Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Jul 24;15:1440678. doi: 10.3389/fneur.2024.1440678
Abstract
Background
Multiple sclerosis (MS) is an inflammatory and degenerative disease of the central nervous system. More than 90,000 Canadians are affected; a cure is yet to be found. Available treatments to manage the disease course are only partially effective. For many years, persons with MS (PwMS) have used cannabis to relax, to reduce pain and spasticity, or to improve sleep and daily functioning, despite the lack of scientific evidence on the efficacy of specific cannabinoids [i.e., tetrahydrocannabinol (THC) and cannabidiol (CBD)] on these MS symptoms. The purpose of this clinical trial is to assess the effectiveness of different doses of these cannabinoids, alone or combined, on spasticity relief, compared to placebo. Moreover, we aim to determine which treatment is best effective to address other key MS conditions.
Methods
A double-blinded, randomized, factorial, placebo-controlled trial will be performed. We intend to include up to 250 PwMS aged over 21 recruited from the Centre hospitalier de l’Université de Montréal MS Clinic. PwMS will be randomly assigned on a 1:1:1:1 ratio to one of the trial arms: THC alone, CBD alone, THC/CBD combination, or placebo, using stratified blocked randomization, with random blocks within each stratum. The primary outcome is a self-assessment of spasticity using the mean Numeric Rating Scale score over 7 days. The main outcome will be the difference in this score at 4 weeks compared to baseline. Secondary outcomes include assessments of spasticity as measured by a clinician, pain, fatigue, sleep, bowel, bladder, and sexual dysfunction, restless legs syndrome, mental health, quality of life, mobility, cognitive functioning, and adverse events. Treatment responders are eligible for a 12-week extension phase, using the same treatment allocation and assessments.
Discussion
Previous clinical studies examined the efficacy of cannabis-based medicines in PwMS, mostly using products with 1:1 THC/CBD ratio. The major barrier to effectively use cannabis in real-world clinical settings is the lack of evidence on benefits of specific cannabinoids and information on possible related risks. The CANSEP study will contribute to overcome these limitations and identify the risks and benefits of cannabis-based treatments in PwMS.
Clinical trial registration
1. Introduction
Multiple sclerosis (MS) is an inflammatory disease of the brain and spinal cord afflicting over 90,000 Canadians (1). MS causes numerous symptoms, such as spasticity, pain, sphincter and sleep dysfunction, fatigue and depression (2). Spasticity has been reported in up to 80% of persons with MS (PwMS) (3). It is described as an involuntary increase in muscle tone, tightness, and spasms of the affected limbs. It is part of the upper motor neuron syndrome, which also comprises weakness, increased deep tendon reflexes, and the Babinski sign (4, 5).
Despite the growing number of available disease-modifying treatments, none are curative (6, 7). PwMS still carry a heavy burden of undermanaged symptoms (8). Current spasticity therapeutic approaches include muscle relaxants such as baclofen, tizanidine, and clonazepam. Injections of botulinum toxin are used for focal spasticity (9, 10). They are effective but must be repeated every 3 to 4 months. Diffuse and extreme spasticity responds well to intrathecal injections of baclofen delivered through an intra-abdominal programmed pump reservoir (11).
Cannabinoids have also been proposed as a potentially useful addition to current therapies to treat MS symptoms. Studies conducted in the United States, where cannabis is legal in most states, have reported that 35 to 40% of PwMS use cannabis (12, 13). Both prescribed and non-therapeutic cannabis products are legally accessible since 2018 to all adults in Canada (14). Sixty-five percent of Canadian PwMS have already been using cannabis to ameliorate mood, improve sleep, and/or relieve pain and spasticity (15). Analgesic, antihyperalgesic, neuro-protective, and anti-inflammatory properties have been attributed to tetrahydrocannabinol (THC) and cannabidiol (CBD) (16). A systematic review concluded that oromucosal nabiximols (~1:1 THC:CBD ratio) are safe and efficient to treat MS spasticity (17–19).
Unfortunately, whether specific cannabis derivatives (CBD vs. THC) or other forms can be specifically optimized to manage MS symptoms remains largely unexplored. A variety of cannabinoid-based products are now available and widely used by PwMS, as mentioned previously, despite the lack of robust scientific evidence to guide their decision. Few studies have systematically compared the two main cannabinoids (THC and CBD) individually or combined for the treatment of spasticity (20), but no Canadian trial has previously compared the cannabinoids. Furthermore, the potential mechanisms mediating the therapeutic and/or adverse events (AEs) (e.g., gastrointestinal and central nervous system such as psychopathologic/cognitive AEs) of cannabis-based medicines in PwMS are poorly documented (20).
In response to this gap in knowledge, this trial will document medical cannabis (THC, CBD, and their combination) as a novel treatment with the potential to improve spasticity and other MS symptoms and produce evidence-based knowledge to guide its use.
1.1. Hypothesis
We hypothesize that the administration of different doses of THC alone, CBD alone, and THC and CBD combined will result in a significant relief of spasticity compared to placebo.
1.2. Study objectives
The main aims of this study are: (1) to compare the efficacy of THC and CBD, alone and in combination, as add-on therapies to the current standard treatments for relief of spasticity in PwMS, (2) to assess the tolerability profile of THC and CBD, alone and in combination, (3) to identify the mechanisms underlying such therapeutic and adverse effects, considering sex, age, pharmacology, and immune profile.
2. Methods/design
2.1. Study overview
CANSEP is the first Canadian randomized clinical trial focusing on interventions with THC, CBD, or their combination, for the treatment of spasticity in PwMS. Our study is part of the Integrated Cannabis Research Strategy funded by the Canadian Institutes of Health Research (21), and MS Canada. The overarching vision of the Integrated Cannabis Research Strategy is to catalyze future research related to the health impacts of cannabis legalization, to validate the potential therapeutic benefits of cannabis, to understand risks and harms, and to support policy and regulatory models for studying cannabis use. Thus, CANSEP will explore in depth the potential therapeutic benefits and harms associated with cannabis use to strengthen the evidence base and to build cannabis-related research capacity in the field of MS. The study duration is 5 years as summarized in the schedule presented in the Supplementary material. Recruitment has begun on November 10, 2022. As of May 29, 2024, 50 participants have been enrolled, and of these, 41 have been randomized.
2.2. Study design
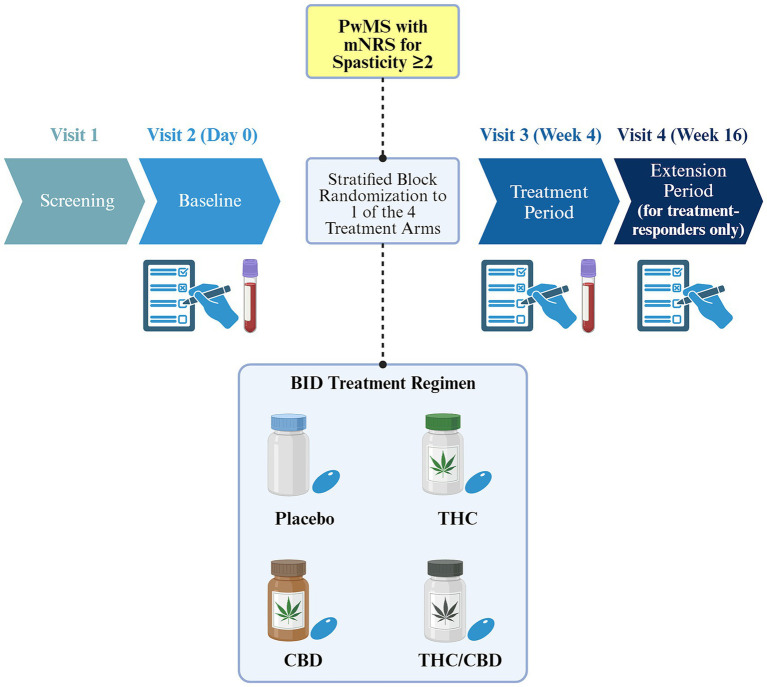
CANSEP is a double-blinded, randomized, factorial, placebo-controlled trial in a cohort of PwMS with an incomplete response to standard treatments, using various measurements, and thorough, multidisciplinary outcomes. Up to 4 visits are scheduled during this trial. Visit 1 is dedicated to the consent process and the confirmation of eligibility criteria. Outcomes assessments are done during visit 2 (baseline) and during visit 3 which takes place after 4 weeks of treatment. Finally, outcomes assessments are also carried out during a fourth visit which is scheduled 12 weeks after the end of the initial treatment period for responders only, i.e., patients who have a decrease in mean Numeric Rating Scale (NRS) of at least one point compared to baseline. Indeed, spasticity will be assessed using the NRS (22), but the spasticity outcome analyzed in this trial will be the mean NRS score over 7 days (mNRS). Blood samples will be collected to measure immunological and neurobiological markers in order to investigate the underlying physiological responses of each treatment administration, and to identify potential mechanisms of their therapeutic effects. The study plan is represented in Figure 1.
Figure 1.
2.3. Study population
The CANSEP study seeks to identify a cohort of PwMS according to the 2017 revised McDonald criteria (23), from the Centre hospitalier de l’Université de Montréal (CHUM) MS Clinic. Our study sample will be representative of the sex distribution of MS, which is 2.6 higher in women than in men in Canada (1). It will recruit adults of at least 21 years of age [the legal age in the province of Quebec to use cannabis products (24)], who reported a mean level of spasticity over 7 days of 2 points or more on the mNRS. All potential participants will be screened to determine their eligibility according to the following inclusion and exclusion criteria:
- Inclusion criteria: (a) Be diagnosed with MS (any subtype), for at least six months, by a MS neurologist, according to the 2017 revised McDonald criteria (23); (b) Spasticity or symptoms related to spasticity due to MS of at least one-month duration and not relieved with current therapy, at a mean level of 2 or more on the mNRS; (c) Have a stable dose of standard therapies for at least 4 weeks prior to the screening visit and willingness to maintain such therapies for the duration of the study; (d) Aged 21 years or older (24); (e) Have the ability, in the investigator’s opinion, and willingness to comply with all study requirements; (f) Able to speak and read French or English (grade nine level of language required).
- Exclusion criteria: (a) Concomitant disease with symptoms of spasticity, or that may have influenced their level of spasticity; (b) Received a botulinum toxin injection within four months prior to the screening visit or unwillingness to stop receiving botulinum toxin injections for the duration of the study; (c) Use of cannabis or cannabinoid-based products within 7 days prior to study entry and unwillingness to abstain from use of cannabinoids for the duration of the study; (d) History of schizophrenia, other psychotic illness, or other significant psychiatric disorder other than anxiety or depression associated with their underlying condition; (e) Alcohol or substance abuse disorder other than nicotine; (f) History of epilepsy or recurrent seizures; (g) Hypersensitivity to cannabinoids or any of the excipients of the study medication; (h) Clinically relevant cardiac dysfunction within the last 12 months or had a cardiac disorder that, in the opinion of the investigator, would put the subject at risk of a clinically relevant arrhythmia or myocardial infarction; (i) Impaired renal function, i.e., serum creatinine clearance lower than 50 mL/min; (j) Significantly impaired hepatic function, at visit 1, in the investigator’s opinion and/or had liver function tests of equal to or greater than three times the upper limit of normal; (k) Pregnancy or breastfeeding; (l) Men with history of fertility problems and who plan to conceive at any time in the future; (m) Any participant who plans to conceive either at screening or while enrolled in the study; (n) Inability (or unwillingness) of women of childbearing potential and men to use a medically acceptable form of contraception throughout the study duration; (o) Any other significant disease or disorder which, in the opinion of the investigator, may either put the subject at risk because of participation in the study, may influence the result of the study, or the subject’s ability to participate in the study; (p) Intention to travel internationally, or to donate blood during the study.
2.4. Randomization
Patients who meet all the inclusion criteria and none of the exclusion criteria and consent to take part in the trial after they have received the study oral and written information, will then be randomly assigned in equal proportions to either THC, CBD, THC + CBD, or placebo using stratified block randomization. Randomization will be stratified by sex and baseline mean spasticity score. In each of the 4 strata, corresponding to different combinations of sex (male vs. female) and baseline mean spasticity score ([2-6[vs. ≥6), randomization will be performed using blocks of size 4. This will minimize the imbalance between group sizes, while preventing unblinding of the treatment allocation of consecutive participants. The randomization schedule will be generated by CHUM’s Centre for the Integration and Analysis of Medical Data (CITADEL), who will send the randomization codes to the CHUM research pharmacy and keep secure digitalized copies. Randomization codes will be maintained throughout the study.
2.5. Intervention
2.5.1. Arms and intervention
An authorization to conduct the CANSEP trial using THC and CBD manufactured by PurCann Pharma was obtained from Health Canada prior to the start of recruitment.
Participants will be allocated to four arms and initially receive THC (4 mg/day), CBD (40 mg/day), THC/CBD combination (THC 4 mg/day and CBD 40 mg/day), or placebo, on the first day. Every two days, the daily quantity will be multiplied by two up to a maximum of 20 mg for THC and 200 mg for CBD, if well tolerated (Table 1). The selection of doses is based on previous studies on cannabinoids in MS (19, 25–34), and on a systematic review indicating that a daily dose of 200 mg of CBD is effective for other neurological conditions and symptoms while being well tolerated (35). One of the authors’ expert opinion on cannabinoids in psychiatric research and clinical practice (36, 37), and the approved and available orally formulated cannabinoids in Canada (38, 39) also accounted for these doses’ selection. THC and CBD will be taken as softgels (cannabis extract; placebo will taste and look exactly the same), in two divided doses per day at 12-h intervals. Participants will receive the allocated treatment for a total of 4 consecutive weeks, followed by an additional 12 weeks of treatment for responders who will be identified as those who have a decrease from baseline in spasticity of at least one point on the mNRS. Participants enrolled in the trial will continue stable doses of other standard treatments for spasticity including muscle relaxants (baclofen, tizanidine, and clonazepam) (18).
Table 1.
Medication dosage.
| Treatment period (4 weeks) | |||||
|---|---|---|---|---|---|
| Week | Day | Daily THC dose (mg)a | Daily CBD dose (mg)a | Daily THC and CBD combined dose (mg)a | |
| THC | CBD | ||||
| W1 | #1 | 4 | 40 | 4 | 40 |
| #2 | 4 | 40 | 4 | 40 | |
| #3 | 8 | 80 | 8 | 80 | |
| #4 | 8 | 80 | 8 | 80 | |
| #5 | 16 | 160 | 16 | 160 | |
| #6 | 16 | 160 | 16 | 160 | |
| #7 | 20 | 200 | 20 | 200 | |
| W2 to W4 included | 20 | 200 | 20 | 200 | |
| Extension period (12 weeks) | |||||
| Week | Daily THC dose (mg)a | Daily CBD dose (mg)a | Daily THC and CBD combined dose (mg)a | ||
| THC | CBD | ||||
| W5 to W16 included | 20 | 200 | 20 | 200 | |
aTreatment will be taken in two divided doses per day at 12-h intervals. CBD, cannabidiol; THC, tetrahydrocannabinol; W, week.
2.5.2. Medical management
Regardless of the assigned arm, participants will receive medical examination and management from the study physician according to usual standards of care. Medication adherence will be monitored using a daily diary. The study pharmacist will count the unused capsules at the end of the 4 initial weeks of treatment and during the additional period of 12 weeks and will check for accuracy by comparing with the numbers mentioned on the daily diary reported by the participants.
2.6. Assessments
2.6.1. Screening
At the screening visit, participants will be asked to provide sociodemographic information, their medication use, and their comorbidities. Furthermore, compulsive use and the individual’s preoccupation with cannabis use will be assessed by the Severity Dependence Scale, a self-reported questionnaire (40). The Structured Clinical Interview for DSM-5 Disorders will be administered to assess substance use disorders, including alcohol and other substances (41). The heart condition will be reviewed by electrocardiography. A pregnancy test and birth control questionnaire will be conducted in women aged ≤50 years with no sign of menopause and who did not undergo surgical contraception. A MS neurologist will evaluate the participants’ eligibility and will assess spasticity and disability with the Modified Ashworth Scale and the Expanded Disability Status Scale, respectively (42, 43).
2.6.2. Primary outcome
The primary outcome (i.e., patient-reported spasticity) will be assessed using the mNRS (22, 44). The main outcome will be the difference in mNRS recorded for 7 days prior to the visit at week 4 and the visit at baseline. NRS is the most used tool to assess spasticity in most previous RCTs of treatment, including cannabinoid-based therapy, for MS-related spasticity (22). It is represented on a 0–10 scale, where 0 means no spasticity and 10, the worst possible spasticity (22). The NRS has been shown to be more robust than the Ashworth Scale for the test–retest reliability and highly correlative of the Patient Global Impression of Change scores (22, 44). Most studies investigating cannabis-derived products enrolled MS patients who have NRS ≥4 on the 0–10 scale (22). To include patients with moderate spasticity, we also enrolled those who have mNRS ≥2 on the 0–10 scale (17, 22, 44).
2.6.3. Secondary and exploratory outcomes
All the secondary – efficacy and safety – outcomes will be assessed at baseline, at week 4 and 12 weeks after the initial treatment period. Clinical efficacy outcomes are presented in Table 2. In addition, we will assess the success of participants’ blinding after the initial treatment period with the James Blinding Index (69). Blood tests will be conducted only at baseline and at week 4 to measure immunological and neurobiological markers. Safety measures will include all reported or observed adverse events and serious adverse events (AEs and SAEs). Pregnancy and birth control will be re-evaluated every 4 weeks.
Table 2.
Secondary and exploratory efficacy assessments.
| Test | Symptom | Type of outcome |
|---|---|---|
| Modified Ashworth Scale (42) | Spasticity, as assessed by a clinician | ClinRO |
| Expanded Disability Status Scale (43) | Disability | |
| Positive and Negative Syndrome Scale (45) | Psychotic symptoms | |
| Multiple Sclerosis Quality of Life Inventory (46, 47), includinga: | PRO | |
| MOS Pain Effects Scale (46, 47) | Pain | |
| Modified Fatigue Impact Scale – 5-Item Version (46, 47) | Fatigue | |
| Bowel Control Scale (46, 47) | Bowel dysfunction | |
| Bladder Control Scale (46, 47) | Bladder dysfunction | |
| Modified Social Support Survey – 5-Item Version (46, 47) | Perceived social support | |
| Sexual Satisfaction Scale (46–48) | Sexual dysfunction | |
| 36-Item Short Form Survey (46, 47) | Quality of life | |
| Perceived Deficits Questionnaire (46, 47, 49) | Subjective cognitive function | |
| Pittsburgh Sleep Quality Index (50) and Epworth Sleepiness Scale (51) | Sleep issues, according to the assessment of sleep quality and sleepiness, respectively | |
| Restless Legs Syndrome Severity Rating Scale (52, 53) | Restless legs syndrome’s severity | |
| Hospital Anxiety and Depression Scale (54, 55) | Anxiety and depression | |
| Cannabis Experience Questionnaire (56, 57)b | Euphoric and paranoid-dysphoric effects of cannabis | |
| Battery of cognitive tests: Montreal Cognitive Assessment (58), Brief Visuospatial Memory Test–Revised (59), D-KEFS Color-Word Interference Test (60), Hopkins Verbal Learning Test–Revised (61), The Trial Making Test A/B (62), and Symbol Digit Modalities Test (63) | Objective cognitive function | PerfO |
| Timed 25 Foot-Walk test (64–66) | Mobility |
ClinRO, clinician-reported outcome; PRO, patient-reported outcome; PerfO, performance outcome. aWe adapted the Multiple Sclerosis Quality of Life Inventory for the CANSEP trial, by excluding two of its ten scales: (1) The Impact of Visual Impairment Scale was not included, because it is not widely used in clinical practice and not relevant to the CANSEP trial; (2) instead of the Mental Health Inventory, we considered the Hospital Anxiety and Depression Scale, a widely used instrument in clinical practice, but also a validated scale in the MS population (55, 67, 68). Furthermore, we added the Cannabis Experience Questionnaire for the subjective effects of cannabinoids (56, 57). bAll tests will be conducted at baseline, at week 4, and at week 16. However, participants who never used cannabis in their lifetime will not complete the Cannabis Experience Questionnaire at baseline.
2.7. Data analyses
The data will be analyzed once all randomized subjects have completed the trial. Analyses of primary and secondary outcomes will rely on the intention-to-treat paradigm. Thus, all initially randomized subjects will be included. Descriptive statistics will be used to compare the baseline characteristics of subjects. They will include means, medians, standard deviations and interquartile ranges for continuous variables, and frequencies and percentages for categorical variables. Sensitivity analysis will be performed to the first few participants who did not complete the NRS scale as per the last version of the protocol and consequently have a different primary outcome measure.
2.7.1. Sample size and power calculation
The sample size for this trial was calculated using ‘pwr.t.test’ (for a Student t-test) in the ‘pwr’ package in R statistical software (70). The required sample size was estimated to ensure at least 80% power, with a two-sided alpha significance level of 5%, to detect clinically important effects of the two factors THC and CBD, on spasticity, which is the primary outcome (71). A target total recruitment of 200 patients would cover scenarios with a mean change from baseline among treated patients ranging from −1.90 to 1.55, where −1.55 is what we consider to be the minimal clinically significant effect (22, 33, 72, 73). However, some power would be lost upon Bonferroni adjustment. Thus, a target total recruitment of 250 patients would be more adequate, after accounting for a potential rate of attrition of 5% over the study period, based on previous studies (22, 33, 72, 73) and the clinical experience at the CHUM MS Clinic.
2.7.2. Primary endpoints
Firstly, an analysis of variance (ANOVA) will be used to assess the treatments effect on the differences in the mNRS from baseline to week 4. The normality of the responses will be assessed using normal-quantile plots. The ANOVA model will only include the factors THC and CBD. Secondly, an analysis of covariance (ANCOVA) will be used to evaluate the treatments effect on the post-intervention mNRS while adjusting for baseline mNRS and sex (stratification variables). The ANCOVA may reduce potential bias present in the ANOVA model (74). Finally, the ANCOVA model described above will be expanded by including potential confounders, such as biomarkers, for which a clinically meaningful difference between any two groups will be revealed by the preliminary descriptive analysis. The estimated adjusted and unadjusted differences in mNRS from baseline to week 4 and their 95% confidence intervals will be reported for all models.
2.7.3. Secondary and exploratory endpoints
The secondary and exploratory outcomes will be analyzed using the same methods as spasticity. Exploratory outcomes and mechanistic factors (sex, neurobiological markers, age, etc.) will be analyzed using ANCOVA models.
2.7.3.1. Sex and gender-based analysis
Numerous publications have documented the impact of sex on multiple aspects of MS (biology, epidemiology, pregnancy) and substance use, respectively (75–78). Our statistical plan will include stratified analyzes to identify the sex-linked immune mechanisms as well as the sex-specific effects of cannabis.
2.7.3.2. Safety analyses
AEs will be analyzed using the Common Terminology Criteria for Adverse Events (version 5.0) according to the Medical Dictionary for regulatory activities (79). The frequency and percentage of participants experiencing each specific AE will be tabulated by severity and treatment. Each AE will be counted once under the maximum severity or the strongest recorded causal relationship to the studied product. For each arm, all AEs will be grouped by organ class and, for each AE, the relative risk and absolute risks between arms with their 95% confidence intervals will be calculated.
2.7.4. Oversight and monitoring
An independent data and safety monitoring board will review the accumulated data to assure that the safety of study participants is protected while the scientific goals of the study are being met. The data and safety monitoring board is responsible for conducting reviews of accumulating safety and efficacy data once a year. It may recommend halting or modifying study procedures if there is a clear and evident reason related to the safety of the study participants including and excess in frequency of any AE (judged by the data and safety monitoring board to be harmful to the participants) in one of the arms, and an excess in frequency of any SAE (grade 3 and higher) in one of the arms.
2.7.5. Missing data and dropouts
Missing data could occur due to two types of reasons: a missing or illegible item response and a missed visit. We will report the percentage of missing values for each variable of interest by visit. Missing values for the primary outcome at follow-up will be imputed by the baseline value resulting in null change from baseline. No imputation for the primary outcome will be needed from the per-protocol analysis. For the ANCOVA models, imputation of missing covariate values will be handled by multiple imputation methods using chained equations. Since we cannot verify the missing at random hypothesis, which is required for the valid use of this imputation method, we will perform a sensitivity analysis with complete cases only.
2.7.6. Sensitivity analysis
To test if our conclusions are robust to the invalidity of the hypothesis of no interaction between the factors, we will use an ANOVA model including THC, CBD, and their interaction. We expect to find a negligible interaction effect.
3. Discussion
Current therapeutic approaches for MS have partial efficacy. Disease-modifying therapies are limited in their action on MS symptoms (11). Both preclinical and clinical data support that analgesic, antihyperalgesic, neuroprotective, and anti-inflammatory properties have been attributed to cannabis-derived cannabinoids, essentially THC and/or CBD (30, 80). The CANSEP trial is part of a large Canadian Institutes of Health Research program on cannabis research in priority areas (21). It was developed in response to the lack of evidence about the safety and the efficacy of specific cannabinoids in MS (20). The originality of our study comes from the fact that we will compare the distinct and combined effects of THC and CBD on spasticity and other symptoms such as pain, sleep, well-being, and quality of life of Canadian PwMS.
CANSEP will fill a knowledge gap since no Canadian study has yet systematically compared the two main cannabinoids, THC and CBD, and their combination, to placebo, as an add-on treatment for spasticity. This will answer the urgent need to assess cannabinoids’ efficacy on spasticity and other symptoms in MS in addition to their acceptability and safety when used as an add-on therapy to usual standard treatments. Additionally, CANSEP can provide explanations for potential therapeutic mechanisms and/or AEs such as gastrointestinal and psychiatric/cognitive effects associated with each type of cannabis-based medication in PwMS that are still poorly understood (18). For example, the polymorphism in cannabinoid receptor 2 associated with reduced endocannabinoid effects is more prevalent in autoimmune diseases, including MS (81). Thus, CANSEP will provide a better characterization of CBD and THC influence on immune and pharmacological factors which could directly influence spasticity to guide both health professionals and PwMS in the decision-making process. Indeed, while our main concern is spasticity, we are also evaluating other MS symptoms (pain, fatigue, bladder, bowel, and sexual dysfunctions, mobility, the restless legs syndrome, mental health, cognition, and quality of life). The CANSEP trial’s results will impact how these different symptoms could be managed and will provide a better grasp of tolerance and toxicity. We have a long experience with MS patients, and the trial’s results will provide guidance to a better control of symptomatic aspects of MS with cannabinoids. It will also guide health professionals and PwMS on the treatment’s dose and frequency.
Another strength of this trial is the development of two ancillary studies. Participants are free to take part or not in these ancillary studies without affecting their participation in the CANSEP clinical trial.
- Ancillary study 1 – Perception of Canadian patients with MS on the use of cannabis for a better management of the disease symptoms (PerSPective Study): The main objective is to conduct a pan-Canadian online survey, co-created for and with PwMS to identify facilitating or limiting factors on the use of medical cannabis by PwMS to anticipate the strategies to be put in place depending on the results of the research.
- Ancillary study 2 – Patient experience assessment of participants in the CANSEP trial and the contribution of patient partner researchers in the implementation work of this project (EXPECT Study): This study includes quantitative questionnaires and semi-structured qualitative interviews to understand how the patient partners involved in CANSEP have been integrated and intervened in the realization of the project (80). A second objective is to assess the experience lived by the PwMS participating in the CANSEP trial and their perceptions related to the use of THC and CBD as a clinical intervention.
The limitations of the CANSEP trial may be related to data collection through patient-reported outcome measurements which could inaccurately estimate problems and not be suitable for all patients (82). However, a daily diary developed to monitor medication adherence and scheduled phone calls between visits will ensure proper data collection. Also, the variability in understanding the definition of spasticity among patients could be a limitation in the assessment of this symptom although we provide the same description to all participants.
4. Knowledge transfer
At the end of the study, we will conduct a robust knowledge transfer. We plan a variety of strategies (e.g., health cards and video clips) in collaboration with patient partners to train PwMS interested in using cannabinoids-based medicines in an appropriate level of literacy. For clinicians, we will create a tool of shared decision-making. To translate results to the health care level, we will build on our involvement in national/international research, clinical and training organizations. For the general population, we plan to organize public conferences to raise awareness of societal issues as well as participation in events organized yearly by the CHUM.
5. Conclusion
CANSEP will provide a better characterization of CBD and THC’s influence on spasticity and on immune and pharmacological factors which could directly influence spasticity treatments and guide both health professionals and PwMS in the decision-making care process.
Ethics statement
The CANSEP study involving humans was approved by the Research Ethics Board of Centre de recherche du Centre hospitalier de l’Université de Montréal, on September 9, 2022 (approval #21.303). The study was conducted in accordance with the local legislation, institutional requirements and the Declaration of Helsinki. The participants provided their written informed consent to participate in this study.
Author contributions
AZ: Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. KAM: Visualization, Writing – original draft, Writing – review & editing. NA: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. DJ-A: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. M-PP: Methodology, Writing – review & editing. IR: Methodology, Writing – review & editing. AP: Writing – review & editing. CL: Writing – review & editing. PB: Writing – review & editing. LC: Writing – review & editing. M-PS: Writing – review & editing. DM: Writing – review & editing. J-SO: Writing – review & editing. NF: Writing – review & editing. PD: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Acknowledgments
The CANSEP study protocol was presented as a poster at the Canadian Association for Population Therapeutics 2023 Annual Conference. This poster’s abstract was published in the Canadian Journal of Health Technologies (83).
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research is jointly supported by the Canadian Institutes of Health Research and MS Canada (Grant number 02088-000 dated 10/03/2020). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Canadian Institutes of Health Research and MS Canada.
Conflict of interest
The investigational products used in the CANSEP trial were purchased from PurCann Pharma, but PurCann Pharma was not involved in conducting nor in funding the CANSEP trial. DJ-A holds a clinical scientist career award from the Fonds de Recherche du Québec. DJ-A received investigational products from Cardiol Therapeutics and Exka for clinical trials funded by the Quebec Ministry of Health and Social Services and the Fonds de recherche du Québec. KAM declares previously receiving employment fees for contracting services from Certara Canada Corporation, before initiating the recruitment process of the CANSEP trial. KAM previously received employment fees for research coordination services from another research team of the Centre de recherche du Centre hospitalier de l’Université de Montréal, for unfunded and funded projects by different grants, including from the Canadian Institutes of Health Research.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1440678/full#supplementary-material
References
- 1.MS Canada . (2020). Prevalence and incidence of MS in Canada and around the world. Available at: https://mscanada.ca/ms-research/latest-research/prevalence-and-incidence-of-ms-in-canada-and-around-the-world (Accessed May 26, 2024).
- 2.Koch MW, Metz LM, Agrawal SM, Yong VW. Environmental factors and their regulation of immunity in multiple sclerosis. J Neurol Sci. (2013) 324:10–6. doi: 10.1016/j.jns.2012.10.021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo MA, Hadjimichael OC, Preiningerova J, Vollmer TL. Prevalence and treatment of Spasticity reported by multiple sclerosis patients. Mult Scler. (2004) 10:589–95. doi: 10.1191/1352458504ms1085oa [DOI] [PubMed] [Google Scholar]
- 4.Urits I, Adamian L, Fiocchi J, Hoyt D, Ernst C, Kaye AD, et al. Advances in the understanding and Management of Chronic Pain in multiple sclerosis: a comprehensive review. Curr Pain Headache Rep. (2019) 23:59. doi: 10.1007/s11916-019-0800-2, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Kister I, Bacon TE, Chamot E, Salter AR, Cutter GR, Kalina JT, et al. Natural history of multiple sclerosis symptoms. Int J MS Care. (2013) 15:146–56. doi: 10.7224/1537-2073.2012-053, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (Fty 720): discovery and development of an Oral drug to treat multiple sclerosis. Nat Rev Drug Discov. (2010) 9:883–97. doi: 10.1038/nrd3248, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. (2018) 97:742–68. doi: 10.1016/j.neuron.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 8.Crabtree-Hartman E. Advanced symptom Management in Multiple Sclerosis. Neurol Clin. (2018) 36:197–218. doi: 10.1016/j.ncl.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 9.Li S, Francisco GE. The use of botulinum toxin for treatment of Spasticity. Handb Exp Pharmacol. (2021) 263:127–46. doi: 10.1007/164_2019_315 [DOI] [PubMed] [Google Scholar]
- 10.Hui D, Argáez C. Onabotulinum toxin a (Botox) for Spasticity associated with multiple sclerosis. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; (2021). [PubMed] [Google Scholar]
- 11.Toosy A, Ciccarelli O, Thompson A. Symptomatic treatment and Management of Multiple Sclerosis. Handb Clin Neurol. (2014) 122:513–62. doi: 10.1016/b978-0-444-52001-2.00023-6 [DOI] [PubMed] [Google Scholar]
- 12.Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, et al. Cannabis use in people with Parkinson’s disease and multiple sclerosis: a web-based investigation. Complement Ther Med. (2017) 33:99–104. doi: 10.1016/j.ctim.2017.07.002, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Weinkle L, Domen CH, Shelton I, Sillau S, Nair K, Alvarez E. Exploring Cannabis use by patients with multiple sclerosis in a state where Cannabis is legal. Mult Scler Relat Disord. (2019) 27:383–90. doi: 10.1016/j.msard.2018.11.022, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Government of Canada. Statutes of Canada (2018). Chapter 16: Cannabis act (bill C-45): Justice Laws website (2018). Available at: https://laws-lois.justice.gc.ca/eng/AnnualStatutes/2018_16/ (Accessed May 26, 2024).
- 15.Santarossa TM, So R, Smyth DP, Gustavsen DS, Tsuyuki DRT. Medical Cannabis use in Canadians with multiple sclerosis. Mult Scler Relat Disord. (2022) 59:103638. doi: 10.1016/j.msard.2022.103638, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Chiurchiù V, van der Stelt M, Centonze D, Maccarrone M. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: clues for other Neuroinflammatory diseases. Prog Neurobiol. (2018) 160:82–100. doi: 10.1016/j.pneurobio.2017.10.007, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Akgün K, Essner U, Seydel C, Ziemssen T. Daily practice managing resistant multiple sclerosis Spasticity with Delta-9-tetrahydrocannabinol: Cannabidiol Oromucosal spray: a systematic review of observational studies. J Cent Nerv Syst Dis. (2019) 11:1179573519831997. doi: 10.1177/1179573519831997, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD Oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. (2013) 260:984–97. doi: 10.1007/s00415-012-6739-4, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Filippini G, Minozzi S, Borrelli F, Cinquini M, Dwan K. Cannabis and cannabinoids for symptomatic treatment for people with multiple sclerosis. Cochrane Database Syst Rev. (2022) 5:CD013444. doi: 10.1002/14651858.CD013444.pub2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav V, Bever C, Jr, Bowen J, Bowling A, Weinstock-Guttman B, Cameron M, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development Subcommittee of the American Academy of neurology. Neurology. (2014) 82:1083–92. doi: 10.1212/wnl.0000000000000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canadian Institutes of Health Research . (2021). Integrated Cannabis research strategy. Available at: https://cihr-irsc.gc.ca/e/50932.html (Accessed May 26, 2024).
- 22.Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of Spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. (2008) 30:974–85. doi: 10.1016/j.clinthera.2008.05.011, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the Mcdonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/s1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 24.Légis Québec . (2018). C-5.3- Cannabis regulation act. Available at: https://www.legisquebec.gouv.qc.ca/en/document/cs/C-5.3 (Accessed May 26, 2024).
- 25.van Amerongen G, Kanhai K, Baakman AC, Heuberger J, Klaassen E, Beumer TL, et al. Effects on Spasticity and neuropathic pain of an oral formulation of Delta 9-tetrahydrocannabinol in patients with progressive multiple sclerosis. Clin Ther. (2018) 40:1467–82. doi: 10.1016/j.clinthera.2017.01.016, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, et al. Efficacy, safety and tolerability of an orally administered Cannabis extract in the treatment of Spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, Crossover Study. Mult Scler. (2004) 10:417–24. doi: 10.1191/1352458504ms1048oa, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG. Multiple sclerosis and extract of Cannabis: results of the Musec trial. J Neurol Neurosurg Psychiatry. (2012) 83:1125–32. doi: 10.1136/jnnp-2012-302468, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Schimrigk S, Marziniak M, Neubauer C, Kugler EM, Werner G, Abramov-Sommariva D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur Neurol. (2017) 78:320–9. doi: 10.1159/000481089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid Dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. (2004) 329:253. doi: 10.1136/bmj.38149.566979.AE, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zajicek J, Ball S, Wright D, Vickery J, Nunn A, Miller D, et al. Effect of Dronabinol on progression in progressive multiple sclerosis (Cupid): a randomised Placebo-Controlled Trial. Lancet Neurol. (2013) 12:857–65. doi: 10.1016/s1474-4422(13)70159-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turcotte D, Doupe M, Torabi M, Gomori A, Ethans K, Esfahani F, et al. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Med. (2015) 16:149–59. doi: 10.1111/pme.12569, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Fox P, Bain PG, Glickman S, Carroll C, Zajicek J. The effect of Cannabis on tremor in patients with multiple sclerosis. Neurology. (2004) 62:1105–9. doi: 10.1212/01.wnl.0000118203.67138.3e, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of Nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory Spasticity caused by multiple sclerosis. Eur J Neurol. (2011) 18:1122–31. doi: 10.1111/j.1468-1331.2010.03328.x, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Messina S, Solaro C, Righini I, Bergamaschi R, Bonavita S, Bossio RB, et al. Sativex in resistant multiple sclerosis Spasticity: discontinuation study in a large population of Italian patients (Sa.Fe. Study). PLoS One. (2017) 12:e0180651. doi: 10.1371/journal.pone.0180651, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millar SA, Stone NL, Bellman ZD, Yates AS, England TJ, O’Sullivan SE. A systematic review of Cannabidiol dosing in clinical populations. Br J Clin Pharmacol. (2019) 85:1888–900. doi: 10.1111/bcp.14038, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manini AF, Yiannoulos G, Bergamaschi MM, Hernandez S, Olmedo R, Barnes AJ, et al. Safety and pharmacokinetics of Oral Cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med. (2015) 9:204–10. doi: 10.1097/ADM.0000000000000118, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mongeau-Perusse V, Brissette S, Bruneau J, Conrod P, Dubreucq S, Gazil G, et al. Cannabidiol as a treatment for craving and relapse in individuals with cocaine use disorder: a randomized placebo-controlled trial. Addiction. (2021) 116:2431–42. doi: 10.1111/add.15417, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Health Canada . (2018). Information for health care professionals: Cannabis (marihuana, marijuana) and the cannabinoids: Government of Canada. Available at: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids.html (Accessed July 05, 2024).
- 39.Health Canada . (2022). Review of Cannabidiol: Report of the science advisory committee on health products containing Cannabis: Government of Canada. Available at: https://www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/external-advisory-bodies/health-products-containing-cannabis/review-cannabidiol-health-products-containing-cannabis.html (Accessed July 05, 2024).‑
- 40.Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, et al. The severity of dependence scale (Sds): psychometric properties of the Sds in English and Australian samples of heroin, Cocaine and Amphetamine Users Addiction. Addiction. (1995) 90:607–14. doi: 10.1046/j.1360-0443.1995.9056072.x, PMID: [DOI] [PubMed] [Google Scholar]
- 41.First MB, Williams JBW, Karg RS, Spitzer RL. The structured clinical interview for Dsm-5 disorders, research version (Scid-5-Rv). Washington, DC: American Psychiatric Association; (2015). [Google Scholar]
- 42.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle Spasticity. Phys Ther. (1987) 67:206–7. doi: 10.1093/ptj/67.2.206 [DOI] [PubMed] [Google Scholar]
- 43.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33:1444–52. doi: 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 44.Hugos CL, Cameron MH. Assessment and measurement of Spasticity in MS: state of the evidence. Curr Neurol Neurosci Rep. (2019) 19:79. doi: 10.1007/s11910-019-0991-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (Panss) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 46.National Multiple Sclerosis Society . The consortium of multiple sclerosis centers health services research subcommittee. Msqli multiple sclerosis quality of life inventory: A User’s manual. New York, NY: National Multiple Sclerosis Society; (1997). [Google Scholar]
- 47.Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Mult Scler. (1999) 5:251–9. doi: 10.1177/135245859900500410 [DOI] [PubMed] [Google Scholar]
- 48.Nowinski JK, LoPiccolo J. Assessing sexual behavior in couples. J Sex Marital Ther. (1979) 5:225–43. doi: 10.1080/00926237908403731 [DOI] [PubMed] [Google Scholar]
- 49.Sullivan MJ, Edgley K, Dehoux E. A survey of multiple sclerosis. Part I: perceived cognitive problems and compensatory strategy use. Can. J Rehabil. (1990) 4:99–105. [Google Scholar]
- 50.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 52.Allen RP, Kushida CA, Atkinson MJ. Factor analysis of the international restless legs syndrome study Group’s scale for restless legs severity. Sleep Med. (2003) 4:133–5. doi: 10.1016/s1389-9457(02)00193-4, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the international restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. (2003) 4:121–32. doi: 10.1016/s1389-9457(02)00258-7, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. (2003) 1:29. doi: 10.1186/1477-7525-1-29, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honarmand K, Feinstein A. Validation of the hospital anxiety and depression scale for use with multiple sclerosis patients. Mult Scler. (2009) 15:1518–24. doi: 10.1177/1352458509347150, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Barkus EJ, Stirling J, Hopkins RS, Lewis S. Cannabis-induced psychosis-like experiences are associated with high Schizotypy. Psychopathology. (2006) 39:175–8. doi: 10.1159/000092678, PMID: [DOI] [PubMed] [Google Scholar]
- 57.Quinn CA, Wilson H, Cockshaw W, Barkus E, Hides L. Development and validation of the Cannabis experiences questionnaire-intoxication effects checklist (Ceq-I) short form. Schizophr Res. (2017) 189:91–6. doi: 10.1016/j.schres.2017.01.048, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, Moca: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x, PMID: [DOI] [PubMed] [Google Scholar]
- 59.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the brief visuospatial memory test: studies of Normal performance, reliability, and validity. Psychol Assess. (1996) 8:145–53. doi: 10.1037/1040-3590.8.2.145 [DOI] [Google Scholar]
- 60.Shunk AW, Davis AS, Dean RS. Test review: Dean C. Delis, Edith Kaplan & Joel H. Kramer, delis Kaplan executive function system (D-Kefs), the psychological corporation, San Antonio, Tx, 2001. $415.00 (complete kit). Appl Neuropsychol. (2006) 13:275–27. doi: 10.1207/s15324826an1304_9 [DOI] [Google Scholar]
- 61.Brandt J. The Hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin Neuropsychol. (1991) 5:125–42. doi: 10.1080/13854049108403297 [DOI] [Google Scholar]
- 62.Arnett JA, Labovitz SS. Effect of physical layout in performance of the trail making test. Psychol Assess. (1995) 7:220–1. doi: 10.1037/1040-3590.7.2.220 [DOI] [Google Scholar]
- 63.Smith A. Symbol digit modalities test [manual]. Torrance, CA: Western Psychological Services; (1973). [Google Scholar]
- 64.Rudick R, Antel J, Confavreux C, Cutter G, Ellison G, Fischer J, et al. Recommendations from the National Multiple Sclerosis Society clinical outcomes assessment task force. Ann Neurol. (1997) 42:379–82. doi: 10.1002/ana.410420318, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The multiple sclerosis functional composite measure (Msfc): an integrated approach to MS clinical outcome assessment. National MS Society clinical outcomes assessment task force. Mult Scler. (1999) 5:244–50. doi: 10.1177/135245859900500409, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Fischer JS, Jak AJ, Kniker JE, Rudick RA, Cutter G. (2001). Multiple sclerosis functional composite (Msfc). Administration and scoring Manuel-revised, October 2001: National Multiple Sclerosis Society. Available at: https://www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/10-2-3-31-msfc_manual_and_forms.pdf (Accessed May 26, 2024).
- 67.Rintala A, Matcham F, Radaelli M, Locafaro G, Simblett S, di San B, et al. Emotional outcomes in clinically isolated syndrome and early phase multiple sclerosis: a systematic review and Meta-analysis. J Psychosom Res. (2019) 124:109761. doi: 10.1016/j.jpsychores.2019.109761, PMID: [DOI] [PubMed] [Google Scholar]
- 68.Peres DS, Rodrigues P, Viero FT, Frare JM, Kudsi SQ, Meira GM, et al. Prevalence of depression and anxiety in the different clinical forms of multiple sclerosis and associations with disability: a systematic review and Meta-analysis. Brain Behav Immun Health. (2022) 24:100484. doi: 10.1016/j.bbih.2022.100484, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.James KE, Bloch DA, Lee KK, Kraemer HC, Fuller RK. An index for assessing blindness in a multi-Centre clinical trial: disulfiram for alcohol cessation–a Va cooperative study. Stat Med. (1996) 15:1421–34. doi: [DOI] [PubMed] [Google Scholar]
- 70.R Core Team . (2019). R: A language and environment for statistical computing Vienna (AT): R foundation for statistical computing. Available at: https://www.R-project.org (Accessed May 26, 2024).
- 71.Montgomery AA, Peters TJ, Little P. Design, analysis and presentation of factorial randomised controlled trials. BMC Med Res Methodol. (2003) 3:26. doi: 10.1186/1471-2288-3-26, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collin C, Davies P, Mutiboko IK, Ratcliffe S. Randomized controlled trial of Cannabis-based medicine in Spasticity caused by multiple sclerosis. Eur J Neurol. (2007) 14:290–6. doi: 10.1111/j.1468-1331.2006.01639.x, PMID: [DOI] [PubMed] [Google Scholar]
- 73.Grimaldi AE, De Giglio L, Haggiag S, Bianco A, Cortese A, Crisafulli SG, et al. The influence of physiotherapy intervention on patients with multiple sclerosis-related Spasticity treated with Nabiximols (THC:CBD Oromucosal spray). PLoS One. (2019) 14:e0219670. doi: 10.1371/journal.pone.0219670, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Senn S. Change from baseline and analysis of covariance revisited. Stat Med. (2006) 25:4334–44. doi: 10.1002/sim.2682, PMID: [DOI] [PubMed] [Google Scholar]
- 75.Duquette P, Girard M. Hormonal factors in susceptibility to multiple sclerosis. Curr Opin Neurol Neurosurg. (1993) 6:195–201. PMID: [PubMed] [Google Scholar]
- 76.Alwan S, Dybalski M, Yee IM, Greenwood TM, Roger E, Nadeau N, et al. Multiple sclerosis and pregnancy: a comparison study. Can J Neurol Sci. (2013) 40:590–6. doi: 10.1017/s0317167100014724 [DOI] [PubMed] [Google Scholar]
- 77.Jobin C, Larochelle C, Parpal H, Coyle PK, Duquette P. Gender issues in multiple sclerosis: an update. Womens Health. (2010) 6:797–820. doi: 10.2217/whe.10.69, PMID: [DOI] [PubMed] [Google Scholar]
- 78.Dugas EN, Sylvestre MP, Ewusi-Boisvert E, Chaiton M, Montreuil A, O’Loughlin J. Early risk factors for daily Cannabis use in young adults. Can J Psychiatr. (2019) 64:329–37. doi: 10.1177/0706743718804541, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.National Institutes of Health, National Cancer Institute . Common Terminology Criteria for Adverse Events (Ctcae) Version 5.0: US Department of Health and Human Services. Bethesdam, MD: National Cancer Institute; (2017). [Google Scholar]
- 80.Maybee A, Clark B, McKinnon A, Angl EN. Evaluating the patient Partnership in Research. Toronto, ON: Ontario SPOR SUPPORT Unit; (2016). [Google Scholar]
- 81.Sipe JC, Arbour N, Gerber A, Beutler E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (Cb2) receptor gene polymorphism: possible risk for autoimmune disorders. J Leukoc Biol. (2005) 78:231–8. doi: 10.1189/jlb.0205111, PMID: [DOI] [PubMed] [Google Scholar]
- 82.Campbell R, Ju A, King MT, Rutherford C. Perceived benefits and limitations of using patient-reported outcome measures in clinical practice with individual patients: a systematic review of qualitative studies. Qual Life Res. (2022) 31:1597–620. doi: 10.1007/s11136-021-03003-z, PMID: [DOI] [PubMed] [Google Scholar]
- 83.Alami Marrouni K, Zertal A, Jutras-Aswad D, Arbour N, Duquette P. Efficacy and safety of cannabinoids in treating Spasticity and other symptoms of multiple sclerosis: a double-blind, randomized, placebo-controlled trial protocol. Can J Health Technol. (2024) 4:26. doi: 10.51731/cjht.2024.897 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.