure 1 showed that the pure CBD and PEO-N80 have endothermic melting peaks at 66°C and 69°C, respectively. DSC thermogram of the PMs showed the presence of CBD endothermic peak in PM4 which verifies that CBD in the crystalline form and the way that used for its mixing with the excipients did not affect its physical state. However, the PM of BF2 (PM2) which contains PEO-N80 as a polymeric carrier, showed a single endothermic peak resulting from the overlapping of the PEO-N80 melting peak and the CBD melting peak. The CBD films developed with the inclusion of PEO-N80 (BF1, BF2, BF3, and BF5) exhibited an endothermic peak in the temperature range of 60°C–65°C; it is an indication of the endothermic behavior of PEO-N80 and suggesting the possible disappearance of CBD endothermic peak. The endothermic peak observed in BF5 was smaller compared to other formulations due to the lower concentration of PEO-N80 at 37.5%. This results align with prior literature showing that PEO-N80 remains crystalline with its endothermic peak in the HME products [38]. Conversely, the absence of endothermic peaks in BF4 suggests the potential amorphization of CBD and supports the capability of HME to convert the drug from its crystalline form to the amorphous form in the presence of a suitable and compatible polymer. It has been reported that changing the drug’s physical state from crystalline to amorphous boosts the solubility of drugs with poor water-solubility [39].
Fig. 1.
In vitro Adhesion
The adhesion test was conducted with a contact time of 60 s to ensure the firm attachment of the CBD film on the adhesive membrane. The difference in the adhesiveness of the developed films was evaluated using a variety of parameters, the most important of which was the force of adhesion, which quantifies the force required to separate the bioadhesive films from the mucosal membrane. Additionally, adhesiveness, which refers to the ability of different surfaces to stick together, and work of adhesion, which represents the energy expended when two adherent surfaces are separated and moved apart.
As shown in Table 3, the results demonstrate higher peak force, work of adhesion, and adhesiveness for the formulation containing two different grades (980 and 934) of Carbopol® (BF2 and BF3) when compared to BF1 which lacks Carbopol® in its composition. It was observed also that BF5 has higher adhesiveness properties than BF4 due to the presence of PEO-N0 in its composition which explained that PEO-N80 has some adhesiveness characteristics. This study was essential since the adhesive characteristics play a pivotal role in mucoadhesive buccal formulations to enhance the adherence of the dosage form to the mucous membrane.
Table 3.
Adhesiveness test measurements for the developed CBD films ± SD (n = 3)
| Test ID | Peak force, N | Adhesiveness | Work of adhesion, Nmm |
|---|---|---|---|
| BF1 | 3.49±0.38 | 0.72±0.13 | 4.6±0.07 |
| BF2 | 4.086±0.17 | 1.97±0.90 | 7.03±0.46 |
| BF3 | 4.00±0.37 | 1.96±1.00 | 7.28±1.10 |
| BF4 | 1.36±0.08 | 1.22±0.32 | 0.85±0.14 |
| BF5 | 3.01±0.36 | 2.06±0.35 | 2.92±0.09 |
Carbopol® was reported as a high molecular weight synthetic resinous copolymer of acrylic acid with attributes resembling water-soluble natural gums including high aqueous solubility, biodegradability, and bio-adhesiveness [40]. Previous studies have consistently supported the utilization of different grades of Carbopol® as mucoadhesive substances; loxoprofen hydrogels was developed with sufficient bioadhesive strength owing to the presence of Carbopol® in the formulation composition [40]. It was also revealed that Carbopol® had high bioadhesive strength in the development of bilayer nicotine mucoadhesive patches [41]. The findings of this study support Carbopol’s ability to adhere to surfaces and its importance as a mucoadhesive substance when used in formulation development.
In vitro Drug Release
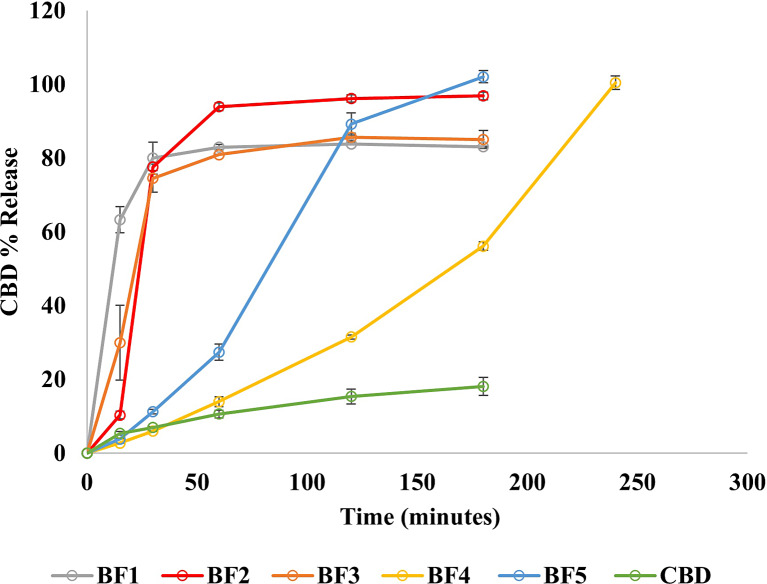
The in vitro drug release results showed an enhancement in CBD release in all the developed CBD buccal films compared to pure CBD as shown in Figure 2. The release study for BF1, BF2, BF3, and BF5 was performed for 180 min to ensure that CBD remained dissolved in the dissolution medium and that saturation solubility was achieved. The release test for BF4 was extended to 240 min as the drug release continued to increase until reaching 100% after 240 min. Each CBD formulation showed significant enhancement in the CBD release compared to pure CBD (p value <0.05). Approximately, 83–100% CBD was released from the developed films compared to 18% CBD dissolved from pure CBD samples under the same condition.
Fig. 2.
The main target of this study was to develop an immediate-release formulation with a high release rate. The amount of the drug released from the developed buccal films was affected by the nature of the polymers and the other excipients in the formulation composition. For this study, Klucel™ EF and PEO-N80 were selected as polymeric carriers. Klucel™ EF was preferred owing to its incorporation in immediate-release formulation development as it has an approximate molecular weight of 80,000 Da and undergoes a faster erosion process [42]. PEO-N80 was also considered as a low molecular weight (200,000 g/mol) water-soluble resin utilized in the preparation of immediate-release dosage forms [43]. The results illustrate that using PEO-N80 and Klucel™ EF as CBD polymeric carriers was successful in the enhancement of CBD solubility and dissolution rate. BF1, BF2, and BF3 showed faster release (within 60 min) than BF4 and BF5 which related to the specific used polymer in each formulation. This difference in the release profile is coming from the difference in the polymer viscosity because Klucel™ EF (in BF4 and BF5) has a higher viscosity (200–600 Cp) than PEO-N80 (65–115 Cp) which was used in other formulations (BF1, BF2, and BF3). Additionally, BF2 showed higher CBD release than other formulations within 60 min. Therefore, it was considered as the lead formulation in this study.
Recent studies have emphasized employing PEO-N80 to achieve an immediate release profile and enhance the drug release for oral films of adipic acid and nifedipine/indomethacin fixed-dose combinations [44, 45]. Despite some reports indicating that Carbopol®, with its high molecular weight and swelling properties, has been employed to induce controlled drug release [46, 47], its incorporation at a low concentration (10%) in formulations BF2, BF3, BF4, and BF5 did not impact or delay the drug release. The difference in the release between BF2 and BF3 resulted from the difference in the viscosity of Carbopol® 980 and Carbopol® 934.
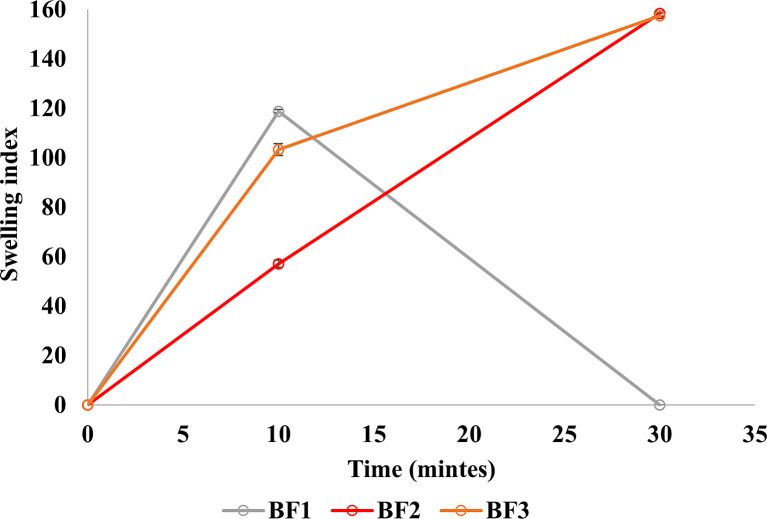
Swelling Index
The swelling characteristics play a crucial role in enhancing hydration and, consequently, the adhesion qualities of mucoadhesive buccal films [19]. The results of the swelling test (Fig. 3) highlight that BF2 and BF3 exhibited maximum swelling capacity at 30 min, attributed to the inclusion of Carbopol® in conjunction with PEO-N80. In contrast, BF1 displays swelling at 10 min due to the presence of PEO-N80 but dissolved after this point so could not be handled and reweighed at 30 min, which may be attributed to the absence of Carbopol®. These findings affirm the importance of Carbopol® in enhancing the swelling characteristics of the formulation, given its capacity to hydrate and swell in an aqueous phase [41].
Fig. 3.
Solubility Studies
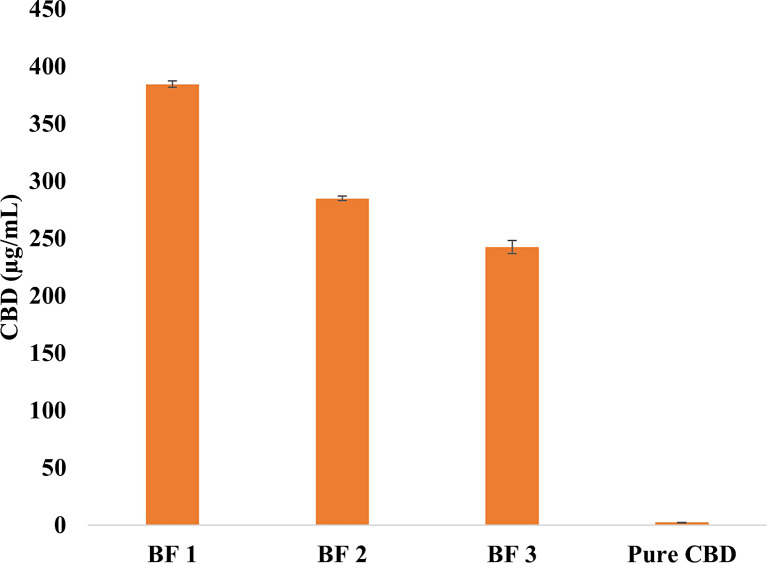
Solubility studies were conducted in both distilled water and dissolution medium (pH 6.8 + 0.1% SLS) at ambient temperature using a shaker. The test was performed on formulations BF1, BF2, and BF3, as these formulations exhibited faster CBD release compared to others (BF4 and BF5). The developed formulations (BF1, BF2, and BF3) demonstrated a significant enhancement in CBD water solubility compared to pure CBD (p value <0.05). Figure 4 illustrates that after 48 h, CBD water solubility was 384, 290, and 242 μg/mL for formulations BF1, BF2, and BF3, respectively, whereas pure CBD had an extremely low solubility of 2.0 μg/mL.
Fig. 4.
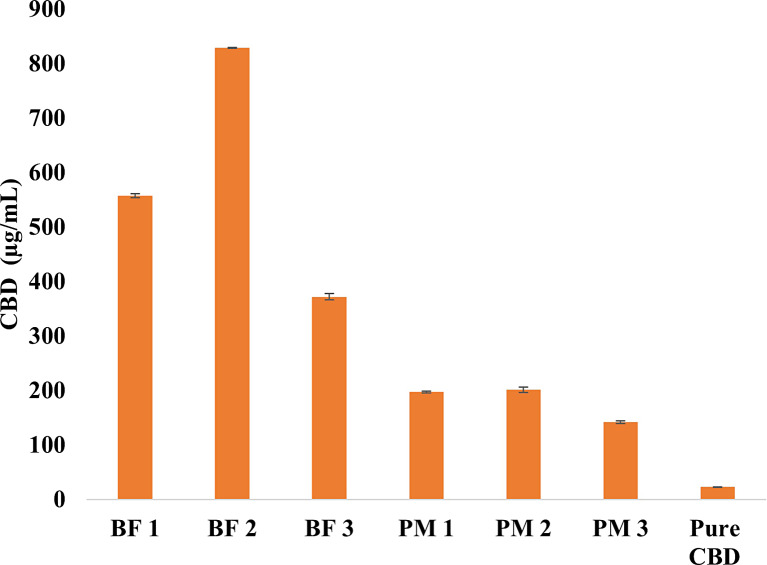
In the dissolution medium, Figure 5 shows that CBD solubility in pH 6.8 + 0.1% SLS was highest in BF2 (828.5 μg/mL) compared to BF1 and BF3 (567 μg/mL and 371 μg/mL, respectively) after 48 h. This result is consistent with the release study, which indicated higher CBD release in BF2. The solubility results for the PMs (PM1, PM2, and PM3) in the dissolution medium (Fig. 5) showed that CBD solubility was lower than in the developed formulations but higher than pure CBD (22.7 μg/mL). The increased CBD solubility in the PMs might be attributed to the presence of other excipients in the formulations rather than a change in the physical state of CBD, as confirmed by DSC results. These findings revealed that, combining HME as a processing method with an appropriate polymeric carrier improved CBD water solubility by converting the drug from its crystalline form to an amorphous form.
Fig. 5.
Fourier Transform Infrared Spectroscopy
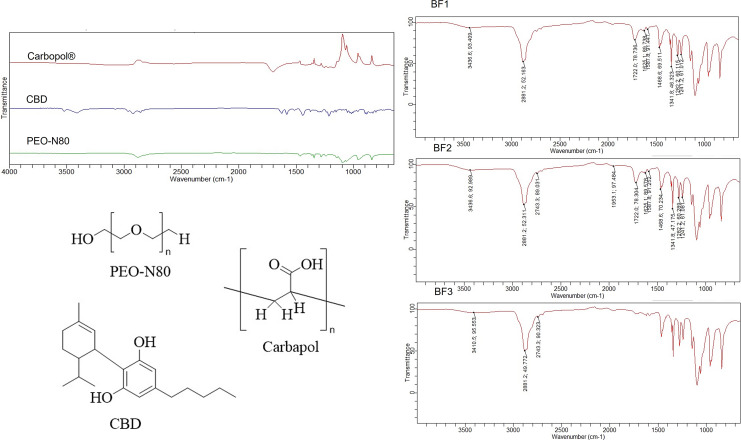
In Figure 6, the FTIR spectrum of PEO-N80 reveal distinctive absorption bands at 1,092.1 cm−1 (C– O–C stretching) and 2,877.5 cm−1 (C–H stretching). Carbopol® showed an absorption band at 1,699.7 cm−1 (C=O). CBD exhibits characteristic absorption bands at 3,522.3 and 3,410.5 cm−1 (O-H stretching). Upon analyzing the developed CBD films using FTIR, a shift in the OH stretching band of CBD at 3,522.3 cm−1 was observed, along with the broadening of the other O-H band at 3,410.5 cm−1 in BF1. For BF2 and BF3, a broad shifted band of -OH stretching at 3,436 cm−1 was also noted. This might indicate the formation of a hydrogen bond between the hydroxyl group of CBD and the ether group in either PEO-N80 or the carbonyl group of Carbopol® which potentially enhanced the solubility and stability of CBD in the developed formulations. This result is supported by previous literature which has highlighted that the establishment of hydrogen bonds between the pharmaceutical active ingredient and the polymer can contribute to improved physical stability and dissolution of products produced through HME [48].
Fig. 6.
Stability Evaluation at Room Temperature
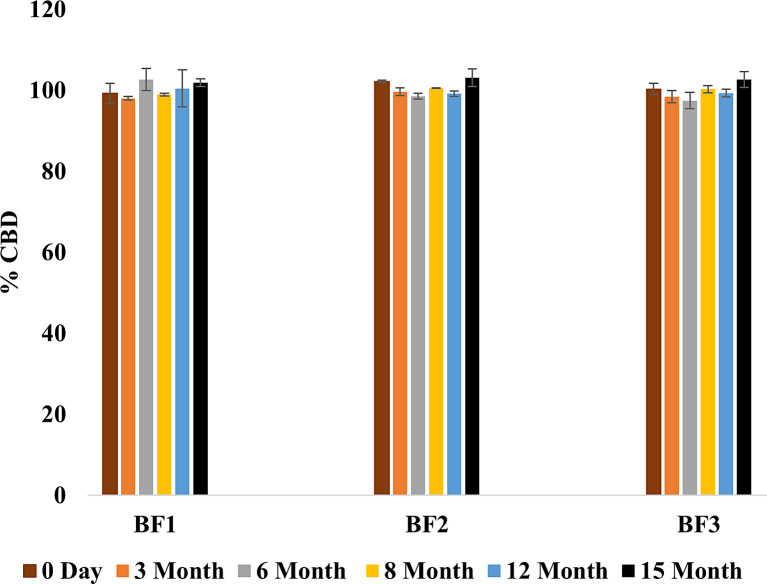
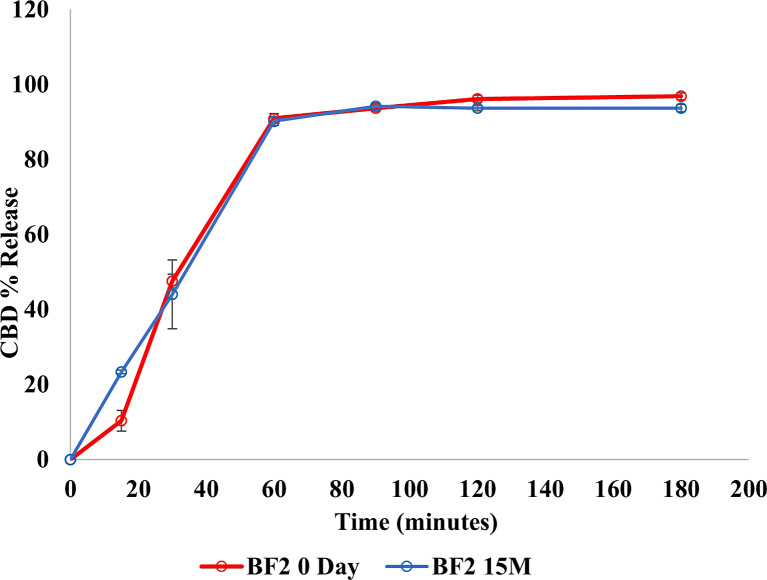
Stability assessments were carried out on BF1, BF2, and BF3 over 15 months at ambient temperature. The findings indicated that no alterations were observed in the developed films throughout the storage period. Notably, no changes were detected in either the color or texture of the films. Analysis of drug content (Fig. 7) exhibited a consistent amount of CBD in the developed formulations (BF1, BF2, and BF3) over the entire 15 months duration. Furthermore, as shown in Figure 8, there was no significant variance in the percentage of CBD released from BF2 (defined as the lead formulation) over the entire storage period (p value = 0.821).
Fig. 7.
Fig. 8.
The lead formulation (BF2) will need to be further evaluated in vivo to determine the extent of improvement in CBD bioavailability over the other formulations. Given the fact that this is a buccal film, the best model for bioavailability evaluation is through clinical studies in humans because there is no good model for buccal (trans-mucosal) studies. GMP for CBD was prepared in our laboratory and we will prepare GMP drug product for clinical evaluation as a part of our drug development efforts.
Conclusion
In this study, HME technology was successfully applied to develop CBD mucoadhesive buccal films. The developed CBD buccal films had a uniform shape and drug content with an enhancement of CBD release and solubility in formulations. The formulations containing PEO-N80 (BF1, BF2, and BF3) demonstrated faster release profile compared to those incorporating klucel™ EF in their composition. Carbopol® was successfully used to increase the adhesiveness properties of the CBD buccal films in BF2, BF3, BF4, and BF5. The stability studies revealed that CBD content in the prepared films (BF1, BF2, and BF3) was consistent within the 15-month storage period in room temperature. Additionally, BF2 also has stable CBD release over the 15 months. This finding provides a path for improvement of CBD aqueous solubility and the development of a stable mucoadhesive buccal films. It is also anticipated that this approach will enhance CBD absorption through buccal mucosa which would bypass the first-pass metabolism, a major issue with oral administration.
Acknowledgments
We would like to thank Dr. Amira Wanas, National Center for Natural Products Research, University of Mississippi for providing the CBD for this research study. We also thank Dr. Mostafa Elhendawy, Department of Chemistry and Biochemistry, University of Mississippi for assisting in the HPLC analysis.
Statement of Ethics
An ethics statement was not required for this study type since no human or animal subjects or materials were used.
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
No external funding.
Author Contributions
Conceptualization: Mahmoud A. ElSohly; data curation and investigation: Iman E. Taha and Eman A. Ashour; formal analysis: Iman E. Taha and Mohamed M. Radwan; funding acquisition: Michael A. Repka and Eman A. Ashour; methodology: Iman E. Taha, Mohamed M. Radwan, and Sundus Omari; project administration: Mahmoud A. ElSohly, Michael A. Repka, and Eman A. Ashour; resources: Mahmoud A. ElSohly, Mohamed M. Radwan, Michael A. Repka, and Eman A. Ashour; supervision: Mahmoud A. ElSohly and Eman A. Ashour; writing – original draft: Iman E. Taha; writing – review and editing: Mahmoud A. ElSohly, Mohamed M. Radwan, Sundus Omari, Michael A. Repka, and Eman A. Ashour. All authors have read and agreed to the published version of the manuscript.
Funding Statement
No external funding.
Data Availability Statement
The data that support the findings of this study are not publicly available but will be available from corresponding author (E.A.) (eashour@olemiss.edu) upon request.
References
- 1. Paonessa R, Nardi M, Di Gioia ML, Olivito F, Oliverio M, Procopio A. Eco-friendly synthesis of lipophilic EGCG derivatives and antitumor and antioxidant evaluation. Nat Prod Commun. 2018;13(9):1934578X1801300–1122. [Google Scholar]
- 2. Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, Dosio F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018;23(10):2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Todorova J, Lazarov LI, Petrova M, Tzintzarov A, Ugrinova I. The antitumor activity of cannabidiol on lung cancer cell lines A549 and H1299: the role of apoptosis. Biotechnol Biotechnol Equip. 2021;35(1):873–9. [Google Scholar]
- 4. Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB 1 and CB 2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mannila J, Järvinen T, Järvinen K, Jarho P. Precipitation complexation method produces cannabidiol/beta-cyclodextrin inclusion complex suitable for sublingual administration of cannabidiol. J Pharm Sci. 2007;96(2):312–9. [DOI] [PubMed] [Google Scholar]
- 6. Grifoni L, Vanti G, Donato R, Sacco C, Bilia AR. Promising nanocarriers to enhance solubility and bioavailability of cannabidiol for a plethora of therapeutic opportunities. Molecules. 2022;27(18):6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perucca E, Bialer M. Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS Drugs. 2020;34(8):795–800. [DOI] [PubMed] [Google Scholar]
- 8. Fraguas-Sánchez AI, Fernández-Carballido A, Martin-Sabroso C, Torres-Suárez AI. Stability characteristics of cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2020;1150:122188. [DOI] [PubMed] [Google Scholar]
- 9. Schwarzenberg A, Carpenter H, Wright C, Bayazeid O, Brokl M. Characterizing the degradation of cannabidiol in an e-liquid formulation. Sci Rep. 2022;12(1):20058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abu-Sawwa R, Chase A, Fowowe O, Park Y. Effects of Epidiolex® (Cannabidiol) on seizure-related emergency department visits and hospital admissions: a retrospective cohort study. Epilepsy Behav. 2022;127:108538. [DOI] [PubMed] [Google Scholar]
- 11. Abu-Sawwa R, Scutt B, Park Y. Emerging use of epidiolex (Cannabidiol) in epilepsy. J Pediatr Pharmacol Ther. 2020;25(6):485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Homayun B, Lin X, Choi HJ. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019;11(3):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kerz T, Paret G, Herff H. Routes of drug administration. Arrest Sci Pract Resusc Med. 2007;I:614–38. [Google Scholar]
- 14. Adhikari SNR, Nayak BS, Nayak AK, Mohanty B. Formulation and evaluation of buccal patches for delivery of atenolol. AAPS PharmSciTech. 2010;11(3):1038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hooda R, Tripathi M, Kapoor K. A review on oral mucosal drug delivery system. Pharma Innov. 2012;1:14–21. [Google Scholar]
- 16. Bhati R, Nagrajan RK. A detailed review on oral mucosal drug delivery system. Int J Pharm Sci Res. 2012;3(3):659–81. [Google Scholar]
- 17. Kaul M. An overview of buccal drug delivery system. Int J Pharm Res. 2021;13:1303–21. [Google Scholar]
- 18. Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery: a promising option for orally less efficient drugs. J Control Release. 2006;114(1):15–40. [DOI] [PubMed] [Google Scholar]
- 19. Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57(11):1666–91. [DOI] [PubMed] [Google Scholar]
- 20. Kurćubić I, Vajić UJ, Cvijić S, Crevar-Sakač M, Bogavac-Stanojević N, Miloradović Z, et al. Mucoadhesive buccal tablets with propranolol hydrochloride: formulation development and in vivo performances in experimental essential hypertension. Int J Pharm. 2021;610:121266. [DOI] [PubMed] [Google Scholar]
- 21. Vasantha PV, Puratchikody A, Mathew ST, Balaraman AK. Development and characterization of Eudragit based mucoadhesive buccal patches of salbutamol sulfate. Saudi Pharm J. 2011;19(4):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jain SK, Jain A, Gupta Y, Kharya A. Design and development of a mucoadhesive buccal film bearing progesterone. Pharmazie. 2008;63(2):129–35. [PubMed] [Google Scholar]
- 23. Takkalkar P, Tobin MJ, Vongsvivut J, Mukherjee T, Nizamuddin S, Griffin G, et al. Structural, thermal, rheological and optical properties of poly(lactic acid) films prepared through solvent casting and melt processing techniques. J Taiwan Inst Chem Eng. 2019;104:293–300. [Google Scholar]
- 24. Panda BP, Dey N, Rao MEB. Development of innovative orally fast disintegrating film dosage forms: a review. PCI Approved IJPSN. 2012;5(2):1666–74. [Google Scholar]
- 25. Anbukarasu P, Sauvageau D, Elias A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci Rep. 2015;5:17884–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palem CR, Kumar Battu S, Maddineni S, Gannu R, Repka MA, Yamsani MR. Oral transmucosal delivery of domperidone from immediate release films produced via hot-melt extrusion technology. Pharm Dev Technol. 2013;18(1):186–95. [DOI] [PubMed] [Google Scholar]
- 27. Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17(1):20–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chokshi R, Zia H. Hot-melt extrusion technique: a review. Iran J Pharm Res (IJPR). 2004;3:3–16. [Google Scholar]
- 29. Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev Ind Pharm. 2007;33(9):909–26. [DOI] [PubMed] [Google Scholar]
- 30. Shi NQ, Wang SR, Zhang Y, Huo JS, Wang LN, Cai JH, et al. Hot melt extrusion technology for improved dissolution, solubility and “spring-parachute” processes of amorphous self-micellizing solid dispersions containing BCS II drugs indomethacin and fenofibrate: profiles and mechanisms. Eur J Pharm Sci. 2019;130:78–90. [DOI] [PubMed] [Google Scholar]
- 31. Sarode AL, Sandhu H, Shah N, Malick W, Zia H. Hot melt extrusion (HME) for amorphous solid dispersions: predictive tools for processing and impact of drug-polymer interactions on supersaturation. Eur J Pharm Sci. 2013;48(3):371–84. [DOI] [PubMed] [Google Scholar]
- 32. Patil H, Feng X, Ye X, Majumdar S, Repka MA. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach. AAPS J. 2015;17(1):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN Pharm. 2012;2012:436763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stanković M, Frijlink HW, Hinrichs WLJ. Polymeric formulations for drug release prepared by hot melt extrusion: application and characterization. Drug Discov Today. 2015;20(7):812–23. [DOI] [PubMed] [Google Scholar]
- 35. Gul W, Gul SW, Radwan MM, Wanas AS, Mehmedic Z, Khan II, et al. Determination of 11 cannabinoids in biomass and extracts of different varieties of cannabis using high-performance liquid chromatography. J AOAC Int. 2015;98(6):1523–8. [DOI] [PubMed] [Google Scholar]
- 36. Vigani B, Rossi S, Sandri G, Bonferoni MC, Caramella CM. Mucoadhesive polymers in substance-based medical devices: functional ingredients or what else? Front Drug Saf Regul. 2023;3:1–13. [Google Scholar]
- 37. Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of Vitamin e TPGS in drug delivery. Eur J Pharm Sci. 2013;49(2):175–86. [DOI] [PubMed] [Google Scholar]
- 38. Crowley MM, Fredersdorf A, Schroeder B, Kucera S, Prodduturi S, Repka MA, et al. The influence of guaifenesin and ketoprofen on the properties of hot-melt extruded polyethylene oxide films. Eur J Pharm Sci. 2004;22(5):409–18. [DOI] [PubMed] [Google Scholar]
- 39. Löbmann K, Grohganz H, Laitinen R, Strachan C, Rades T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs: Part 1: preparation, stability and dissolution enhancement. Eur J Pharm Biopharm. 2013;85(3 Pt B):873–81. [DOI] [PubMed] [Google Scholar]
- 40. Bonacucina G, Martelli S, Palmieri GF. Rheological, mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int J Pharm. 2004;282(1–2):115–30. [DOI] [PubMed] [Google Scholar]
- 41. Abu-Huwaij R, Obaidat RM, Sweidan K, Al-Hiari Y. Formulation and in vitro evaluation of xanthan gum or carbopol 934-based mucoadhesive patches, loaded with nicotine. AAPS PharmSciTech. 2011;12(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mohammed NN, Majumdar S, Singh A, Deng W, Murthy NS, Pinto E, et al. KlucelTM EF and ELF polymers for immediate-release oral dosage forms prepared by melt extrusion technology. AAPS PharmSciTech. 2012;13(4):1158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Ascentiis A, deGrazia JL, Bowman CN, Colombo P, Peppas NA. Mucoadhesion of poly(2-hydroxyethyl methacrylate) is improved when linear poly(ethylene oxide) chains are added to the polymer network. J Control Release. 1995;33(1):197–201. [Google Scholar]
- 44. Althobaiti AA, Ashour EA, Almotairy A, Almutairi M, AlYahya M, Repka MA. Development and characterization of different dosage forms of nifedipine/indomethacin fixed-dose combinations. J Drug Deliv Sci Technol. 2023;80:104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elkanayati RM, Chambliss WG, Omari S, Almutairi M, Repka MA, Ashour EA. Mucoadhesive buccal films for treatment of xerostomia prepared by coupling HME and 3D printing technologies. J Drug Deliv Sci Technol. 2022;75:103660. [Google Scholar]
- 46. Muramatsu M, Kanada K, Nishida A, Ouchi K, Saito N, Yoshida M, et al. Application of Carbopol to controlled release preparations I. Carbopol as a novel coating material. Int J Pharm. 2000;199(1):77–83. [DOI] [PubMed] [Google Scholar]
- 47. Sahoo S, Chakraborti C, Mishra S. Qualitative analysis of controlled release ciprofloxacin/carbopol 934 mucoadhesive suspension. J Adv Pharm Technol Res. 2011;2(3):195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Y, Pang H, Guo Z, Lin L, Dong Y, Li G, et al. Interactions between drugs and polymers influencing hot melt extrusion. J Pharm Pharmacol. 2014;66(2):148–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available but will be available from corresponding author (E.A.) (eashour@olemiss.edu) upon request.