Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Nov 29;14:29656. doi: 10.1038/s41598-024-77796-7
Abstract
The pharmaceutical industry has been primarily focused on developing synthetic drugs to address orofacial pain (OFP)-related conditions. There is limited knowledge regarding the efficacy of the use of herbal extracts in treating OFP. A systematic review and a meta-analysis of 62 randomized controlled trials assessing the analgesic effects of herbal extracts on pain intensity in various orofacial conditions was conducted. The intervention comprised the use of herbal extracts compared with a placebo and/or standard treatment. The primary outcome was pain intensity assessed before and after the intervention. The pain scores were compared with the baseline scores in each treatment. When compared with standard therapy, the pooled results of the patients who received herbal extracts revealed lower pain intensity in periodontal pain (MD = -0.92[-6.69, 4.85]), oral surgery pain (MD = 18.80[8.80, 28.79]), oral neuropathic pain (MD = 20.34[6.16, 34.52]), endodontic pain (MD = -8.04[-11.72, -4.37]), oral mucosal pain (MD = 8.74[2.76, 14.73]), and temporomandibular pain (MD = 30.94[6.04, 55.83]). The findings indicated a pain-attenuating effect of herbal extracts such as cannabis, turmeric, capsaicin, licorice, ginger, chamomile, clove, Hypericum perforatum, and Arnica montana. These findings revindicate that herbal extracts may be valuable alternatives to traditional pain medications and promising source for the development of new active ingredients for pharmaceuticals.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77796-7.
Introduction
Orofacial pain (OFP) refers to a range of painful conditions affecting the soft and hard tissues of the mouth, jaw, and face1. It poses a significant burden worldwide, affecting approximately 32.2% of the global population on average, ranging between 15.1% and 74.9%2. The diverse etiology and involvement of multiple anatomical structures often require a multidisciplinary management including both non-pharmacological and pharmacological treatments3. Recently, there has been a growing interest in exploring alternative and natural therapies to alleviate OFP4.
The pharmaceutical industry has primarily focused on developing synthetic drugs to address OFP-related conditions5. However, the adverse effects, drug interactions, and the need for personalized treatments due to the lack of effectiveness has caused researchers and healthcare professionals to seek alternative options4,6,7. Therefore, herbal extracts have gained attention as potentially valuable resources for pain management because of their natural origins, perceived safety profiles, and potential synergistic effects8.
Herbal extracts have been used for centuries in different cultures to alleviate orofacial pain; numerous plants have clinical applications after being constantly used by indigenous tribes worldwide9. These extracts possess pharmacodynamic characteristics and interact with receptors in a similar way as conventional drugs do. Nevertheless, one of the major concerns is the limited knowledge regarding their impact on oral tissues, mechanisms of action, and potential interactions10.
Several studies evaluating plants and their phytochemicals have revealed promising results regarding their analgesic effects11. Herbal extracts can be quite helpful for alleviate mild to moderate pain, and some of their bioactive constituents elicit analgesic and anti-inflammatory activities12. The mechanisms of action of these herbal extracts are often multifactorial and involve interactions with neurotransmitter receptors, modulation of inflammatory mediators, and inhibition of pain-related enzymes13.
Moreover, herbal extracts have shown promise as adjuncts in oral health care. Their potential to relieve OFP makes them attractive options for both patients and healthcare providers. The rising demand for natural and plant-based products in oral care has stimulated research and development in the use of herbal extracts for oral health promotion and disease prevention14.
This systematic review and meta-analysis aimed to comprehensively evaluate the literature on the use of herbal extracts for the management of OFP in humans. By synthesizing the available evidence, we sought to elucidate the potential benefits, efficacy, and safety of herbal extracts as adjunct therapies for OFP. Furthermore, this study aimed to identify knowledge gaps and provide valuable insights into future research directions and the integration of herbal extracts into OFP management.
Methods
Protocol and registration
This Systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline15. The review protocol was registered (CRD42022367553) in the International Prospective Register of Systematic Reviews (PROSPERO) system (https://www.crd.york.ac.uk/prospero/).
Eligibility criteria
This article addresses the following PICO question: What kinds of herbal extracts (intervention) are used as analgesics (outcome) to treat OFP in humans (population)?. Randomized controlled trials (RCT) (center, multicentered, parallel, and crossover design) and systematic reviews, both with and without meta-analyses, comparing the effectiveness and/or safety of herbal extracts for treating patients with OFP of any age against placebo or standard treatments were screened for inclusion. Perspective, opinion, commentary articles, case reports, and grey literature were excluded. Titles, abstracts, and full texts were reviewed according to the following criteria:
Inclusion Criteria: (a) original research studies published in any language; (b) studies including human subjects; (c) review articles on the use of herbal extracts to treat OFP. Exclusion Criteria: (a) studies not reporting a significant difference in the analgesic property; (b) studies in which the details of the study samples were not mentioned (dosage, timing, frequency, or administration route); (c) studies with no clear explanation of the herbal extracts used; (d) studies with missing data necessary for assessment in the meta-analysis.
Search strategy
The electronic databases were screened without time or language restrictions. The final refresh search was conducted on August 11, 2023. The terms used were medical subject heading (MeSH) terms, and the key words are available in the Supplementary File.
Study selection
Retrieved articles with abstracts were compiled in Mendeley software (Elsevier, New York, NY, USA) and uploaded to Rayyan.ai website for systematic literature reviews. After removing duplicate papers, the titles and abstracts of each study were independently screened by two authors. Following this initial evaluation, the full-text assessment of all potentially relevant publications was retrieved, and data from all relevant studies were extracted using a customized data extraction spreadsheet (Excel, Microsoft Corporation, Redmond WA, USA). Any disagreements regarding study eligibility of studies were resolved through consultation with a third author.
Data extraction and quality assessment
Data extractions was undertaken independently by the authors (SD, LB, JK, and AK) through a full-text assessment of the articles. Disagreements during study selection and data extraction were resolved by a third reviewer (JC). For each paper, the extracted data contained information on the study design, sample size, intervention, and measures of the effects and outcomes. Following data extraction, one author (SD) checked all the data entry fields for reliability. .
The exclusion criteria were as follows (i) trials using herbal synthetic chemicals and natural extracts from fungi, algae, and honey; (ii) studies using unvalidated outcome measurements, (iii) observational non-randomized and/or non-controlled studies; and, (iv) studies with measurements not expressed as mean and standard deviation or median and interquartile range.
For the quality assessment, one reviewer (SD) independently assessed the risk of bias for each study using the Cochrane risk of bias tool for randomized trials (RoB2)16. The evaluated domains included outcome measurements, random sequence generation, allocation concealment, selective reporting, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data and selective reporting were the domains evaluated. Each research result was classified as “low risk of bias”, “high risk of bias”, or “some concerns”.
The meta-analysis was performed by two reviewers (SD and DB) with the data from results involving a minimum of two measurements presented as a mean and standard deviation. These measurements were taken at specific timepoints when the pain symptoms were representative of each individual condition. Before the analysis, all the plant species were grouped into their respective plant families as shown in the doughnut chart in the Supplementary File. RevMan version 5.4.1 (Cochrane Collaboration, London, UK) was used to perform pairwise meta and subgroup analyses, and Stata 17.0 (StataCorp, College Station, TX, USA) was used to perform network meta-analyses.
Data synthesis
This study included only randomized controlled clinical trials that reported sample size, pain condition, herbal extract, administration route, dosage-time, clear data analysis, and validated and comparable scales of pain, such as the visual analogue scale (VAS), faces pain scale (FPS), numeric rating scale (NRS) and visual numeric scale (VNS).
Results
Study selection
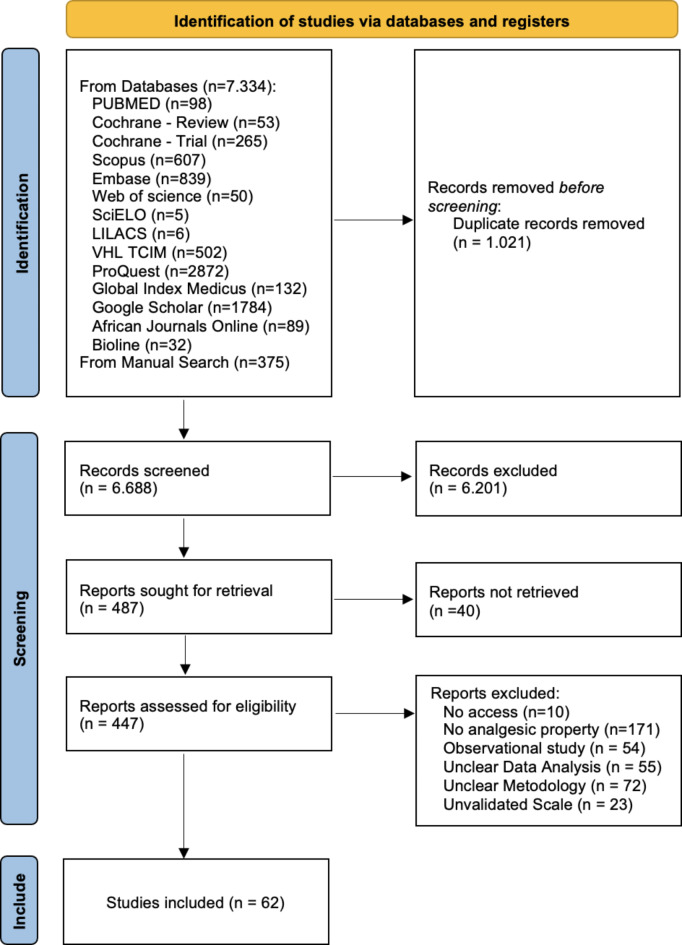
The database search identified 7709 studies. After deduplication, screening, and full-text assessment, 62 papers were included for data extraction (Fig. 1).
Fig. 1.
Study characteristics
Most studies were blinded (n = 47), and the sample sizes ranged from 15 to 270 patients. The study characteristics are shown in Table 1; 70 different plants from 44 plant families were identified (Supplementary File).
Table 1.
Characteristics of included clinical trials investigating herbal extracts for orofacial pain treatment categorized by type of orofacial pain.
| Refs. | Author, Year | Type of study | Condition | Plant | Administration route | Comparator | Dosage – Time |
|---|---|---|---|---|---|---|---|
| Periodontal Pain | |||||||
| 18 | Shahakbari et al., 2014 | SBRCT | Pericoronitis | Camellia sinensis | Green tea Mouthwash | Clorhexidine | Each patient received a 250-ml dark bottle containing the mouthwash (green tea 5%) and was instructed to rinse with this mouthwash twice a day for 7 days. |
| 23 | Keceli et al., 2015 | DBRCT | Free gingival graft | Glycyrrhiza glabra, Alpinia officinarum, Vitis vinifera,
Urtica dioica, Thymus vulgaris |
topical Ankaferd Blood Stopper | Placebo | After removing the graft, wet gauze with the herbal extract was compressed to the donor site by the surgeon during 60 s with moderate finger pressure. six-month follow-up |
| 33 | Patil et al., 2018 | RCT | Orthodontic pain | Atropa belladonna | Systemic Belladonna globules | Ibuprofen | In the Belladonna 6 C group, four globules given to patient. Patients were given two doses of medication of their respective groups, 1 h before placement of elastomeric separators which was administered in the department and one dose 6 h after the placement. |
| 25 | Das et al., 2019 | TBRCT | Periodontal flap | Arnica montana, Calendula officinalis, Matricaria chamomilla, Hypericum perforatum, Aconitum napellus, Bellis perennis, Atropa Belladonna, Echinacea purpurea, Echinacea angustifolia, Hamamelis virginiana, Achillea millefolium, Symphytum officinale | Systemic Traumeel tablets | Ibuprofen | Ibuprofen, 600 mg and traumeel, 600 mg (up to three tablets) every 8 h for first 24 h and SOS (Si Opus Sit/if needed) thereafter for a period of 1 week as pain medication, respectively. |
| 34 | Anil et al., 2019 | RCT | Periodontal flap | Curcuma longa | Topical Curcumin mucoadhesive film | Placebo | Curcumin mucoadhesive films of 0.5% were cut into smaller rectangular strips of 4–5 mm width and the length depending on the extent of flap surgery in each patient. UKirkland flap surgery was performed. After suturing, the preformed films were adapted on the gingiva in the test and control sites, respectively, over which periodontal pack (Coe-Pack) was placed |
| 39 | Alshibani et al., 2022 | SBRCT | Periodontal Therapy | Zingiber officinale | Systemic Ginger tablets | Ibuprofen | ginger tablets (400 mg) every 12 h for 3 days and then as needed for pain |
| 40 | Al-Askar et al., 2022 | SBRCT | Periodontal Therapy | Curcuma longa | Systemic Curcumin capsules | Mefenamic acid | Test group: patients received curcumin capsules (200 mg). The participants in the test and control groups were advised to orally take 1 MA tablet and 2 curcumin capsules, respectively, immediately after the procedure and then every 8 h for 3 days. After the third day, participants in the test and control groups were advised to take the respective analgesics as needed for pain. |
| Oral Surgery Pain | |||||||
| 44 | Alqareer et al., 2006 | SBRCT | Anesthesia infiltration pain (pre-surgery) | Syzygium aromaticum | Topical Clove gel | 1: Benzocaine
2: Placebo |
home made clove gel. Approximately 2 g of material were applied to the buccal mucosa superior to the gingiva over the canine prominence, covering an area of about 1.5 cm in diameter for 4 min and then was reapplied for another minute. The material was reapplied because the authors were concerned about material washout by saliva. |
| 21 | Mohite et al., 2020 | SBRCT | Anesthesia infiltration pain (pre-surgery) | Anacyclus pyrethrum, Spilanthes acmella | Topical herbal anesthesic in gel | Lignocaine | After isolation, the test region was dried by utilizing a sterile cotton gauze. The topical anesthetic to be tested was drawn for each participant and applied using a cotton applicator stick. After 10 min, a 26gauge sterile needle was inserted . |
| 42 | Jesudasan et al., 2015 | DBRCT | Alveolar osteitis (post-surgery) | Syzygium aromaticum | Topical Eugenol paste | 1: Chlorhexidine
2: No-treatment |
After the third molar was extracted, eugenol-based paste was applied to the socket |
| 41 | Rayati et al., 2017 | DBRCT | Extraction (surgery) | Zingiber officinale | Systemic ginger capsules | 1: Ibuprofen
2: Placebo |
one capsule of ginger containing 500 mg of ginger rhizome powder (Zintoma; Goldaru Co., Iran) All medications were administered orally and 6 hourly, 500 mg of ginger rhizome powder |
| 43 | Komasawa et al., 2018 | SBRCT | Extraction (surgery) | Cinnamomi Cassiae, Syzygium aromaticum, Glycyrrhiza Uralensis, Ligusticum wallichii, Nuphar japonica, Quercus robur, Rheum rhabarbarum | Systemic Jidabokuippo granules | No-Treatment | Patients were given three oral doses (2.5 g each) of JDI (TJ-89, Tsumura Co, Tokyo, Japan) just before falling asleep the night before surgery, and in the morning and around noon on the day of surgery (total 7.5 g). |
| 22 | De Souza et al., 2021 | TBRCT | Extraction (surgery) | Arnica montana, Calendula officinalis, Matricaria chamomilla, Hypericum perforatum, Aconitum napellus, Bellis perennis, Atropa Belladonna, Echinacea purpurea, Echinacea angustifolia, Hamamelis virginiana, Achillea millefolium, Symphytum officinale | Traumeel S intramuscular injection | Dexamethasone | Right after anesthezing an intramuscular injection in the masseter muscle of 2 mL at three different points |
| Oral Neuropathic Pain | |||||||
| 45 | Marino et al., 2010 | DBRCT | Burning mouth syndrome | Capsicum annuum | Capsaicin mouthwash | 1: Alpha-lipoic
2: Lysozymelactoperoxidase 3: Boric acid |
daily oral rinses with capsaicin, 250 mg of red pepper emulsion in 50 ml of water for |
| 24 | Spanemberg et al., 2012 | DBRCT | Burning mouth syndrome | Paullinia cupana, Trichilia catigua, Zingiber officinale, Ptychopetalum
olacoides |
Systemic Catuama capsules | Magnesium silicate | take 2 capsules a day, before lunch and dinner, for 8 weeks after the first evaluation. |
| 46 | Pakfetrat et al., 2019 | DBRCT | Burning mouth syndrome | Saffron | Systemic Crocin Tablets | Citalopram | For one group, citalopram (Sobhan Darou, Iran) was given orally once daily with an initial dose of 10 mg that increased to 20 mg after a week. For the other group, crocin tablets 15 mg (prepared by a pharmacologist) was prescribed twice daily. Both groups received the treatments for 11 weeks. |
| 47 | Bessho et al., 1998 | RCT | Burning mouth syndrome | Bupleurum chinense, Pinellia ternata, Scutellaria baicalensis, Magnoliae Officinalis, Ziziphus mauritiana, Panax ginseng, Glycyrrhiza Glabra, Perilla frutescens, Zingiber officinale | Systemic Sai-boku-to Tablets | Diazepam + Vitamin B | Oral administration of 2.5 g of Sai-boku-to (TJ-96, Tsumura & Co, Tokyo, Japan) 3 times per day (before meals) for 3 months was prescribed. |
| 48 | Lee et al., 2007 | DBRCT | Facial sensitivity and pain | Capsicum annuum | Topical Capsaicin cream | No-Treatment | Topical capsaicin cream (0·075%), which was applied to the mental area unilaterally, four times daily for 2 weeks. Around 40 µL of capsaicin cream (0·075%)* was applied topically four times daily for 2 weeks on a 4-cm2 area in the treatment side of the mental area. Subjects were instructed to squeeze a 6-mm long strip of capsaicin cream onto a cotton swab and vigorously apply it over the area |
| Endodontic Pain | |||||||
| 49 | Majji & Murthy, 2016 | SBRCT | Dentinal hypersensitivity | Calendula officinalis, Plantago major | Calendula and plantago Toothpaste | 1: Potassium nitrate
2: Calcium sodium phosphosilicate 3: Strontium chloride |
Each patient was advised to brush their teeth in the usual manner for 3 min, twice daily, with soft bristle toothbrush, and to apply the dentifrice in an amount equal to about half the length of the bristle head. |
| 20 | Kar et al., 2019 | DBRCT | Dentinal hypersensitivity | Spinacia oleracea, Syzygium aromaticum, Terminalia chebula, Terminalia bellirica, Phyllanthus emblica | Topical paste of palakya, lavanga, and triphala. | 1: Potassium salt
2: Arginine |
Desensitizing paste was applied over the isolated hypersensitive area. Using a disposable applicator tip, pea-sized amount of the toothpaste was applied over the isolated hypersensitive area of the tooth for 5 s, and a rotary polishing cup at moderate-to-high speed was used to polish the paste over this surface for 1 min. |
| Oral Mucosal Pain | |||||||
| 17 | Liu et al., 2022 | DBRCT | Orthodontic wounds | Glycyrrhiza Glabra | Licorice mouthwash | Placebo | 200 cc mouthwash bottle at the start of the study and after 2 days. The patients rinsed with the mouthwash for 10–20 s and four times daily. |
| 50 | Mansour et al., 2014 | DBRCT | Aphthous ulcers | Commiphora myrrh | topical myrrh gel | Placebo | apply the drug to the ulcer four times a day (after meals and before bedtime) for 5 days using finger or cotton tip applicator and to refrain from eating and drinking for 30 min after application |
| 28 | Tadbir et al., 2015 | DBRCT | Aphthous ulcers | Matricaria chamomilla | Topical chamomile application | 1: Placebo
2: Triamcinolone |
Chamomile in Orabase |
| 51 | Motallebnejad et al., 2008 | DBRCT | Aphthous ulcers | Hypericum perforatum | Hypericum perforatum mouthwash | 1: No-treatment
2: Placebo |
topical hypericum containing mouthwash (0.5%) for seven days |
| 26 | Jiang et al., 2012 | DBRCT | Aphthous ulcers | Allium sativum | Allicin Garlic oral adhesive tablets | Placebo | Subjects were instructed to apply 1 Allicin oral adhesive tablets 5 mg to the appointed ulcer 4 times a day (after meals and before bedtime) for 5 days (day 1 to day 5). |
| 121 | Deshmukh & Bagewadi, 2014 | DBRCT | Aphthous ulcers | Curcuma longa | Topical Curenext gel | Triamcinolone | apply the gel three times a day on each ulcer after meals and not to consume food or water for half an hour after application. All patients were provided with same measuring applicator and were instructed about the quantity and method of gel application |
| 52 | Pourahmad et al., 2010 | DBRCT | Aphthous ulcers | Vachellia erioloba | Camel thorn swish and swallow mouthwash | Placebo | (distillate) The patients were instructed to use 40 milliliters of the solution 4 times a day until they experienced complete resolution of their symptoms. The patients were instructed to keep the drug in their mouths for one minute and then swallow it. The camel thorn dose administered to patients was based on the normal dose used in Iranian folk medicine. |
| 53 | Babaee et al., 2012 | DBRCT | Aphthous ulcers | Aloe vera | Topical Aloe vera gel | Placebo | To apply the gel on the lesions three-times a day by the patients for at
least ten days. |
| 122 | Kia et al., 2020a | DBRCT | Aphthous ulcers | Curcuma longa | topical Curcumin orabase paste | Triamcinolone | 5% of Curcumin orabase apply the orabase three times a day after eating meals, for a 10-day period. The patients were asked to clean the lesion by soft and dry clean
gauze and then put 1 cm of the orabase on the wet tip of their fingers and dab it on the lesion with no rubbing action. Patients were advised to avoid eating and drinking for at least half an hour after drug application |
| 123 | Halim et al., 2013 | SBRCT | Aphthous ulcers | Curcuma longa | Topical turmeric powder | Triamcinolone | apply the medication twice per day for 5 days |
| 54 | Yang et al., 2016 | DBRCT | Aphthous ulcers | Taraxacum mongolicum, Isatis indigotica, Corydalis bungeana, Scutellaria baicalensis | Pudilan Keyanning toothpaste | Placebo | brush their teeth for 2–3 min, twice a day (in the morning and evening), each time covering two thirds the length of the toothbrush provided. After brushing the participants were asked to apply a little toothpaste to cover the ulcer surface with a cotton swab provided for 6 days |
| 29 | Ghalayani et al., 2013 | DBRCT | Aphthous ulcers | Punica granatum | Topical Punica granatum gel | Placebo | apply the gel three times daily by placing a small sterile cotton pad impregnated with gel on the lesions for 1 min. They were asked not to eat for at least 30 min after administering the preparations. |
| 55 | Babaee et al., 2010 | DBRCT | Aphthous ulcers | Myrtus communis | Topical Myrtle paste | Placebo | The paste was applied by subjects themselves four times a day for 6 days |
| 30 | Jin et al., 2017 | RCT | Aphthous ulcers | Taraxacum mongolicum, Isatis indigotica, Corydalis bungeana, Scutellaria baicalensis | Systemic Pudilan oral solution | Placebo | Pudilan oral solution for 8 days at a dosage of 10 mL three times per day |
| 124 | Thomas et al., 2017 | DBRCT | Oral lichen planus | Curcuma longa | Topical curcumin gel | Triamcinolone | Group 2 (curcumin oral gel thrice daily) and Group 3 (curcumin oral gel six times daily). Curenext Oral Gel (Piramel, Health Care, India) each gram of which contains curcuma longa extracts 10 mg having 1% Curcuminoids. |
| 125 | Kia et al., 2020b | DBRCT | Oral lichen planus | Curcuma longa | Systemic Nano-Curcumin capsule | Prednisolone | take one capsule of Nano-Curcumin 80 mg after having their breakfast |
| 56 | Jornet & Aznar-Cayuela, 2016 | RCT | Oral lichen planus | Matricaria chamomilla | Topical Chamomile gel | Placebo | The patients received Topical Chamomile gel 2% together with instructions for their correct use. The preparations (0.5 mL/3 times a day) were applied uniformly to the oral cavity in the areas that presented symptoms, spreading the gel with the finger. |
| 31 | Keshari et al., 2015 | RCT | Oral lichen planus | Curcuma longa | Topical curcumin ointment | Triamcinolone | Topical curcumin ointment (commercially available as Curenext Oral gel-Abbott Pharmaceuticals, India) each to be applied thrice daily for 2 weeks. |
| 32 | Chainani-Wu et al., 2012 | RCT | Oral lichen planus | Curcuma longa | Systemic Curcumin C3 complex softgel | Placebo | 6000 mg/d in 3 divided doses for 12 days |
| 57 | Mansourian et al., 2011 | DBRCT | Oral lichen planus | Aloe vera | Aloe vera mouthwash | Triamcinolone | rinse the mouth with 2 tablespoons of AV mouthwash for 2 min, 4 times a day and expectorate. |
| 58 | Hasheminasab et al., 2020 | DBRCT | Oral Mucositis | Plantago ovata | Plantago ovata mouthwash | 1:Placebo
2: No-Treatment |
The herbal compound consisted of a mixture of 500 mg of P. ovate husk in 30 mL water plus three drops of vinegar per dose, three times per day during their next chemotherapy cycle (cycle 1 of treatment) |
| 63 | Elhadad et al., 2020 | SBRCT | Oral Mucositis | Matricaria chamomilla | Topical chamomile gel | Miconazol + Benzocaine | chamomile topical oral gel 3% alone which was prescribed three times daily, one day prior to the scheduled cycle of chemotherapy, lasting for 3 weeks |
| 65 | De Cássia Dias Viana Andrade et al., 2022 | SBRCT | Oral Mucositis | Curcuma longa | Topical Curcumin photosensitizing agent | 1: Photobiomodulation
2: Nystatin |
The curcumin solution (10 mL) was sprayed inside the oral cavity remained for 10 min for impregnation with the mouth closed then the oral cavity was illuminated with a blue diode light emitter for 10 min. This was performed 1 time a week for 30 days, totalized 4 applications, during the period in which the patient was undergoing radiation and/or chemotherapy. |
| 59 | Aghamohammadi et al., 2018 | DBRCT | Oral Mucositis | Zataria multiflora | Zataria Mouthwash | Placebo | Patients gargled the ZM mouthwash or a placebo before the beginning of the treatment three times daily and before each radiotherapy session. |
| 60 | Soltani et al., 2020 | DBRCT | Oral Mucositis | Plantago major | oral consumption of plantago major syrup | Placebo | take plantago major L syrup 7.5 cc, three times a day for 7 weeks, from three days before the start of radiotherapy to the end of it. Patients were advised not to rinse their mouth for half an hour after taking the drug |
| 61 | Kia et al., 2021 | DBRCT | Oral Mucositis | Curcuma longa | Systemic Curcumin Capsules | Placebo | The study group was received Curcumin nanomicelle capsules 80 mg twice a day and the control group took placebo two times a day for 7 weeks. |
| 35 | Patil et al., 2015 | RCT | Oral Mucositis | Curcuma longa | Curcumin mouthwash | Clorhexidine | curcumin mouthrinse 0.004% to be used in 1:5 dilution for 1 min, three times daily for twenty days |
| 36 | Mansouri et al., 2016 | RCT | Oral Mucositis | Aloe vera | Aloe vera mouthwash | Placebo | 5 ml of aloe vera solution for two minutes three times a day for 14 days. |
| 37 | Hussain et al., 2019 | RCT | Oral Mucositis | Nigella sativa | Nigella sativa oil Mouthwash | Nystatin + Tetracycline + Lidocaine + Dexamethasone | The patients in both groups received the NS oil mouth rinse and the magic mouthwash topically as a mouth rinse (10 ml each 6 h) daily, starting from the first day after the initiation of CT up to day 28 (the end of the CT). |
| 62 | Monsen et al., 2021 | SBRCT | Oral Mucositis | Salvia officinalis | Salvia officinalis Mouthwash | Placebo | Salvia officinalis solution consisting of 2.5 g SO herbal tea/100 ml water. The SO herbal tea solution was based on dry extract of Salvia officinalis leaves from the Hospital Pharmacy (Sanivo Pharma AS), which were steeped for 2 min in boiled water. rinsing
with the assigned solution (10–15 ml) twice for 30s four times a day, and after each rinsing. |
| 38 | Wang et al., 2018 | RCT | Oral Mucositis | Rheum rhabarbarum, Glycyrrhiza glabra, Mentha piperita, Scutellaria baicalensis, Liriope, Paeonia lactiflora, Scrophularia ningpoensis, Forsythia | Systemic Chining decoction | Epidermal growth factor | mixed with hot water, 200 mL daily, taken morning and evening, 100 mL each time, from the first day of radiotherapy until the completion of radiotherapy |
| 64 | Reis et al., 2016 | SBRCT | Oral mucositis | Matricaria chamomilla | Chamomile infusion Cryotherapy | Cryotherapy | Patients in the chamomile group received a cup of ice chips made with chamomile infusion at 2.5%. Patients were instructed to swish the ice around in their oral cavity for atleast 30 min, starting 5 min before the chemotherapy infusion. |
| 19 | Najafi et al., 2017 | DBRCT | Oral Mucositis | Glycyrrhiza Glabra | Glycyrrhiza mouthwash | Placebo | Glycyrrhiza or placebo were given to patients and they were asked to use 20 cc twice per day for 14 days after starting radiotherapy |
| 66 | Piyush et al., 2018 | DBRCT | Oral submucous fibrosis | Curcuma longa | Systemic Curcumin tablet | Placebo | Curcumin tablet (300 mg) twice daily or Lycopene capsules (8 mg) twice daily for six months |
| 126 | Jiang et al., 2013 | RCT | Oral submucous fibrosis | Salvia Miltiorrhiza | Salvianolic acid B Intralesional injection | Triamcinolone | All patients received an intralesional injection after 5 min of local anesthetic cream application (20% Topcaine; Medental, Balama City, USA) at weekly intervals for 20 weeks. Salvianolic acid B (4 mg). |
| 27 | Yadav et al., 2014 | RCT | Oral submucous fibrosis | Curcuma longa | Systemic Turmix tablets | Dexamethasone + Hialuronic Acid + Lignocaine | oral administration of 2 tablets of Turmix given once daily for a period of 3 months |
| 67 | Hazarey et al., 2015 | RCT | Oral submucous fibrosis | Curcuma longa | Longvida Curcumin tablets | Clobethasol | Longvida lozenges (Mfg Lic.: GA/1482) (400 mg lozenges) manufactured by Pharmanza Herbal Pvt. Ltd. The total daily dose decided was 2 g of Longvida lozenges. |
| 68 | Srivastava et al., 2021 | RCT | Oral submucous fibrosis | Curcuma longa, Syzygium aromaticum | Systemic TurmNova lozenges and Clove oil | Dexamethasone | Patients were administered curcumin lozenges (TurmNova®, Gelnova Laboratories Pvt. Ltd, Navi Mumbai, India) containing turmeric extract 100 mg along with clove oil 10 mg three times daily for 3 months. Patients were advised to chew these lozenges slowly followed by swallowing |
| Temporomandibular Disorder Pain | |||||||
| 71 | Chaimano et al., 2021 | DBRCT | Temporomandibular disorder | Zingiber cassumunar, Curcuma longa, Cinnamomum camphora | Thai herbal compress ball | Placebo | The compress balls are applied on the painful muscle at least once a day. Two compress balls should be steamed in a stacked electric steamer pot for twenty minutes. After that, the first warm ball (approx 40ºC) was applied to the jaw muscle, then replaced with the second one when it was slightly lukewarm. The two herbal balls were alternately steamed and alternately used for twenty minutes. After each application, the balls were wrapped in a plastic bag and kept in the freezer until they could be reused. This study employed the reuse of herbal balls daily for one month |
| 69 | Li et al., 2009 | DBRCT | Temporomandibular disorder | Mentha piperita, Cinnamomum camphora, Gaultheria fragrantissima, Santalum album, Eucalyptus | Topical Ping On ointment | Placebo | rub the ointment over the painful area and then to massage in a circular motion for 5 min twice a day. The area of application was just on the skin around the TMJ and affected muscles, which were usually the temporalis and masseters. |
| 72 | Nitecka-Buchta et al., 2019 | DBRCT | Temporomandibular disorder | Cannabis | Topical CBD oil | Placebo | Oleum CBD 2.0 g (20% CBD oil). Group1 received CBD formulation. topical use to be applied on the skin surface of the masseter muscle, at the right and left side. Each patient had been taught on the procedure to apply the formulation in equal amounts (the size of peas) on both sides. Patients were informed that the formulation should be applied and rubbed gently into the skin surface (approximately 4 × 4 cm) and were supposed to apply it twice a day for up to a period of 14 days before the follow-up visit. |
| 70 | Campbell et al., 2016 | DBRCT | Temporomandibular disorder | Capsicum annuum | Topical Capsaicin cream | Placebo | Topical capsaicin cream (8%), The investigator spread a 0.1-mL dollop of cream to a standardized area overlying the affected TMJ and superficial masseter and then covered the site with a cotton gauge for 2 h. This area extended 3 cm anteriorly and inferiorly from the posterior aspect of the TMJ. A square 3 × 3-cm template was cut into a rubber dental dam to standardize the application site. The drug was applied to the right or left side randomly for subjects without TMD, whereas for the TMD sufferers, the side that was reported as more painful was studied. |
Risk of bias
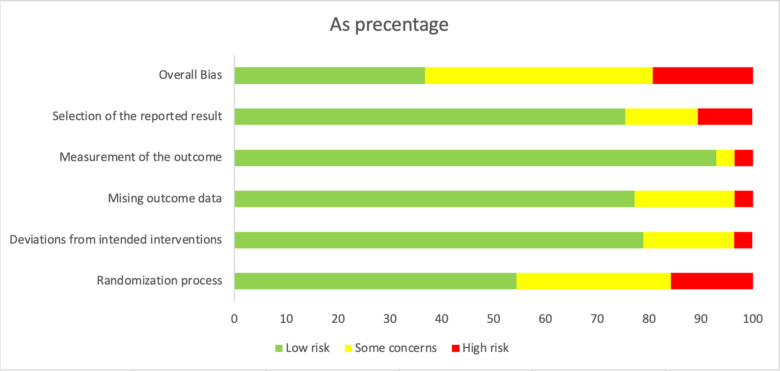
Regarding the risk of bias assessment, the majority of the studies had some concerns (n = 27), followed by those with low risk (n = 22) and high risk (n = 13). Six studies had a high risk in selection of the reported results17–22; 2, outcome measurement23,24; 2, missing outcome data19,25; 2, deviation from intended intervention26,27; and, 11, randomization process28–38 (Fig. 2).
Fig. 2.
Synthesis of results
The 62 papers identified 17 painful orofacial conditions, which were categorized into six distinct groups based on their origin: periodontal pain, endodontic pain, oral mucosal pain, oral neuropathic pain, oral surgery pain, and temporomandibular disorder (TMD) pain (Table 1).
Periodontal pain
The periodontal pain group included patients with five conditions: pericoronitis, free gingival graft, orthodontic pain, periodontal flap, and both surgical and non-surgical periodontal therapy.
In the study led by Shahakbari et al. (2014), the pain associated with pericoronitis notably reduced in 97 patients treated with green tea compared with that in those treated with 0.12% chlorhexidine. Similarly, Keceli et al., (2015) conducted a clinical trial involving 33 patients with free gingival grafts, where they noted a significant improvement in pain relief when a topical Ankaferd Blood Stopper was administered compared to placebo.
Based on a study of 80 patients experiencing orthodontic pain, Patil et al., (2018) revealed that belladonna exhibited superior analgesic properties compared to that of ibuprofen. Meanwhile, Das et al., (2019) clinical trial involving 20 patients with periodontal flap showed that those treated with Traumeel exhibited lower pain scores than those treated with ibuprofen. Additionally, in a study conducted by Anil et al., (2019) involving 15 patients (30 sites) with periodontal flaps, significant analgesic properties were observed for curcumin when compared to placebo.
Alshibani et al., (2022) examined the effects of ginger tablets in a cohort of 44 patients, whereas Al-Askar et al., (2022) administered curcumin capsules to 76 patients, all of whom had undergone periodontal therapy. In both investigations, no statistically significant differences were observed between the intervention and control groups which were given ibuprofen and mefenamic acid, respectively.
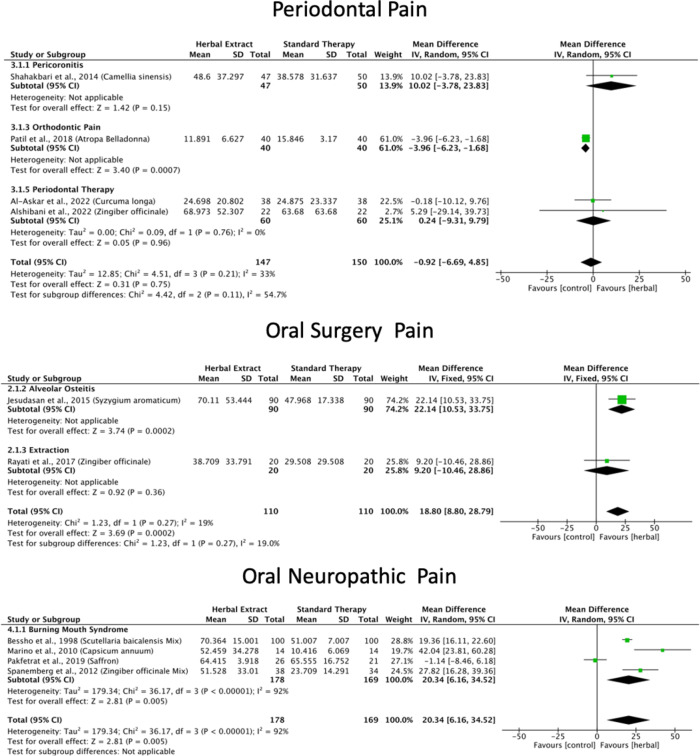
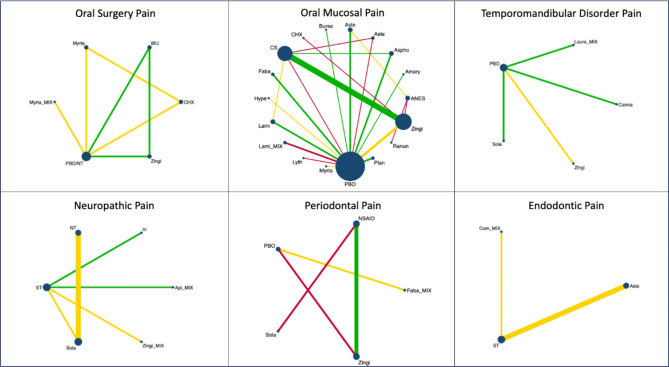
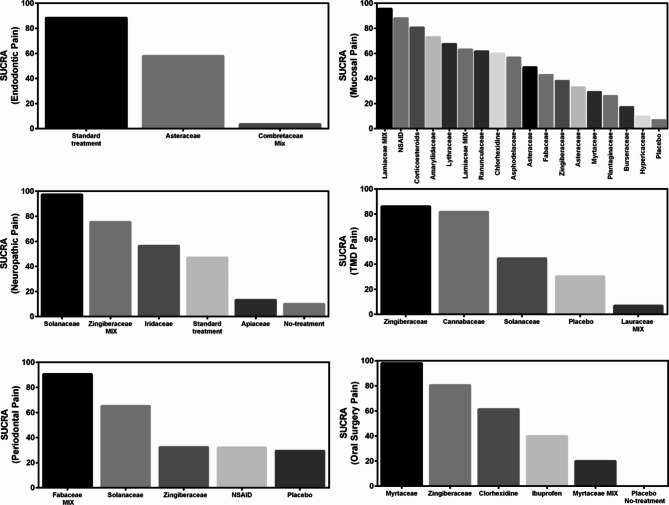
A meta-analysis of six studies18,23,33,34,39,40 revealed that green tea, Atropa belladonna, curcumin and ginger were more effective in reducing periodontal pain as compared to standard therapies (Fig. 3), and Ankaferd Blood Stopper and curcumin were more effective in reducing periodontal pain as compared to the placebo (Supplementary File). Network meta-analysis and ranking based on the probability of each treatment being the best were performed among the five interventions (Fig. 5). The surface under the cumulative ranking curve (SUCRA) of the treatment with Fabaceae combination and Solanaceae was 90.6% and 65.3%, respectively, confirming that these plant families are the top two best interventions for periodontal pain over Zingiberaceae (32.2%), NSAID’s (32.4%) and placebo (29.5%) interventions (Fig. 6).
Fig. 3.
The other two studies could not be included in the meta-analysis owing to insufficient measurements over time25, and lack of transitivity18.
Oral surgery pain
Among the included studies, three conditions were identified: anesthesia infiltration pain, alveolar osteitis, and extraction.
Jesudasan et al. (2015) examined the efficacy of a eugenol paste derived from cloves in 270 patients with alveolar osteitis. Patients treated with eugenol experienced significantly greater relief in postoperative pain, inflammation, infection, and wound healing than that of those treated with 0.2% chlorhexidine gel. Various topical formulations have been investigated in clinical studies to address the pain caused by anesthetic infiltration. For instance, Alqareer et al. (2006) evaluated a gel containing clove in 73 patients, whereas Mohite et al. (2020) used interventions with Anacyclus pyrethrum and Spilanthes acmella gels in 30 patients. In both studies, no statistically significant differences in pain were found in comparison with the control groups (benzocaine and lignocaine gels).
In a clinical trial involving 60 patients41, ginger powder proved to be as effective as ibuprofen for managing postsurgical sequelae after extraction. In a study conducted by Komasawa et al. (2018) involving 60 patients, the researchers investigated the efficacy of preoperative administration of Jidabokuippo, a combination of botanical extracts, including Cinnamomi cassiae, Clove, Licorice, Ligusticum wallichii, Nuphar japonica, Quercus robur, and Rheum rhabarbarum. They compared the effects of Jidabokuippo in the management of pain after tooth extraction with those of a no-treatment group. The study’s findings revealed that the severity of postoperative pain was significantly reduced in the Jidabokuippo group at 3 and 24 h after anesthesia recovery.
A homeopathic medicine known as Traumeel (that containing Arnica montana, Calendula, Chamomile, St. John’s wort, Aconitum napellus, Bellis perennis, Atropa Belladonna, Echinacea purpurea, Echinacea angustifolia, Hamamelis virginiana, Achillea millefolium, Symphytum officinale) has been used for pain, edema, and trismus relief after third molar surgery in 17 patients22, suggesting that Traumeel might be a good alternative which is comparable to dexamethasone.
The meta-analysis of the three studies41–43 found that clove and ginger were more effective in reducing oral surgery pain than standard therapies (Fig. 3), and Jidabokuippo, clove and ginger were more effective in reducing oral surgery pain than the negative control or placebo (Supplementary File). The network meta-analysis and ranking based on the probability of each treatment being the best were performed for the six interventions (Fig. 5). The SUCRA of the treatment with Myrtaceae and Zingiberaceae was 98% and 80.6%, respectively, confirming that these two plant families are the best interventions for oral surgical pain over chlorhexidine (61.4%), Myrtaceae combination (20%), and placebo/no-treatment (0%) interventions (Fig. 6). The other three studies could not be included in the meta-analysis because of insufficient measurements over time21,44, and data not being expressed as mean and standard deviation22.
Oral neuropathic pain
Two conditions were identified in the studies included in the oral neuropathic pain group: burning mouth syndrome (BMS) and facial pain and sensitivity to mechanical, cold, and heat stimuli.
Marino et al. (2010) conducted a study of 56 individuals diagnosed with BMS who were treated with capsaicin, alpha-lipoic acid, lysozyme-lactoperoxidase, or boric acid. The results revealed a significant symptom score reduction in patients treated with capsaicin, alpha-lipoic acid, and lysozyme-lactoperoxidase, showing higher effectiveness than boric acid treatment. Spanemberg et al. (2012) examined the impact of Catuama, a herbal treatment containing Paullinia cupana, Trichilia catigua, ginger, and Ptychopetalum olacoides, in 72 patients with BMS. The results revealed a notable enhancement in the test group compared to magnesium silicate after a 4-week treatment period. Furthermore, this considerable improvement persisted even 12 weeks after treatment initiation.
Pakfetrat et al. (2019) conducted a clinical trial involving 47 patients with BMS treated with crocin, a herbal extract derived from saffron. The results of an 11-week trial demonstrated that crocin significantly decreased the severity of BMS symptoms, comparable to the effects of citalopram. In a study involving 200 patients, Bessho et al. (1998) employed sai-boku-to, a herbal extract derived from Bupleurum chinense, Pinellia ternata, Scutellaria baicalensis, Magnoliae Officinalis, Ziziphus mauritiana, Panax ginseng, licorice, Perilla frutescens, and ginger, for treating BMS. The results demonstrated that sai-boku-to exhibited effectiveness comparable to Diazepam + Vitamin B in reducing pain, burning sensations, and discomfort.
In a clinical trial conducted by Lee et al. (2007), the application of capsaicin to the facial skin of 40 patients resulted in decreased sensitivity to mechanical, heat, and cold-induced pain. Interestingly, this reduction in pain sensitivity occurred without affecting non-painful tactile sensations, as evidenced by a comparison with the pain sensitivity in the control group that did not receive capsaicin treatment.
According to the comprehensive meta-analysis of the five studies24,45–48, Catuama, crocin, Sai-boku-to, and capsaicin exhibited greater efficacy in reducing oral neuropathic pain compared to conventional treatment methods (Fig. 3). Moreover, capsaicin showed superior effectiveness in alleviating facial pain compared to that of the negative control (Supplementary File). Network meta-analysis and the ranking based on the probability of each treatment being the best was performed among the six interventions (Fig. 5). The SUCRA of the treatment with Solanaceae was 97.4%; Zingiberaceae combination, 75.6%; and Iridaceae, 56.5%, confirming that these three plant families are the best interventions for oral neuropathic pain over standard treatment (47.1%), Apiaceae combination (13.3%) and no-treatment (10.1%) interventions (Fig. 6).
Endodontic pain
The included studies focused on dentinal hypersensitivity (DH) and used tactile and air stimuli to measure DH levels.
In a study conducted by Majji and Murthy (2016), 160 patients were divided into four groups, each assigned to a different type of desensitizing toothpaste. The toothpaste formulations were evaluated. The findings indicated that all four toothpaste types (5% potassium nitrate, 5% CSPS (NovaMin), 10% strontium chloride, and a herbal formulation containing Calendula and Plantago major), effectively relieved dentinal hypersensitivity. Notably, the CSPS group demonstrated the most favorable clinical response at the end of the two-month period.
On the other hand, Kar et al. (2019) conducted a study with 45 adults, dividing them into three groups, each using a different type of toothpaste: potassium salt, 8% arginine, or a herbal desensitizing paste containing Spinacia oleracea, Clove, Terminalia chebula, Terminalia bellirica, and Phyllanthus emblica. The results of this study showed that the herbal toothpaste was more effective than the potassium nitrate-containing toothpaste in reducing dentinal hypersensitivity. However, the toothpaste containing 8% arginine was found to be the most effective in reducing DH.
According to the meta-analysis conducted in the two studies20,49, the standard therapies, typically found in commercially available desensitizing toothpastes, proved to be more effective in reducing endodontic pain than the Calendula, plantago, palakya, lavanga, and triphala toothpastes (Fig. 4). Network analysis and the ranking based on the probability of each treatment being the best were performed for the three interventions (Fig. 5). The SUCRA of the standard treatment was 88.3%, and for Asteraceae was 58%, confirming that these two treatments were the best interventions for endodontic pain over the Combretaceae combination (3.6%) intervention (Fig. 6).
Fig. 4.
Fig. 5.
Fig. 6.
Oral mucosal pain
Out of the 38 studies included into the oral mucosal pain category, five conditions were identified: aphthous ulcers, oral mucositis induced by chemotherapy and/or radiotherapy, oral submucous fibrosis, oral lichen planus, and oral mucosal wounds resulting from orthodontic treatment.
Numerous clinical trials have investigated diverse herbal topical formulations to alleviate pain and discomfort associated with aphthous ulcers. For instance, the effects of turmeric have been examined by Deshmukh and Bagewadi (2014), Kia et al. (2020a), and Halim et al. (2013), whereas those of chamomile have been studied by Tadbir et al. (2015). These studies found no statistically significant differences in the alleviation of pain when compared with triamcinolone. Conversely, other studies have compared multiple herbal extracts, such as myrrh50, Hypericum perforatum51, allicin26, camel thorn52, Aloe vera53, pudilan30,54, Punica granatum29, and myrtle55. These studies found significantly better analgesic properties compared with placebos.
Curcumin’s efficacy as a pain-relieving remedy for patients with oral lichen planus was studied by Thomas et al. (2017), Kia et al. (2020b), Keshari et al. (2015), and Chainani-Wu et al. (2012). The results demonstrated significant analgesic properties, in comparison to triamcinolone, prednisolone, and placebo. Furthermore, the analgesic attributes of patients with oral lichen planus treated with chamomile56 or Aloe vera57 where compared with those treated with placebo and triamcinolone, respectively.
Multiple herbal extracts have been studied for the treatment of oral mucositis caused by chemotherapy and/or radiotherapy. For instance, significant analgesic properties have been reported for Plantago ovata58, Zataria multiflora59, Plantago mayor60, curcumin61, Aloe vera36, Salvia officinalis62 and licorice19 when compared with placebo. Other studies have found pain-relieving properties of chamomile63,64, curcumin35,65, Nigella sativa37, and chinning decoctions38 when compared to standard therapies.
The efficacy of curcumin as an analgesic has been studied in patients with oral submucous fibrosis27,66–68 when compared to placebo or standard therapies. Jiang et al. (2013) reported the analgesic properties of salvianolic acid in pain related to oral submucous fibrosis. In contrasts, Liu et al. (2022) found that licorice exerts analgesic effects on oral mucosal wounds resulting from orthodontic treatment.
According to a comprehensive meta-analysis of 36 studies, Curcuma longa, chamomile, Aloe vera, Nigella sativa, chinning decoction, Salvia miltiorrhiza, and clove exhibited greater efficacy in reducing oral mucosal pain than that of standard therapies (Fig. 4). Moreover, licorice, myrtle, Aloe vera, Punica grantum, allicin, pudilan, myrrh, St. John’s wort, camel thorn, chamomile, Zataria multiflora, Plantago ovata, Curcuma longa, Salvia officinalis and Plantago major had superior effectiveness in alleviating oral mucosal pain in comparison to placebo (Supplementary File).
The network meta-analysis and the ranking based on the probability of each treatment being the best were performed for the 18 interventions (Fig. 5). The SUCRA of the treatment with Lamiaceae was 95.6%; anesthetics, 88.1%; corticoiesteroids, 80.7; Amaryllidaceae, 73.1%; Lythraceae, 67.5%; Lamiaceae combination, 63.2%; Ranunculaceae, 61.6%; and, chlorhexidine, 60%, confirming that these are the best eigth interventions for oral mucosal pain over Asphodelaceae (57%), Asteraceae (49%), Fabaceae (43%), Zingiberaceae (38.3%), Asteraceae (33.2%), Myrtaceae (29.4%), Plantaginaceae (26.3%), Burseraceae (17.4%), Hypericaceae (9.8%), and placebo (7%) interventions (Fig. 6). Two studies were not included in the meta-analysis because the data were not expressed as mean and standard deviation32,67.
Temporomandibular disorder pain
Four studies about TMD pain were included. In a clinical trial by Li et al. (2009), 55 subjects with temporomandibular joint (TMJ) pain receivied Ping On ointment containing Mentha piperita, Cinnamomum camphora, Gaultheria fragrantissima, Santalum album, and eucalyptus or a placebo for 4 weeks. Patients reported that Ping On ointment significantly reduced the painful symptoms of the TMJs, and they felt more comfortable opening their mouths than the placebo group. In another study by Campbell et al. (2016), 15 patients with TMD were treated with a high-concentration capsaicin (8%) cream or placebo for a week, and the results showed a significantly higher pain-relief response in the week after application in the capsaicin-treated subjects with TMD.
Chaimano et al. (2021) showed that the subjects with myogenic TMD pain who underwent pain treatment with a herbal compress ball, containing Cassumunar ginger, turmeric, and camphor, had greater pain-free maximum opening compared to those who only used the warm placebo. Nitecka-Buchta et al. (2019) investigated the myorelaxant properties of cannabidiol (CBD) administered topically to the masseter muscle of 60 patients who experiencied myofascial pain. The results revealed a significant reduction of 70.2% in pain intensity in the CBD-treated group as compared to that in the placebo group which exhibited only a 9.81% reduction. Moreover, CBD application led to decreased activity and enhanced condition of the masticatory muscles.
The comprehensive meta-analysis of all four studies69–72 revealed that capsaicin, cannabis, Ping on ointment and cassumunar ginger, turmeric, and camphor exhibit greater efficacy in diminishing TMD pain compared to placebo (Fig. 4). Network meta-analysis and the ranking based on the probability of each treatment being the best were performed for the 5 interventions (Fig. 5). The SUCRA of the treatment with Zingiberaceae was 86.1%, and that for Cannabaceae was 81.8%, confirming that these two treatments are the best interventions for TMD pain over the Solanaceae (44.7%), placebo (30.4%), and Lauraceae combination (7%) interventions (Fig. 6) (Table 2).
Table 2.
Ranking from best to worst herbal treatments for orofacial pain categorized by type of orofacial pain and plant family, using the surface under the cumulative ranking curve (SUCRA).
| Rank | Best | 2nd | 3rd | 4th | 5th | Worst | SUCRA | |
|---|---|---|---|---|---|---|---|---|
| Periodontal Pain | ||||||||
| 1 | Fabaceae Mix | 52.8 | 7.5 | 7.7 | 21.5 | N.C | 10.5 | 90.6 |
| 2 | Solanaceae | 0 | 9.5 | 33.2 | 43.7 | N.C | 13.6 | 65.3 |
| 3 | NSAID | 24.7 | 39 | 30 | 6.3 | N.C | 0 | 32.4 |
| 4 | Zingiberaceae | 22.2 | 41 | 23.3 | 12.7 | N.C | 0.8 | 32.2 |
| 5 | Placebo | 0.3 | 3 | 5.8 | 15.8 | N.C | 75.1 | 29.5 |
| Endodontic Pain | ||||||||
| 1 | Standard Treatment | 16.1 | 83.9 | N.C | N.C | N.C | 0 | 88.3 |
| 2 | Asteraceae | 0 | 7.3 | N.C | N.C | N.C | 92.7 | 58 |
| 3 | Combretaceae Mix | 83.9 | 8.8 | N.C | N.C | N.C | 7.3 | 3.6 |
| Oral Neuropathic Pain | ||||||||
| 1 | Solanaceae | 88.1 | 10.7 | 1.2 | 0 | 0 | 0 | 97.4 |
| 2 | Zingiberaceae Mix | 11.6 | 60.5 | 22.2 | 5.7 | 0 | 0 | 75.6 |
| 3 | Iridaceae | 0 | 0 | 0 | 6.8 | 36.9 | 56.3 | 56.5 |
| 4 | Standard Treatment | 0 | 0 | 0 | 4.4 | 57.6 | 38 | 47.1 |
| 5 | Apiaceae Mix | 0.3 | 14.2 | 53.3 | 32.2 | 0 | 0 | 13.3 |
| 6 | No Treatment | 0 | 14.6 | 23.3 | 50.9 | 5.5 | 5.7 | 10.1 |
| Temporomandibular Disorder Pain | ||||||||
| 1 | Zingiberaceae | 0.7 | 0.6 | 12.6 | 25.7 | N.C | 60.4 | 86.1 |
| 2 | Cannabaceae | 79.7 | 13.4 | 6.1 | 0.8 | N.C | 0 | 81.8 |
| 3 | Solanaceae | 12.5 | 21.5 | 48.4 | 10.1 | N.C | 7.5 | 44.7 |
| 4 | Placebo | 0 | 0 | 4.8 | 63.1 | N.C | 32.1 | 30.4 |
| 5 | Lauraceae Mix | 7.1 | 64.5 | 28.1 | 0.3 | N.C | 0 | 7 |
| Oral Surgery Pain | ||||||||
| 1 | Myrtaceae | 0 | 100 | 0 | 0 | 0 | 0 | 98 |
| 2 | Zingiberaceae | 0 | 0 | 0 | 4.1 | 88.9 | 7 | 80.6 |
| 3 | Clorhexidine | 0 | 0 | 100 | 0 | 0 | 0 | 61.4 |
| 4 | Ibuprofen | 0 | 0 | 0 | 1.4 | 7.2 | 91.4 | 40 |
| 5 | Myrtaceae Mix | 100 | 0 | 0 | 0 | 0 | 0 | 20 |
| 6 | Placebo/No Treatment | 0 | 0 | 0 | 94.5 | 3.9 | 1.6 | 0 |
| Rank | Best | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | 13th | 14th | 15th | 16th | 17th | Worst | SUCRA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral Mucosal Pain | ||||||||||||||||||||
| 1 | Lamiaceae | 0 | 0 | 0.2 | 0.3 | 1.3 | 2.8 | 3 | 6 | 7.2 | 10.5 | 12.1 | 13.4 | 14.8 | 11.9 | 9.4 | 4.2 | 2.7 | 0.2 | 95.6 |
| 2 | Anesthetic | 0 | 0 | 0 | 0.3 | 0.7 | 1.7 | 1.3 | 2.9 | 2.6 | 4 | 7.5 | 9.4 | 14.4 | 16.3 | 15.3 | 11.9 | 9.1 | 2.6 | 88.1 |
| 3 | Corticoesteroids | 0.1 | 0.1 | 0.7 | 2.4 | 5.5 | 11.2 | 18 | 15.3 | 17.7 | 13.1 | 8.7 | 5 | 1.6 | 0.4 | 0.2 | 0 | 0 | 0 | 80.7 |
| 4 | Amaryllidaceae | 0.3 | 0.9 | 1.6 | 2.9 | 4.9 | 5.2 | 5.5 | 6.1 | 8.2 | 9.5 | 9.7 | 9.1 | 9.8 | 10.1 | 8.8 | 4.8 | 1.7 | 0.9 | 73.1 |
| 5 | Lythraceae | 7.4 | 11.7 | 10.8 | 12.6 | 11.2 | 8.5 | 6.7 | 6.2 | 5.1 | 4.7 | 4.9 | 4.2 | 2.7 | 1 | 1.4 | 0.5 | 0.3 | 0.1 | 67.5 |
| 6 | Lamiaceae Mix | 0 | 0 | 0.2 | 0.6 | 1.3 | 1.9 | 3 | 3.5 | 4.7 | 6.7 | 9.4 | 13.1 | 15.7 | 14.8 | 12.1 | 7.4 | 4.4 | 1.2 | 63.2 |
| 7 | Ranunculaceae | 1 | 1.4 | 2.1 | 2.9 | 4.3 | 6 | 4.1 | 3.4 | 5.1 | 5.6 | 7.5 | 8.3 | 10.4 | 10.3 | 8 | 8.3 | 7.2 | 4.1 | 61.6 |
| 8 | Clorhexidine | 0.1 | 0.5 | 0.6 | 1.1 | 0.9 | 1.1 | 0.8 | 2.8 | 2.5 | 3.6 | 3.3 | 5.3 | 5.1 | 7 | 9 | 12.5 | 21.2 | 22.6 | 60 |
| 9 | Asphodelaceae | 0 | 0.3 | 1 | 1.8 | 5.1 | 9.4 | 10.3 | 12.1 | 13 | 11.8 | 12.2 | 9.2 | 6.2 | 5 | 1.4 | 0.9 | 0.3 | 0 | 57 |
| 10 | Asteraceae | 2.3 | 6 | 9.8 | 13.6 | 12.7 | 11 | 9.4 | 7.4 | 4.8 | 7.1 | 5.3 | 4.4 | 2.8 | 1.9 | 1 | 0.2 | 0.3 | 0 | 49 |
| 11 | Fabaceae | 22.3 | 31.9 | 22.7 | 12 | 6.7 | 2.2 | 1.1 | 0.5 | 0.5 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43 |
| 12 | Zingiberaceae | 0 | 0.4 | 1.2 | 4.4 | 9.5 | 16.6 | 17.8 | 19.8 | 13.4 | 9.8 | 4.7 | 1.8 | 0.6 | 0 | 0 | 0 | 0 | 0 | 38.3 |
| 13 | Asteraceae | 9.4 | 16.5 | 21.6 | 20.5 | 13 | 6.8 | 5.2 | 2.8 | 2.4 | 0.4 | 0.9 | 0.3 | 0.2 | 0 | 0 | 0 | 0 | 0 | 33.2 |
| 14 | Myrtaceae | 51.5 | 20.5 | 11.7 | 7 | 3.8 | 1.8 | 0.7 | 1 | 0.8 | 0.4 | 0.4 | 0.1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 29.4 |
| 15 | Plantaginaceae | 5.5 | 9.7 | 14.5 | 15.9 | 14.7 | 9.3 | 7.6 | 4.9 | 4.8 | 4.7 | 3.2 | 2.4 | 1.1 | 1.1 | 0.6 | 0 | 0 | 0 | 26.3 |
| 16 | Burseraceae | 0.1 | 0.1 | 1.3 | 1.7 | 4.4 | 4.5 | 5.5 | 5.2 | 7.1 | 7.9 | 9.8 | 13.2 | 10.8 | 10.3 | 8.4 | 5.9 | 3 | 0.8 | 17.4 |
| 17 | Hypericaceae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0.4 | 0.6 | 1.4 | 2.8 | 4 | 7.3 | 20.7 | 62.5 | 9.8 |
| 18 | Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | 2.1 | 7.1 | 20.4 | 36.1 | 29.1 | 5 | 7 |
Discussion
Compared with synthetic drugs, which often carry concerns related to side effects, trustworthiness, and potential drug interactions, herbal extracts have a significant advantage in terms of patient adherence73. This inherent specificity sets them apart from synthetic drugs, making them an appealing choice for individuals who may not tolerate the side effects associated with conventional pain-relief medications. Despite the common belief that natural remedies are inherently safe for the general population, there is still a substantial gap in our understanding of their mechanisms of action, potential adverse effects, and interactions with pharmaceutical drugs74. Safety remains a significant concern when it comes to herbal extracts, particularly in cases where their use is inadequately monitored or not monitored at all, highlighting deficiencies in pharmacovigilance in most countries75.
As herbal extracts are derived from living organisms, they inherently exhibit characteristics optimized through evolution to serve various specific biological functions76, giving them multipotent properties that allow for simultaneous targeting77. Among the 44 plant families identified, 10 had exceptional pain-relieving properties in OFP, as indicated by SUCRA score > 50%. These families have been the subject of extensive research and have provided compelling evidence for their ability to provide pain relief, which is primarily attributed to the presence of various bioactive components. For instance, alkaloids found in Amaryllidaceae78, Ranunculaceae79, and Solanaceae80, flavonoids in Asteraceae81, Fabaceae82, and Zingiberaceae83; tannins in Iridaceae84; and, terpenoids in Lamiaceae85, Lythraceae86, and Myrtaceae87. Each of these secondary metabolites potentially contribute to their analgesic, antioxidant, and anti-inflammatory properties.
The mechanisms of action of these herbal extracts can be categorized into three main groups based on the most common molecular mechanisms targeted by their bioactive substances. The first group includes extracts that target inflammatory mediators like COX-2, TNF-α, IFN-γ, NO, and various interleukins88–90. The second group comprises extracts that target receptors such as TRPV1, TRPA1, 5-HT, GABA, σ/µ-opioid, and cannabinoid receptors91–95. The third group includes extracts that modulate neurotransmitters like glutamate, glutathione, substance P, N-methyl-D-aspartate (NMDA), and monoamine oxidase (MAO)96–100. Herbal extracts often contain numerous bioactive compounds that allow them to simultaneously target multiple molecular mechanisms. This multifaceted approach may explain why, in certain cases, they exhibit superior efficacy compared with NSAIDs, topical anesthetics, corticosteroids, and other synthetic drugs.
Of the 71 plants identified, the most extensively studied plants were Curcuma longa (turmeric), Zingiber officinale (ginger), Aloe vera, Arnica montana, Calendula officinalis, Matricaria chamomilla (chamomile), Glycyrrhiza glabra (licorice), Hypericum perforatum (St. John’s wort), Mentha piperita (peppermint), Syzygium aromaticum (clove), and Capsicum annuum (chili pepper). In addition to their potent analgesic, anti-inflammatory, and antioxidant properties, familiarity also plays a significant role, primarily because these herbal extracts are widely recognized across various cultures. This sense of familiarity cultivates trust and safety101 making them potentially more appellant to study.
Despite their notable in-vitro potential, herbal extracts often exhibit limited in-vivo activity because of their inadequate lipid solubility and irregular molecular sizes, resulting in poor absorption and low bioavailability. Certain natural compounds such as piperine, curcumin, naringin, quercetin, and genistein demonstrate to improve that bioavailability102. Therefore, it is essential to acknowledge the potential increase in bioavailability of herbal combinations containing Curcuma longa or plants rich in quercetin. Additionally, absorption, among other parameters, can be modified by a proper formulation for the oral cavity, which should be characterized by adequate dispersion, retention, release, and bioadhesivity103,104. This is a major concern because the use of numerous herbal extracts in the oral cavity does not result in adequate formulations, mostly due to the lack of commercially available presentations, also bringing a considerable obstacle to therapeutic applications.
Our results revealed that the progress in studying herbal extracts to address OFP primarily centers on challenging-to-manage disorders, such as BMS105, oral mucositis106, oral submucous fibrosis107, oral lichen planus108, and TMD109, all of which commonly lack effective conventional treatment options. Additionally, two challenges that we identified regarding some of these conditions were the frequent lack of consensus regarding their differential diagnosis and the use of unvalidated scales for evaluating pain, sensitivity, burning sensation, and discomfort.
Although there is substantial evidence highlighting the potential of herbal extracts in managing pain11, it is crucial to prioritize the development of clinical practice guidelines because healthcare practitioners often lack proper information, contained in scientific evidence110. Consequently, these guidelines play a pivotal role in integrating scientific findings into healthcare decision-making recommendations111. The proper clinical application of these guidelines could potentially reduce the recently increasing concern regarding drug-plant interactions. These interactions occur when certain herbal products interact with pharmaceutical drugs, either enhancing or diminishing their effects, and potentially leading to adverse outcomes112. For example, there are reports on herb-drug interactions with Allium sativum, Salvia miltiorrhiza, Hypericum perforatum, Glycyrrhiza glabra, and Zingiber officinale113, among many others, that have not been studied or reported. Healthcare professionals must be aware of these interactions, and open communication and informed decision-making is essential to ensure the safe and effective use of both pharmaceuticals and natural remedies114.
Herbal medicine continues to play a significant role in healthcare worldwide, with an estimated 80% of the world’s population (approximately 4 billion people) relying on it as a primary healthcare resource115. This practice is particularly prevalent in developing countries where traditions have persisted, with the percentage of the population using herbal medicines varying across regions: 53%, Mexico; 68%, India; 75%, South Africa; and, 81%, Ghana. The demand for plant-based medicines is also increasing rapidly in industrialized nations because these remedies are increasingly valued for their safety, affordability, and accessibility116,117. The Western world has shown notable shift towards herbal medicines. For instance, 70% of Canadian individuals have used herbal medicines at least once118, and in Germany, the prevalence of herbal medicine users increased from 52% in 1970 to 70% in 2010119. Similarly, in the United States, the use of herbal medicines increased from 12.1% in 1997 to 18.6% in 2002120, whereas in the European Union, the prevalence stands at 48.3%121. This trend highlights the increasing acceptance and integration of herbal medicine worldwide.
There is a general confusion between herbal medicines and homeopathic herbal medicines despite significant differences in preparation, dosage, and safety. In homeopathy, remedies like Belladonna are prepared through serial dilution and vigorous shaking122. For instance, the homeopathic medicine Belladonna 6 C, has been diluted 1 part in 100, six times over. At this level of dilution, the remedy is considered safe and nontoxic, with little to no active alkaloids remaining. In contrast, herbal medicine uses crude plant extracts, such as the mother tincture of Belladonna, which contains a much higher concentration of toxic alkaloids, such as atropine, that can cause severe side effects123. Therefore, homeopathic medicines can be safely used, even when the crude herbal form is considered toxic. Western pharmaceutical practices often shift from whole plants to specific isolates of bioactive compounds, such as polyphenols and quercetin, to harness the therapeutic potential with greater precision, controlled dosing, and less variability associated with whole-plant preparations. Liquid chromatography-mass spectrometry is often used to identify and quantify these isolated molecules124. This approach contrasts with traditional medicine, which utilizes whole plants and values the synergy of multiple constituents for therapeutic effects, although Western methods offer more standardized and reproducible results122.
Herbal extracts are invaluable sources of bioactive compounds in the pharmaceutical industry. This is partly due to their chemical diversity, complexity, and composition, as well as their specific biological properties. The support of a solid base of ethnopharmacological information servs as a starting point for the development of new drugs76,125,126. Other perspectives suggest that as soon as the search for pharmacotherapy from natural compounds proves to be a sustainable and economically viable source, comparable to or even superior to synthetic medicines, the pharmaceutical industry will increase its investments in this field127. Furthermore, to reduce costs, time, and development cycle as well as improve the success rate of the discovery and development of this drugs, it is crucial to incorporate new standards and regulations, improved analytical tools, and biosynthetic engineering strategies76,125. Furthermore, the imminent threats on natural products in drug discovery need to be considered. However, there is the risk of losing traditional knowledge due to modernization128, and the extinction of natural species129,130.
Conclusions
The use of herbal extracts is an effective approach in the management of OFP in humans. Randomized controlled clinical trials have confirmed the significant analgesic properties of 71 plants species including Curcuma longa, Zingiber officinale, Aloe vera, Arnica montana, Calendula officinalis, Matricaria chamomilla, Glycyrrhiza glabra, Hypericum perforatum, Mentha piperita, Scutellaria baicalensis, Syzygium aromaticum, Plantago major, Atropa belladonna, Cannabis and Capsicum annuum, among many others. Fourty-four plant families were identified including Amaryllidaceae, Ranunculaceae, Solanaceae, Asteraceae, Fabaceae, Zingiberaceae, Iridaceae, Lamiaceae, Lythraceae, and Myrtaceae. All were used for 17 painful conditions: endodontic pain, periodontal pain, oral neuropathic pain, oral mucosal pain, temporomandibular disorder pain, and oral surgery pain.
Nevertheless, given their promising properties, herbal extracts may play an increasingly important role in the treatment of OFP and provide a valuable option for individuals seeking alternative or complementary therapies. However, clinical practice guidelines and adequate formulations for orofacial tissues must be developed, particularly topical formulations, and potential drug-herb interactions must be identified.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
N/A.
Author contributions
S.D.B. and L.J.B.C. and D.A.D.B wrote the main manuscript text and J.K., A.S. and J.E.C.P. prepared figures, A.C. and A.M. prepared tables and helped revise the manuscript. All authors reviewed the manuscript.
Data availability
All data related to the study can be provided on reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anand Marya, Email: amarya@puthisastra.edu.kh.
Akhilanand Chaurasia, Email: chaurasiaakhilanand49@gmail.com.
References
- 1.De Rossi, S. S. <ArticleTitle Language=”En”>Orofacial Pain. Dental Clin. N. Am.57 (3), 383–392. 10.1016/j.cden.2013.04.001 (2013). [Google Scholar]
- 2.Raiyani, P. P. Systematic Review and Meta-analysis of Incidence and Prevalence of Orofacial Pain- Global Burden of Diseases. Washington.edu. (2020). https://doi.org/Raiyani_washington_0250O_21653.pdf
- 3.Romero-Reyes, M. & Uyanik, J. M. Orofacial pain management: current perspectives. J. Pain Res.10.2147/jpr.s37593 (2014). 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhalla, K., Kamarthi, N., Malik, S., Goel, S. & Gupta, S. Comparison of conventional pharmacological therapy and holistic approaches (Naturopathy and Yoga) in the management of chronic orofacial pain: A randomized controlled study. J. Indian Acad. Oral Med. Radiol.31 (1), 29. 10.4103/jiaomr.jiaomr_3_19 (2018). [Google Scholar]
- 5.Patil, S. Pain Management in Dentistry: A Review and Update. J. Neuroinfectious Dis.07 (01). 10.4172/2314-7326.1000199 (2015).
- 6.Carter, G. et al. Side Effects of Commonly Prescribed Analgesic Medications. Phys. Med. Rehabil. Clin. North Am.25 (2), 457–470. 10.1016/j.pmr.2014.01.007 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Moreno, G. Pharmacological interactions of anti-inflammatory-analgesics in odontology. (2009). https://www.semanticscholar.org/paper/Pharmacological-interactions-of-in-odontology.-G%C3%B3mez-Moreno-Guardia/796ac394b9338f7506e40bed869c4cdb7aaa7a21 [PubMed]
- 8.Jahromi, B., Pirvulescu, I., Candido, K. D. & Knezevic, N. N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics. 13 (2), 251. 10.3390/pharmaceutics13020251 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colvard, M. D. & Cordell, G. A. Rationalizing the Study of Plants for the Treatment of Oral Pain. Curr. Chem. Biol.2 (2), 140–152 (2008). [Google Scholar]
- 10.Taheri, J. B., Azimi, S., Rafieian, N. & Zanjani, H. A. Herbs in dentistry. Int. Dent. J.61 (6), 287–296. 10.1111/j.1875-595x.2011.00064.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rauf, A., Jehan, N., Ahmad, Z. & Mubarak, M. S. Analgesic Potential of Extracts and Derived Natural Products from Medicinal Plants. InTech eBooks. (2017). 10.5772/intechopen.68631
- 12.Weiner, D. K. & Ernst, E. Complementary and Alternative Approaches to the Treatment of Persistent Musculoskeletal Pain. Clin. J. Pain. 20 (4), 244–255 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Yunes, R. A., Filho, V. C., Ferreira, J. & Calixto, J. B. The use of Natural Products as Sources of New Analgesic Drugs191–212 (Elsevier eBooks, 2004). 10.1016/s1572-5995(05)80033-x
- 14.Zare, P., Saeedi, M., Akbari, J. & Morteza-Semnani, K. A review on herbal oral care products. J. Mazandaran Univ. Med. Sci.26(144), 394–410 (2017). [Google Scholar]
- 15.Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol.62 (10), e1–e34. 10.1016/j.jclinepi.2009.06.006 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Sterne, J. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. l4898. 10.1136/bmj.l4898 (2019). [DOI] [PubMed]
- 17.Liu, H. L., Hsu, P. Y., Chung, Y. C., Lin, C. H. & Lin, K. Y. Effective licorice gargle juice for aphthous ulcer pain relief: A randomized double-blind placebo-controlled trial. Pak. J. Pharm. Sci.35 (5), 1321–1326 (2022). [PubMed] [Google Scholar]
- 18.Shahakbari, R. et al. Effectiveness of green tea mouthwash in comparison to chlorhexidine mouthwash in patients with acute pericoronitis: a randomized clinical trial. Int. J. Oral Maxillofac. Surg.43 (11), 1394–1398. 10.1016/j.ijom.2014.05.017 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Najafi, S. et al. Preventive Effect of Glycyrrhiza Glabra Extract on Oral Mucositis in Patients Under Head and Neck Radiotherapy: A Randomized Clinical Trial. J. dentistry (Tehran Iran). 14 (5), 267–274 (2017). [PMC free article] [PubMed] [Google Scholar]
- 20.Kar, P. P., Shaikh, Z. A., Hiremath, A. M. & Murugaboopathy, V. Comparison of the effectiveness of three different desensitizing toothpastes in reducing dentin hypersensitivity: A 4-week clinical study. J. Conservative Dentistry. 22 (2), 181. 10.4103/jcd.jcd_304_18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohite, V. et al. Comparative evaluation of a novel herbal anesthetic gel and 2% lignocaine gel as an intraoral topical anesthetic agent in children: Bilateral split-mouth, single-blind, crossover in vivo study. J. Indian Soc. Pedod. Prev. Dentistry. 38 (2), 177. 10.4103/jisppd.jisppd_226_20 (2020). [DOI] [PubMed] [Google Scholar]
- 22.De Souza, G. M., Fernandes, I. A., Pinheiro, M. L. P. & Falci, S. G. M. Comparative Effectiveness of the Homeopathic Preparation Traumeel S in Third Molar Extraction Surgery: A Preliminary Triple-Blind Clinical Trial. Homeopathy. 110 (04), 229–235. 10.1055/s-0041-1725038 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Keceli, H. G., Aylıkçı, B. U., Köseoğlu, S. & Dolgun, A. Evaluation of palatal donor site haemostasis and wound healing after free gingival graft surgery. J. Clin. Periodontol.42 (6), 582–589. 10.1111/jcpe.12404 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Spanemberg, J. C. et al. Effect of an herbal compound for treatment of burning mouth syndrome: randomized, controlled, double-blind clinical trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol.113 (3), 373–377. 10.1016/j.oooo.2011.09.005 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Das, R. et al. Comparative evaluation of analgesic and anti-inflammatory efficacy of ibuprofen and traumeel after periodontal flap surgery: A randomized triple-blind clinical trial. J. Indian Soc. Periodontology. 23 (6), 549. 10.4103/jisp.jisp_85_19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang, X. et al. Clinical evaluation of allicin oral adhesive tablets in the treatment of recurrent aphthous ulceration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol.113 (4), 500–504. 10.1016/j.oooo.2011.09.007 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Yadav, M. et al. Comparison of curcumin with intralesional steroid injections in Oral Submucous Fibrosis – A randomized, open-label interventional study. J. Oral Biology Craniofac. Res.4 (3), 169–173. 10.1016/j.jobcr.2014.11.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadbir, A. A. et al. The effect of Matricaria chamomilla (chamomile) extract in Orabase on minor aphthous stomatitis, a randomized clinical trial. J. Herb. Med.5 (2), 71–76. 10.1016/j.hermed.2015.05.001 (2015). [Google Scholar]
- 29.Ghalayani, P., Zolfaghary, B., Farhad, A. R., Tavangar, A. & Soleymani, B. The efficacy of Punica granatum extract in the management of recurrent aphthous stomatitis. J. Res. Pharm. Pract.2 (2), 88. 10.4103/2279-042x.117389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin, Y. et al. The effect of Pudilan Anti-Inflammatory Oral Liquid on the treatment of mild recurrent aphthous ulcers. Evidence-based Complement. Altern. Med.2017, 1–6. 10.1155/2017/6250892 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keshari, D., Patil, K. & Mahima, V. Efficacy of topical curcumin in the management of oral lichen planus: A randomized controlled-trial. J. Adv. Clin. Res. Insights. 2, 197–203. 10.15713/ins.jcri.78 (2015). [Google Scholar]
- 32.Chainani-Wu, N., Madden, E., Lozada-Nur, F. & Silverman, S. High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J. Am. Acad. Dermatol.66 (5), 752–760. 10.1016/j.jaad.2011.04.022 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Patil, H. et al. Comparison of the efficacy of ibuprofen and belladonna in the control of orthodontic separator pain. Indian J. Res. Homoeopathy. 12 (1), 29. 10.4103/ijrh.ijrh_64_17 (2018). [Google Scholar]
- 34.Anil, A., Gujjari, S. K. & Venkatesh, M. P. Evaluation of a curcumin-containing mucoadhesive film for periodontal postsurgical pain control. J. Indian Soc. Periodontology. 23 (5), 461. 10.4103/jisp.jisp_700_18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil, K., Guledgud, M. V., Kulkarni, P. K., Keshari, D. & Tayal, S. Use of curcumin mouthrinse in Radio-Chemotherapy induced oral mucositis patients: a pilot study. J. Clin. Diagn. Res.10.7860/jcdr/2015/13034.6345 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansouri, P., Haghighi, M., Beheshtipour, N. & Ramzi, M. The Effect of Aloe Vera Solution on Chemotherapy-Induced Stomatitis in Clients with Lymphoma and Leukemia: A Randomized Controlled Clinical Trial. Int. J. Community Based Nurs. Midwifery. 4 (2), 119–126 (2016). [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain, S. A. et al. Nigella sativa Oil Mouth Rinse Improves Chemotherapy-Induced Oral Mucositis in Patients with Acute Myeloid Leukemia. Biomed. Res. Int.2019, 1–10. 10.1155/2019/3619357 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, C. et al. Efficacy of traditional Chinese medicine in treatment and prophylaxis of Radiation-Induced oral mucositis in patients receiving radiotherapy: a randomized controlled trial. Integr. Cancer Ther.17 (2), 444–450. 10.1177/1534735417725578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alshibani, N. et al. Postoperative Analgesic and Anti-inflammatory Effectiveness of Ginger (Zingiber officinale) and NSAIDs as Adjuncts to Nonsurgical Periodontal Therapy for the Management of Periodontitis. PubMed. 20 (1), 227–232. 10.3290/j.ohpd.b3125633 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Al-Askar, M. et al. Analgesic Efficacy of Curcuma longa (Curcumin) after Surgical Periodontal Therapy. PubMed. 20 (1), 19–26. 10.3290/j.ohpd.b2572979 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Rayati, F., Hajmanouchehri, F. & Najafi, E. Comparison of anti-inflammatory and analgesic effects of Ginger powder and Ibuprofen in postsurgical pain model: A randomized, double-blind, case–control clinical trial. Dent. Res. J.14 (1), 1. 10.4103/1735-3327.201135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jesudasan, J. S., Wahab, P. U. A. & Sekhar, M. Effectiveness of 0.2% chlorhexidine gel and a eugenol-based paste on postoperative alveolar osteitis in patients having third molars extracted: a randomised controlled clinical trial. Br. J. Oral Maxillofacial Surg.53 (9), 826–830. 10.1016/j.bjoms.2015.06.022 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Komasawa, N. et al. Preoperative Administration of Jidabokuippo, a Kampo Medicine, Alleviates Postoperative Pain after Tooth Extraction with Mandible Bone Removal under General Anesthesia: A Prospective, Single-Blind, Randomized Controlled Trial. J. Altern. Complement. Med.10.1089/acm.2018.0244 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Alqareer, A., Alyahya, A. & Andersson, L. The effect of clove and benzocaine versus placebo as topical anesthetics. J. Dent.34 (10), 747–750. 10.1016/j.jdent.2006.01.009 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Marino, R. L., Torretta, S., Capaccio, P., Pignataro, L. & Spadari, F. Different therapeutic strategies for burning mouth syndrome: preliminary data. J. Oral Pathol. Med.39 (8), 611–616. 10.1111/j.1600-0714.2010.00922.x (2010). [DOI] [PubMed] [Google Scholar]
- 46.Pakfetrat, A. et al. Evaluation of the effectiveness of crocin isolated from saffron in treatment of burning mouth syndrome: A randomized controlled trial. PubMed. 9 (6), 505–516. 10.22038/ajp.2019.12764 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bessho, K., Okubo, Y., Hori, S., Murakami, K. & Iizuka, T. Effectiveness of Kampo medicine (Sai-boku-to) in treatment of patients with glossodynia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology. 86 (6), 682–686. 10.1016/s1079-2104(98)90204-9 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Lee, Y., Kho, H., Kim, Y. & Chung, S. Influence of topical capsaicin on facial sensitivity in response to experimental pain. J. Rehabil.34 (1), 9–14. 10.1111/j.1365-2842.2006.01639.x (2007). [DOI] [PubMed] [Google Scholar]
- 49.Majji, P. & Murthy, K. R. V. Clinical efficacy of four interventions in the reduction of dentinal hypersensitivity: A 2-month study. Indian J. Dent. Res.27 (5), 477. 10.4103/0970-9290.195618 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Mansour, G., Ouda, S., Shaker, A. & Abdallah, H. M. Clinical efficacy of new aloe vera- and myrrh-based oral mucoadhesive gels in the management of minor recurrent aphthous stomatitis: a randomized, double-blind, vehicle-controlled study. J. oral Pathol. &. 43 (6), 405–409. 10.1111/jop.12130 (2014). medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. [DOI] [PubMed] [Google Scholar]
- 51.Motallebnejad, M., Moghadamnia, A. A. & Talei, M. The Efficacy of Hypericum perforatum Extract on Recurrent Aphthous Ulcers. J. Med. Sci.8 (1), 39–43. 10.3923/jms.2008.39.43 (2008). [Google Scholar]
- 52.Pourahmad, M., Rahiminejad, M., Fadaei, S. & Kashafi, H. Effects of camel thorn distillate on recurrent oral aphthous lesions. J. Der Deutschen Dermatologischen Gesellschaft. 8 (5), 348–352. 10.1111/j.1610-0387.2010.07316.x (2010). [DOI] [PubMed] [Google Scholar]
- 53.Babaee, N., Zabihi, E., Mohseni, S. & Moghadamnia, A. A. Evaluation of the therapeutic effects of Aloe vera gel on minor recurrent aphthous stomatitis. Dent. Res. J.9 (4), 381–385 (2012). [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, Y. et al. Short-Term Efficacy of Pudilan Keyanning Toothpaste in Treatment of Minor Recurrent Aphthous Ulcers. Evidence-based complementary and alternative medicine: eCAM, 2016, 9125327. (2016). 10.1155/2016/9125327 [DOI] [PMC free article] [PubMed]
- 55.Babaee, N., Mansourian, A., Momen-Heravi, F., Moghadamnia, A. A. & Momen-Beitollahi, J. The efficacy of a paste containing Myrtus communis (Myrtle) in the management of recurrent aphthous stomatitis: a randomized controlled trial. Clin. Oral Invest.14 (1), 65–70. 10.1007/s00784-009-0267-3 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Jornet, L., Aznar-Cayuela, C. & P., & Efficacy of topical chamomile management vs. placebo in patients with oral lichen planus: a randomized double-blind study. J. Eur. Acad. Dermatol. Venereol.30 (10), 1783–1786. 10.1111/jdv.13770 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Mansourian, A. et al. Comparison of aloe vera mouthwash with triamcinolone acetonide 0.1% on oral lichen planus: a Randomized Double-Blinded Clinical trial. Am. J. Med. Sci.342 (6), 447–451. 10.1097/maj.0b013e3182171164 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Hasheminasab, F. S. et al. Effects of a Plantago ovata-based herbal compound in prevention and treatment of oral mucositis in patients with breast cancer receiving chemotherapy: A double-blind, randomized, controlled crossover trial. J. Integr. Med.18 (3), 214–221. 10.1016/j.joim.2020.02.008 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Aghamohammadi, A. et al. The effectiveness of Zataria extract mouthwash for the management of radiation-induced oral mucositis in patients: a randomized placebo-controlled double-blind study. Clin. Oral Invest.22 (6), 2263–2272. 10.1007/s00784-017-2324-7 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Soltani, G. M. et al. Efficacy of the plantago major L. syrup on radiation induced oral mucositis in head and neck cancer patients: A randomized, double blind, placebo-controlled clinical trial. Complement. Ther. Med.51, 102397. 10.1016/j.ctim.2020.102397 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Kia, S. J., Basirat, M., Saedi, H. S. & Arab, S. A. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: a randomized clinical trial. BMC Complement. Med. Ther.21 (1). 10.1186/s12906-021-03400-4 (2021). [DOI] [PMC free article] [PubMed]
- 62.Monsen, R. E. et al. A mouth rinse based on a tea solution of Salvia officinalis for oral discomfort in palliative cancer care: a randomized controlled trial. Support. Care Cancer. 29 (9), 4997–5007. 10.1007/s00520-021-06021-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elhadad, N. M. A., El-Negoumy, E., Taalab, M. R., Ibrahim, R. S. & Elsaka, R. The effect of topical chamomile in the prevention of chemotherapy‐induced oral mucositis: A randomized clinical trial. Oral Dis.28 (1), 164–172. 10.1111/odi.13749 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Reis, P. E. D. D. et al. Chamomile infusion cryotherapy to prevent oral mucositis induced by chemotherapy: a pilot study. Support. Care Cancer. 24 (10), 4393–4398. 10.1007/s00520-016-3279-y (2016). [DOI] [PubMed] [Google Scholar]
- 65.De Cássia, D. V. et al. Comparative randomized trial study about the efficacy of photobiomodulation and curcumin antimicrobial photodynamic therapy as a coadjuvant treatment of oral mucositis in oncologic patients: antimicrobial, analgesic, and degree alteration effect. Support. Care Cancer. 30 (9), 7365–7371. 10.1007/s00520-022-07127-x (2022). [DOI] [PubMed] [Google Scholar]
- 66.Piyush, P., Mahajan, A., Singh, K., Ghosh, S. & Gupta, S. Comparison of therapeutic response of lycopene and curcumin in oral submucous fibrosis: A randomized controlled trial. Oral Dis.25 (1), 73–79. 10.1111/odi.12947 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Hazarey, V. K., Sakrikar, A. R. & Ganvir, S. M. Efficacy of curcumin in the treatment for oral submucous fibrosis – A randomized clinical trial. J. Oral Maxillofacial Pathol.19 (2), 145. 10.4103/0973-029x.164524 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava, R. et al. A Comparative Study to Evaluate the Efficacy of Curcumin Lozenges (TurmNova®) and Intralesional Corticosteroids with Hyaluronidase in Management of Oral Submucous Fibrosis. J. Contemp. Dent. Pract.22 (7), 751–755 (2021). [PubMed] [Google Scholar]
- 69.Li, L. C., Wong, R. W. & Rabie, A. B. M. Clinical effect of a topical herbal ointment on pain in temporomandibular disorders: a Randomized Placebo-Controlled trial. J. Altern. Complement. Med.15 (12), 1311–1317. 10.1089/acm.2009.0129 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Campbell, B. et al. Effects of High-Dose Capsaicin on TMD subjects. JDR Clin. Translational Res.2 (1), 58–65. 10.1177/2380084416675837 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaimano, S. et al. A Randomized Controlled Trial on Short-term Therapeutic Effects of Thai Herbal Compresses versus Warm Placebo Compresses on Myogenous Temporomandibular Disorder Pain. CM Dent J, 42(2), 114–119. Retrieved from: (2021). https://www.dent.cmu.ac.th/cmdj/frontend/web/?r=site/viewarticle&id=11
- 72.Nitecka-Buchta, A. et al. Myorelaxant Effect of Transdermal Cannabidiol Application in Patients with TMD: A Randomized, Double-Blind Trial. J. Clin. Med.8 (11), 1886. 10.3390/jcm8111886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karimi, A. Herbal versus synthetic drugs; beliefs and facts. PubMed Central (PMC). (2015). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5297475/ [PMC free article] [PubMed]
- 74.Ekor, M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol.10.3389/fphar.2013.00177 (2013). 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olsson, S., Pal, S. N. & Dodoo, A. Pharmacovigilance in resource-limited countries. Expert Review of Clinical Pharmacology. Jun 2;8(4):449–60. (2015). 10.1586/17512433.2015.1053391 [DOI] [PubMed]
- 76.Atanasov, A. G. et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv.33 (8), 1582–1614. 10.1016/j.biotechadv.2015.08.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ibrahim, S. R. M. et al. Phytoconstituents and Pharmacological Activities of Indian Camphorweed (Pluchea indica): A Multi-Potential Medicinal Plant of Nutritional and Ethnomedicinal Importance. Molecules. 27 (8), 2383. 10.3390/molecules27082383 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He, M., Qu, C., Gao, O., Hu, X. & Hong, X. Biological and pharmacological activities of amaryllidaceae alkaloids. RSC Adv.5 (21), 16562–16574. 10.1039/c4ra14666b (2015). [Google Scholar]
- 79.Goo, Y. Therapeutic potential of Ranunculus species (Ranunculaceae): A literature review on traditional medicinal herbs. Plants. 11 (12), 1599. 10.3390/plants11121599 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Atul, T. Certain medicinal plants of Solanaceae and their alkaloids screening. (2014). https://www.semanticscholar.org/paper/Certain-Medicinal-Plants-of-Solanaceae-and-Their-Atul-Ray/4f649f62fc1c8657ad1f3f5b359aeb3e83505661
- 81.Bessada, S. M., Barreira, J. C. & Oliveira, M. B. P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crops Prod.76, 604–615. 10.1016/j.indcrop.2015.07.073 (2015). [Google Scholar]
- 82.Mans, D. R., Friperson, P., Pawirodihardjo, J. & Djotaroeno, M. Phenolic compounds and antioxidant activities of eight species of fabaceae that are commonly used in traditional medical practices in the Republic of Suriname. In IntechOpen eBooks. (2022). 10.5772/intechopen.106076
- 83.Alolga, R. N. et al. Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants. 11 (7), 1281. 10.3390/antiox11071281 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mykchailenko, O. O. & Kovalyov, M. V. Phenolic compounds of the genus Iris plants (Iridaceae). Fenolické sloučeniny rostlin rodu Iris (Iridaceae). Ceska Slov. farmacie: casopis Ceske farmaceuticke spolecnosti Slovenske farmaceuticke spolecnosti. 65 (2), 70–77 (2016). [PubMed] [Google Scholar]
- 85.Abdelhalim, A. & Hanrahan, J. R. Biologically active compounds from Lamiaceae family: Central nervous system effects. In Studies in natural products chemistry (pp. 255–315). (2021). 10.1016/b978-0-12-819485-0.00017-7
- 86.Florence, A., Sukumaran, S., Joselin, J., Brintha, T. & Jeeva, S. Phytochemical screening of selected medicinal plants of the family Lythraceae. (2015). https://www.semanticscholar.org/paper/Phytochemical-screening-of-selected-medicinal-of-Florence-Sukumaran/90b5f5d840e6f935518d01ea530f89213c06ee55
- 87.Stefanello, M. É. A., Pascoal, A. C. R. F. & Salvador, M. J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers.8 (1), 73–94. 10.1002/cbdv.201000098 (2011). [DOI] [PubMed] [Google Scholar]
- 88.Aminuddin, M., Sargowo, D., Sardjono, T. & Widjiati, W. Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates. Open. Veterinary J.13 (1), 11. 10.5455/ovj.2023.v13.i1.2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong, S. S. & Oh, J. S. Phenylpropanoid ester from Zingiber officinale and their inhibitory effects on the production of nitric oxide. Arch. Pharm. Res.35 (2), 315–320. 10.1007/s12272-012-0211-y (2012). [DOI] [PubMed] [Google Scholar]
- 90.Radjabian, T. & Ghazanfari Tooba. Hosseinpur Yektaei Zahra., Naghizadeh Mohammad Mehdi.,. Immunomodulatory Impacts of Bulbs Extracts From Five Allium Species on IFN-γ, IL-4, and IL-17 Cytokines. ImmunoRegulation. 4. 91–100. (2022). 10.32598/IMMUNOREGULATION.4.2.4
- 91.Liu, J. Y. et al. Involvement of TRPV1 and TRPA1 in the modulation of pacemaker potentials in the mouse ileum. Cell. Calcium. 97, 102417. 10.1016/j.ceca.2021.102417 (2021). [DOI] [PubMed] [Google Scholar]
- 92.Sloley, B. D. et al. Chemical and pharmacological evaluation of Hypericum perforatum extracts. Acta Pharmacol. Sin.21 (12), 1145–1152 (2000). [PubMed] [Google Scholar]
- 93.Avallone, R. et al. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol.59 (11), 1387–1394. 10.1016/s0006-2952(00)00264-1 (2000). [DOI] [PubMed] [Google Scholar]
- 94.Kim, H. et al. Pharmacological action ofPanax Ginseng on the behavioral toxicities induced by psychotropic agents. Arch. Pharm. Res.28 (9), 995–1001. 10.1007/bf02977391 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Adel, Y. & Alexander, S. Neuromolecular mechanisms of cannabis action. In Advances in Experimental Medicine and Biology (pp. 15–28). (2020). 10.1007/978-3-030-57369-0_2 [DOI] [PubMed]
- 96.Mahmoud, A. M. et al. Commiphora molmolModulates Glutamate-Nitric Oxide-cGMP and Nrf2/ARE/HO-1 Pathways and Attenuates Oxidative Stress and Hematological Alterations in Hyperammonemic Rats. Oxidative Med. Cell. Longev.2017, 1–15. 10.1155/2017/7369671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Basu, A. et al. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr. Res.33 (3), 180–187. 10.1016/j.nutres.2012.12.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shahane, K. et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals. 16 (4), 611. 10.3390/ph16040611 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burks, T. F., Buck, S. H. & Miller, M. S. Mechanisms of depletion of substance P by capsaicin. Federation Proc.44 (9), 2531–2534 (1985). [PubMed] [Google Scholar]
- 100.Mallozzi, C. et al. Curcumin modulates the NMDA receptor subunit composition through a mechanism involving CAMKII and SER/THR protein phosphatases. Cell. Mol. Neurobiol.38 (6), 1315–1320. 10.1007/s10571-018-0595-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alghadir, A. H., Iqbal, A. & Iqbal, Z. A. Attitude, beliefs, and use of herbal remedies by patients in the Riyadh region of Saudi Arabia. Healthcare. 10 (5), 907. 10.3390/healthcare10050907 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ara, N., Sultana, T., Bolleddu, R., Venkatesh, S. & Kiran, A. A strategy to enhance bioavailability of drug candidates: natural bioenhancers. SunText Rev. Pharm. Sci.02 (01). 10.51737/2766-5232.2021.008 (2021).
- 103.Sontakke, R., Singhal, R., Jain, N. K. & Sontakke, S. Formulation and Evaluation of Polyherbal Gel containing Ethanolic Extract used as Local Anesthetics in Oral Cavity. Int. J. Drug Delivery Technol.12 (02), 85–88. 10.25258/ijddt.12.1.16 (2022). [Google Scholar]
- 104.Pistone, S., Goycoolea, F. M., Young, A., Smistad, G. & Hiorth, M. Formulation of polysaccharide-based nanoparticles for local administration into the oral cavity. Eur. J. Pharm. Sci.96, 381–389. 10.1016/j.ejps.2016.10.012 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Tan, H. L., Smith, J. G., Hoffmann, J. & Renton, T. A systematic review of treatment for patients with burning mouth syndrome. Cephalalgia. 42 (2), 128–161. 10.1177/03331024211036152 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Daugėlaitė, G., Užkuraitytė, K., Jagelavičienė, E. & Filipauskas, A. Prevention and treatment of chemotherapy and radiotherapy induced oral mucositis. Medicina-lithuania. 55 (2), 25. 10.3390/medicina55020025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Warnakulasuriya, S., Kerr, A. R. & Medicine, O. Oral submucous fibrosis: a review of the current management and possible directions for novel therapies. Oral Surgery, Oral Pathology, and Oral Radiology, 122(2), 232–241. (2016). 10.1016/j.oooo.2016.02.020 [DOI] [PubMed]
- 108.Rogulj, A. A. et al. Different Treatment Modalities of Oral Lichen Planus—A Narrative Review. Dentistry J.11 (1), 26. 10.3390/dj11010026 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gil-Martínez, A., Paris-Alemany, A., López‐de‐Uralde‐Villanueva, I. & La Touche, R. Management of pain in patients with temporomandibular disorder (TMD): challenges and solutions. J. Pain Res.11, 571–587. 10.2147/jpr.s127950 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woolf, S. H., Grol, R., Hutchinson, A., Eccles, M. & Grimshaw, J. Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ. 318 (7182), 527–530. 10.1136/bmj.318.7182.527 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guerra-Farfán, E. et al. Clinical practice guidelines: The good, the bad, and the ugly. Injury-International J. Care Injured. 54, S26–S29. 10.1016/j.injury.2022.01.047 (2023). [DOI] [PubMed] [Google Scholar]
- 112.Chhabra, A., Singh, G. & Upadhyay, Y. A review on herbal drug interaction. Asian J. Pharm. Res. Dev.8 (1), 94–99. 10.22270/ajprd.v8i1.663 (2020). [Google Scholar]
- 113.Fugh-Berman, A. Herb-drug interactions. Lancet. 355 (9198), 134–138. 10.1016/s0140-6736(99)06457-0 (2000). [DOI] [PubMed] [Google Scholar]
- 114.Greener, M. Drug-plant interactions: Herbs and beyond. Nurse Prescribing. 10.12968/npre.2017.15.1.38 (2017). [Google Scholar]
- 115.Fabricant, D. S. & Farnsworth, N. R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect.109 (suppl 1), 69–75. 10.1289/ehp.01109s169 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oyebode, O., Kandala, N., Chilton, P. J. & Lilford, R. J. Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Policy Plann.31 (8), 984–991. 10.1093/heapol/czw022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khan & Ahmad, I. Herb. Med.10.1016/B978-0-12-814619-4.00001-X. (2019). [Google Scholar]
- 118.Gunjan, M. et al. Marketing Trends & Future Prospects of Herbal Medicine in the Treatment of Various Disease. World J. Pharm. Res.4 (9), 132–155 (2015). [Google Scholar]
- 119.Allensbach Institute for Opinion Research. Naturheilmittel. https://www.ifd-allensbach.de/uploads/tx_studies/7528_Naturheilmittel_2010.pdf (2010).
- 120.Tindle, H. A., Davis, R. B., Phillips, R. S. & Eisenberg, D. M. Trends in use of complementary and alternative medicine by us adults: 1997–2002. Altern. Ther. Health Med.11 (1), 42–49 (2005). [PubMed] [Google Scholar]
- 121.Eardley, S. et al. A Systematic literature review of complementary and Alternative medicine prevalence in EU. Complement. Med. Res.19 (Suppl. 2), 18–28. 10.1159/000342708 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Frye, J. C. Herbal and homeopathic medicine: understanding the difference. Seminars Integr. Med.1 (3), 158–166. 10.1016/s1543-1150(03)00030-9 (2003). [Google Scholar]
- 123.Almubayedh, H. & Ahmad, R. Clinical uses and toxicity of Atropa belladonna; an evidence based comprehensive retrospective review (2003–2017). Biosci. Biotechnol. Res. Comm.10.21786/bbrc/11.1/6 (2018). [Google Scholar]
- 124.Sasidharan, S., Chen, Y., Saravanan, D., Sundram, K. M. & Latha, L. Y. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. PubMed Cent.8 (1), 1–10 (2011). [PMC free article] [PubMed] [Google Scholar]
- 125.Atanasov, A. G., Zotchev, S. B., Dirsch, V. M. & Supuran, C. T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discovery. 20 (3), 200–216. 10.1038/s41573-020-00114-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koehn, F. E. & Carter, G. T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discovery. 4 (3), 206–220. 10.1038/nrd1657 (2005). [DOI] [PubMed] [Google Scholar]
- 127.Moors, E. H., Cohen, A. F. & Schellekens, H. Towards a sustainable system of drug development. Drug Discovery Today. 19 (11), 1711–1720. 10.1016/j.drudis.2014.03.004 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Vedavathy, S. SCOPE AND IMPORTANCE OF TRADITIONAL MEDICINE. Indian J. Traditional Knowl.2(3) (July 2003), pp236–239 (2012). [Google Scholar]
- 129.Rosenzweig, M. L. Reconciliation ecology and the future of species diversity. Oryx. 37 (2), 194–205. 10.1017/s0030605303000371 (2003). [Google Scholar]
- 130.Moritz, C. & Agudo, R. The future of species under climate change: resilience or decline? Science. 341 (6145), 504–508. 10.1126/science.1237190 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to the study can be provided on reasonable request from the corresponding author.