Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Jun 29;13:101685. doi: 10.1016/j.toxrep.2024.101685
Abstract
For millennia, various cultures have utilized cannabis for food, textile fiber, ethno-medicines, and pharmacotherapy, owing to its medicinal potential and psychotropic effects. An in-depth exploration of its historical, chemical, and therapeutic dimensions provides context for its contemporary understanding. The criminalization of cannabis in many countries was influenced by the presence of psychoactive cannabinoids; however, scientific advances and growing public awareness have renewed interest in cannabis-related products, especially for medical use. Described as a ‘treasure trove,’ cannabis produces a diverse array of cannabinoids and non-cannabinoid compounds. Recent research focuses on cannabinoids for treating conditions such as anxiety, depression, chronic pain, Alzheimer’s, Parkinson’s, and epilepsy. Additionally, secondary metabolites like phenolic compounds, terpenes, and terpenoids are increasingly recognized for their therapeutic effects and their synergistic role with cannabinoids. These compounds show potential in treating neuro and non-neuro disorders, and studies suggest their promise as antitumoral agents. This comprehensive review integrates historical, chemical, and therapeutic perspectives on cannabis, highlighting contemporary research and its vast potential in medicine.
1. Introduction
Cannabis is one of the most ancient plants recognized by humans, widely cultivated, consumed, and utilized several millennia ago in eastern and central Asia [103], [34]. Historically, it was a source for food, and fiber for tissue and rope as some archaeological artifacts suggests [153], historical records dating back to at least the third millennium BC mentioned its use for religious or spiritual purposes [146]. Several scholars postulate that Cannabis’s existence goes as far back as the Pleistocene epoch. Paleo-botanical research provides evidence of its presence during the Holocene epoch approximately 11,700 years ago, in the regions around East and Central Asia, near the Altai Mountains [146]. First traces were found in a cave in China in 1900; hemp tissue dating around 12.000 BCE [67]. and its fibers were unearthed in clay vessels from graves dating as far back as 10,000 BCE [161]. For its medicinal and psychotropic properties, it has spread worldwide nowadays due to human cultivation [100], [146].
Cannabis was used in shamanistic rituals and in Chinese medicine around 2700–2300 BC and it is documented in written records of Ayurvedic medicine, and by archaeobotanical evidence found in Western China, precisely in the Yanghai Tombs dating back over 4500 years [146], [161]. Furthermore, it is documented during the Han Dynasty (between about 206 BC and 220 AD) by the botanist Shen-Nung in the first known Pharmacopoeia; the “Shen Nung Pen Ts’ao Ching”, where it is recommended to treat gout, rheumatism, malaraia, alleviate weight loss and weakness, referred to as waste diseace, constipation, and female’s disorders or menstrual issues [100]. This founder of Chinese medicine has discovered the first therapeutic interests of the plant by experimenting it on himself [100], [180], while the physician Hua T’o used cannabis as anesthetic on his patient undergoing surgical operations on abdominal organs [100]. While in India, using this plant was common for both therapeutic and recreational purposes [121], [181] and became part of the Hindu religion around 1000-year BC, making its way to Europe sometime between 1000 and 2000 B.C [73]. Various forms of archaeobotanical evidence, encompassing chemical compounds, macrofossils (seeds, fibers…), as well as microfossils (pollen, trichomes, phytoliths…) have been retrieved from multiple archaeological sites spanning the regions of Asia to Europe [146]. which proves the spreading use of cannabis from Asia, traversing the Silk Road, and extending to Europe and Africa [173]. According to a papyrus that presents cannabis as a sacred plant of the pharaohs, Africa discovered cannabis after Asia and Europe, Egypt being its first destination, under the reign of Amenhotep 1st in the 16th century BC [15]. Its spreading across Africa was slow, it was introduced to the Maghrib region in the 7th century CE during the Arab invasions of North Africa. Bioactive Traces dating from the beginning of the 13th century AC were found in Kenya and Ethiopia, and from the 15th century in South Africa [11] where it was used to treat snake bites, facilitate childbirth, fight malaria, fever, or dysentery [52]. Around this time, cannabis was implanted in Morocco, precisely in the region Sanhaja, in the Rif [78]. Furthermore, it became a very important economic stake, which explains its entry to the American territory, starting with South America (mainly Chile) in the mid-1500s, and later on, its cultivation reached North America in the early 1600 s, where it was used for religious rituals and to treat some pathologies, such as toothache, and stomachache [180], [47], [73]. The plant was also used as a fiber, thus the declaration of Independence of the United States was written on hemp paper in 1776 [191]. During the colonial era (1882–1960), hemp cultivation was encouraged in Latin America by Spanish kings for its versatile fibers used in fabric and rope production [123].
In the slow progression of Western Medicine, limited cannabis usage was recognized during the first half of 19th century, thanks to the work of the Irish doctor William B. O’Shaughnessy and the French psychiatrist Jacques-Joseph Moreau. The former discovered in 1839 the potential of this plant to stimulate appetite, relieve pain, and prevent convulsions, he also widely described its use in different countries, as well as different efficacious human trials employing cannabis formulations against rheumatism and muscle contractions, especially those caused by tetanus and rabies [13], [146]. Moreau, for his part, published “Du Hachisch et de l′Aliénation mentale: Etudes psychologiques“ in 1845, a book containing thorough explanations of cannabis’s short-term impacts in humans, after conducting several experiments using different preparations of cannabis: first on himself and later on his students [126], [198], [47], [52]. Furthermore, in the mid-19th century, cannabis extracts were included in the American pharmacopoeia [161], and Queen Victoria was advised to use it as an herbal tea as a remedy for dysmenorrhea [13].
The ancient records spanning from ancient China to Africa, documenting cannabis’s therapeutic and psychoactive properties laid the groundwork for recognizing its potential benefits, and informed Western interest and medieval medicine in its potential for pain management and mental health applications, guiding subsequent research into its analgesic and anti-inflammatory properties. Moreover, the increasing comprehension of cannabis characteristics contributed to its broader use for both medicinal and recreational purposes during the 20th century. Nevertheless, research progress regarding its medicinal beneficial effects was hindered due to prejudice and misinformation, [111], [66], [73] and the financial significance of its seeds became negligible by the mid-20th century, as they were mainly utilized as animal feed and, sometimes, consumed by humans [161]. Due to its high abuse liability, its utilization was strongly prohibited and was removed from the British pharmacopeia [32]. Later on, in 1937, all its medicinal uses were functionally ended by the USA federal legalization: the “Marihuana Tax Act”, before removing it from the “National Formulary and Pharmacopoeia” in 1941 [139], [32]. During the latter part of the 20th century, cannabis acquired a significant societal significance as its use for epicurean pursuits surged significantly. Since the 1960s, the young population of the Western world commonly used it as recreational drug, and the rise of hedonism and psychedelia contributed to its widespread internationally in the illicit market, making it the world’s top prohibited entertaining drug [161]. Throughout this surge, In Morocco, cannabis cultivation extended from the Rif region westward to Laarache and southward to Taounate [78]. However, cultivated areas declined after the United Nations ratified the single convention on narcotic drugs in 1961 [190], [55].
In 1964, Professor Raphael Mechoulam discovered and isolated the psychoactive molecule; tetrahydrocannabinol THC, thus promoting an increase in research initiatives focused on active components of this plant from that date on [119], [70]. In 1985, the United States Food and Drugs Administration (US FDA) reconsidered using cannabinoids as medicines, and granted approval for Marinol (dronabinol) and Cesamet (nabilone) as synthetic counterparts of THC, specifically to manage nausea and vomiting induced by cancer chemotherapy [32]. This approval marked a significant step in the historical use of cannabis, as nowadays, integrating traditional knowledge of cannabis with modern scientific methods can enhance treatment approaches, while cultural sensitivity ensures ethical considerations in cannabis’s medical use and commercialization. Broadening research beyond conventional domains may unveil new therapeutic applications, leveraging historical insights into safe usage and optimal dosage to maximize cannabis’s benefits and mitigate risks in clinical practices. However, according to Wilkinson and D’Souza; the nature of the plant, and the limited understanding of its properties and therapeutic impacts makes its medicalizing and/or incorporating into a pharmaceutical composition rather complex [73].
Since the enactment of the 2014 Farm Bill, there has been a gradual easing of restrictions on the cultivation and production of cannabis in the United States [89], from 2010 to 2015, cannabis was cultivated in 135 countries [68]. By then, the European Food Safety Authority (EFSA) set 0.3 wt% as a threshold of Δ9-THC content in edible items, aiming to ensure public health safety [43]. Afterwards, the Agricultural Improvement Act of 2018, commonly known as the 2018 Farm Bill, brought about a significant change by excluding hemp from the federal Schedule I substances as per the Controlled Substances Act. Subsequently, in June 2018, the introduction of the first legitimate medical product laid the foundation for a thriving CBD market [93], [185]. Nowadays, the Cannabis plant has achieved global distribution, flourishing in a variety of altitudes, well adapting to diverse soil types and different climatic zones, even the most unfavorable (Suman [40], [46]). According the latest World Drug Report (2022) China is considered the biggest producer of cannabis internationally, however, the largest production of cannabis resin occurs in Morocco [184].
Current regulatory frameworks influence profoundly the pace and direction of scientific research into cannabis-based therapies. Regulatory frameworks dictate funding availability, access to research materials, and the feasibility of conducting studies. Clear and consistent laws that differentiate between medical and recreational cannabis uses are essential. These frameworks should streamline processes for obtaining research licenses and accessing cannabis-derived compounds, while also addressing misconceptions, reducing stigma, and promoting informed discussions. Standardizing cannabis cultivation, extraction, and product manufacturing protocols ensures research consistency and product quality. Adequate governmental and private sector funding is crucial to support comprehensive clinical trials, longitudinal studies, and basic research. Furthermore, fostering international collaboration among researchers, policymakers, and healthcare professionals is needed to accelerate scientific discovery and achieve regulatory alignment globally. Overcoming these regulatory barriers through comprehensive reforms, increased funding, stringent quality control standards, and collaborative efforts can fully unlock the therapeutic potential of cannabis for treating a variety of medical conditions.
In the grey literature, the reviews on cannabis are existing but often fragmented, with papers typically focusing on specific aspects such as isolated compounds, cultivation techniques, extraction methodologies, or particular therapeutic applications, comparing in vivo or in vitro studies. In contrast, this comprehensive review aims to consolidate a wide array of information into a single document. By integrating a wide array of aspects including historical and legislative context, botanical classification and variety descriptions, chemical composition highlighting cannabinoids, terpenes, and phenolic compounds, insights into biosynthetic pathways, and exploration of documented therapeutic potentials across diverse medical disciplines, this review serves as cornerstone for future research endeavors. It provides researchers and clinicians alike with a holistic overview of existing data and insights into the multifaceted aspects of cannabis science. This synthesis not only bridges gaps in current knowledge but also offers a cohesive framework that encourages further exploration and hypothesis-driven studies in cannabis research (Table 1, Table 2, Table 3).
Table 1.
Different names of Cannabis.
| Danish | English | Russian | Indian | Arabic | Chinese | Dutch | French | German | Spanish |
|---|---|---|---|---|---|---|---|---|---|
| hemp | hemp, marihuana | Kannabis, sativa | ganja, bhang, charas | Al-Bhango, Al-Hashish, Al-Qanaap | Xian ma, Ye ma | Hennep | chanvre, chanvre d’inde, chanvre indien, chanvrier | Hanf, indischer hanf, gras | Mariguana, marijuana |
Table 2.
taxonomic classification of Cannabis sativa.
| Kingdom | Plantae |
| SubKingdom | Tracheobionta |
| Phylum | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Hamamelididae |
| Order | Urticales // Rosales |
| Family | Cannabaceae |
| Genus | Cannabis |
| Species | Cannabis sativa, Cannabis indica, Cannabis ruderalis |
Table 3.
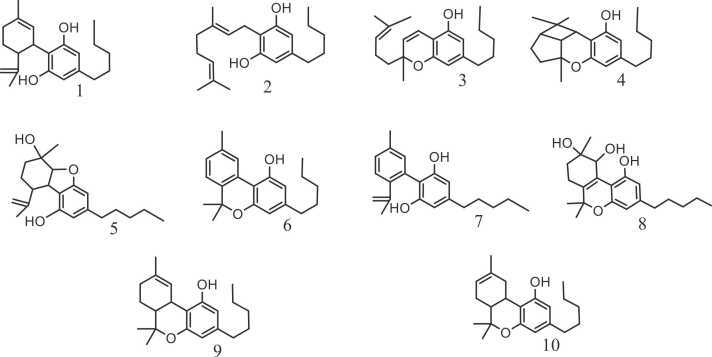
Sub-categories of cannabinoids and the compounds identified up to date with their common abbreviations.
| Sub-category | Compounds |
|---|---|
| Cannabidiol (CBD) |
|
| Cannabigerol (CBG) |
|
| Cannabichromene (CBC) |
|
| Cannabicyclol (CBL) |
|
| Cannabielsoin (CBE) |
|
| Cannabinol (CBN) |
|
| Cannabinodiol (CBND) |
|
| Cannabitriol (CBT) |
|
| Δ9- tetrahydrocannabinol (Δ9-THC) |
|
| Δ8-tetrahydrocannabinol (Δ8-THC) |
|
2. Origin and botanical classification of Cannabis
The plant’s name originated from the Scythians, adopted into Persian as ‘kanab,’ Greek as ‘κάνναβις’ (kánnabis), and Latin as ‘cannabis’ [13]. From an etymological point of view, the genus name “Cannabis,” signifying “cane-like,” and the epithet name “sativa” meaning “planted or sown,” indicates that cannabis grew from its seeds rather than its roots [139], [146], [51]. It bears various common names across different languages, some of these are mentioned below [138], [51], [53]:
2.1. Botanical Classification
Up until the second half of the 18th century, the taxonomic classification of Cannabis has remained a topic of continuous debate regardless of its use for millennia for diverse application [51], 1753 marked a pivotal moment for its modern botanical nomenclature upon its initial classification by the Swedish botanist Carl Linnaeus considered the father of modern taxonomy in the initial documented publication employing the scientific species name “Species Plantarum” (Latin for “The species of plants) [26], [59]. Currently, according to the phylogenetic classification APG IV (Angiosperm Phylogeny Group) of 2016, and to the USA Department of Agriculture (2021) [51], Cannabis sativa is currently classified as follows:
2.2. Genetic crosses and varieties
The number of cannabis species has been debated for a long time, up until the early 70 s when Small and Cronquist [172] categorized it as a genus having two subspecies, namely Indica or Marijuana englobing two under subspecies -variety indica which is domesticated, and variety kafiristanica considered wild- and Sativa which can also be broken down to domesticated var. sativa and wild var. spontanea [156]. This early classification simply distinguished two species of Cannabis: marijuana (cannabis indica) and hemp (Cannabis sativa) [62], then in 1974, Schultes, Klein, Plowman, and Lockwood have divided the cannabis genus into three distinct subspecies according to their cannabinoid composition, morphological, geographical, ecotypic or chemo-typic differences: Sativa L, Indica Lam, and Ruderalis. This last one only englobes wild varieties [146], [74]. The differences between the species primarily revolve around the general morphology of the plant, their intended uses, and the optimal climatic conditions for cultivation [58]. Nevertheless, these distinctions are increasingly blurred due the endless possibilities presented by numerous hybrid varieties [156].
Many scientists still consider the genus Cannabis to contain only two major species: Sativa and Indica, given their economic importance besides their broad abundance [91], while others acknowledge cannabis as monotypic, while the remaining species are variations of sativa L (Suman [41], [199]).
The three species have noticeable morphological differences:
Sativa L: also known as industrial hemp, originated from Europe, this variety is slender and tall, with a fibrous stem, quite long flowering, and narrow leaflets. It usually blooms later than indicas, taking approximately 120 days to mature. While exhibiting an exceptionally low concentration of the psychoactive compound ∆9-tetrahydrocannabinol (∆9-THC) <0.3 %, C. sativa L exhibits a greater abundance of cannabidiol (CBD) compared to indica and ruderalis -usually CBDA is the most abundant subsequently accompanied by CBD in a proportion that could progressively diminish due to decarboxylation-[5], [30], [182].
The letter “L” represents Linnaeus as a tribute to his contributions as he was the first one to name the species as Cannabis sativa [186].
Indica Lam: Originated from Asia, this drug-type plant is shorter with a woody stem, broad leaflets, and compact habits. It matures early producing a powerful distinct smell that causes intoxication if inhaled [68]. This variety exhibit a more important concentration of THC (∆9-THC>0.3 %), therefore commonly known as a medicinal and recreational drug [156], [30], [5].
Ruderalis: Cultivated in the Eurasian regions, it exhibits a sparse and invasive growth pattern, varied leaflets, shorter stature, and small size which gives it a wild looking. It contains intermediate levels of CBD and ∆9-THC [1], [193], [51]. Ruderalis is characterized by its early bloom, some of its representatives’ bloom even independently of the photoperiod, which makes it an interesting variety for the acquisition of the trait “auto-bloom”, inexistent in the other varieties. It tolerates colder climates and difficult environmental conditions.
Hybrids: Furthermore, apart from the main varieties; indica, sativa and ruderalis, these cultivars are commonly interbred and propagated to generate hybrid phenotypes possessing various proportions of all three types and desired characteristics [81]. exceeding 2300 diverse strains or “chemovars” [1].
2.3. Botanical description of the plant
Botanically speaking, cannabis is mainly a dioecious plant, rarely monoecious. Given the right environment, it exhibits rapid growth and can attain a height of up to five meters within the span of 4–8 months, typically from spring through autumn (Suman [41], [51], [56], [114]). The external environment factors and the genetic code influence the plant phenotype (leaf shape, flower color…) [139].
Morphologically, C. sativa’s characteristics (size, shape…) depends on its genetics, mainly it is a plant with a fluted woody branched stem, in its lower part, there are opposite, palmate stipulate leaves that include up to 7 unequal toothed and elongated segments, as it approaches the apex of the stem, the leaves transition to a tri-segmented pattern or adopt either a simple or an alternate arrangement [29]. The roots of the plant are usually branched about 30–60 cm deep in wet soils. Staminate or pistillate inflorescences consists of multiple flower heads arranged along elongated leafy stalks, each individual flower yields a singular brownish fruit, typically measuring 2–5 mm in length, encapsulating a single seed encased in a robust shell [139]. Pistillate are short, dense, with candlelike floral aggregates, while staminate are characterized by their height, slim profile, a lower leaf density, and they bloom earlier than pistillate [58], [62]. These seeds are round dark red, or brownish, tightly covered with a rigid shell, and they germinate after 8–12 days [51], [55].
On a microscopic scale, the main structure is the trichome, glandular and non-glandular protuberance spiked on the leafy surface of flowers, leaves, and stem of the plant [8]. These resinous structures are the reservoir of phytocannabinoid and terpenoids, and they are accountable for botanical interactions, shielding the plant, and its characteristic odor [139].
Male flower plays a role in fertilization by pollinating the female flower. While this phenomenon naturally transpires in the wild, deliberately generating new variations entails meticulous breeding within a regulated setting. In the event that a male plant from one strain fertilizes a female plant from a different one, the resultant seeds are hybrids, and inherits traits of both parent types [157], [74]. Through repetitive breeding, specific characteristics can become more consistent.
3. The plant’s chemical constitution
Cannabis as a polymorphic plant have very complex composition, producing different chemicals components that can be located in various plant components (flowers, leaves, stalks, and seeds). The biggest main group is cannabinoids- an exceptional category of terpenophenolic active compounds, primarily limited to cannabis, plus other compounds: terpenoids, flavonoids, alkaloids, stilbenoids, steroids, carbohydrates, polysaccharides, fatty acids, and their esters, amides, amin-acids, phytosterols, polyphenols, sterols, oils, waxes, proteins… Ashton [10], [103], [106], [114], [144], [150], [17], [49], [79]. This chemical composition is affected mainly by geographical conditions, variety, stage of the plant’s development, the growth settings ((nutriments, humidity, light settings…), harvest times, and storeroom conditions [114], [125], [20], [43].
The number of isolated or identified compounds increases over the years, starting with 423 in 1980, to more than 750 currently. Researchers isolated 42 phenolics, 34 flavonoids, 120 terpenes and over 125 cannabinoids [150], [161], [193], [36], [49]. Some non-cannabinoid compounds create “the entourage effect”, exerting a synergy between them and cannabinoids to enhance the overall effects [51], [130] (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
3.1. Cannabinoids
3.1.1. Overview of cannabinoids
Cannabinoids are oxygenated tricyclic compounds with a benzopyran structure [51]. In their natural form, their consist of 21 carbon atoms, with variations in the side chain length linked to the aromatic ring [161], [25], [51]. They are non-polar lipophilic compounds, typically categorized into 3 types: endocannabinoids, synthetic cannabinoids, and phytocannabinoids [1]. The first ones are mainly fund in the brain, they are endogenous lipids that can affect physiological functions by interacting with cannabinoid receptors [115], they are synthesized by nearly all organisms within the Animalia kingdom [183]. Synthetic cannabinoids have similar capacities to phytocannabinoids, as they can bind with the same affinity to human cannabinoid receptors [168]. As for phyto-cannabinoid, they are a group of oxygenated aromatic metabolites found in in C sativa, which has been classified into 11 different categories [103], [124], [144], [25]:
- (i)
Cannabidiol (CBD): This sub-category contains 7 known compounds currently. It is a non-psychoactive cannabinoid that was first discovered in 1940 by Roger, then in 1963, Mechoulam updated and corrected its structure.
- (ii)
Cannabigerol (CBG): It is a non-psychoactive cannabinoid isolated by Mechoulam et al. in 1964. This sub-category currently englobes 16 compounds.
- (iii)
Cannabichromene (CBC), englobing 9 cannabinoids, this compound is non-psychoactive detected only in small quantities. It was first discovered in 1966 by Mechoulam et al.
- (iv)
Cannabicyclol (CBL), with 3 compounds, first mentioned in 1964 by Korte et al. and was named “cannabipinol” by the same group. Then in 1967 it was updated to “cannabicyclol” by Mechoulam et al.
- (v)
Cannabielsoin (CBE), englobing 5 compounds, first mentioned in 1973 (Johnson et al., 2020).
- (vi)
Cannabinol (CBN), in this psychoactive sub-group, 11 compounds were isolated until now. Robert S. Cann. marked the initial discovery and isolation of cannabinoids, as he identified CBN as the first naturally occurring one in 1896 (Nahar et al., 2021; [144]).
- (vii)
Cannabinodiol (CBND), a psychoactive sub-category containing 2 compounds, first mentioned in 1972 and updated in 1977 (Johnson et al., 2020).
- (viii)
Cannabitriol (CBT), with 9 compounds, this sub-group was isolated it 1966, and its structure was reported in 1976 (Johnson et al., 2020; Mechoulam and Hanuš, 2000).
- (ix)
Δ9- tetrahydrocannabinol (Δ9-THC), containing 23 compounds, its chemical structure initially reported in 1942, then its accurate structure was subsequently documented in 1964 ([70]; Mechoulam, 1986). Being the main psychoactive compound in plant, it induces a sense of euphoria ([51]; Sirangelo et al., 2022), the content of this volatile lipophilic compound varies across distinct plant sections [81], resulting in the highest concentration within flowers (10–12 %), lower amounts in leaves (1–2 %), and minimal levels in stems (0.1–0.3 %) and roots (<0.03 %) [51].
- (x)
Δ8-tetrahydrocannabinol (Δ8-THC) englobing 5 compounds, it was first identified in 1966, then its structure was updated in in 1970.
- (xi)
Miscellaneous, currently, this sub-category englobe 30 compounds discovered by different researchers up until 2005.
3.1.2. The biosynthesis of cannabinoids
Cannabinoids are biosynthesized and found within the living plant in their respective carboxylic acid state, precisely in resin secreted within pistillate’ s glands (Nahar et al., 2021; Sirangelo et al., 2022). Due to their instability, they undergo a non-enzymatic decarboxylation to form neutral homologues that are more active and efficient pharmacologically, because of combustion (smoking), drying, light or storage conditions after harvesting [139], [149], [161], [49], [51], [61].
Cannabis trichomes are either glandular or non-glandular; these last ones serve to protect the plant tissues against biotic (caused by living organism: pathogens, pests, or weed) and abiotic stresses (due to environmental factors: heat or cold stress, salt stress, water stress…) [71], while the gland-rich ones are in charge of producing and storing bioactive compounds mainly cannabinoids and terpenes. They can be categorized as stalked, sessile, or bulbous. Among these, bulbous trichomes yield fewer cannabinoids compared to the other varieties [80], As the flowers and seeds mature, the cannabinoid levels in the resin undergoes alterations, with the highest levels occurring at flower maturity and in warmer temperatures (Sirangelo et al., 2022).
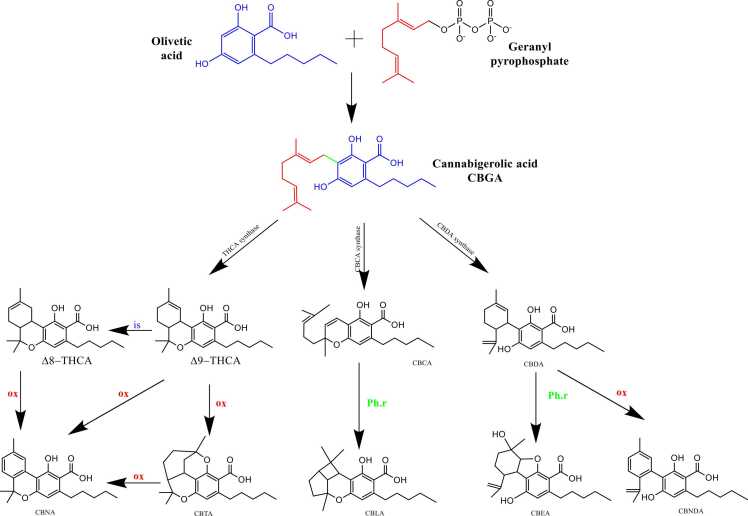
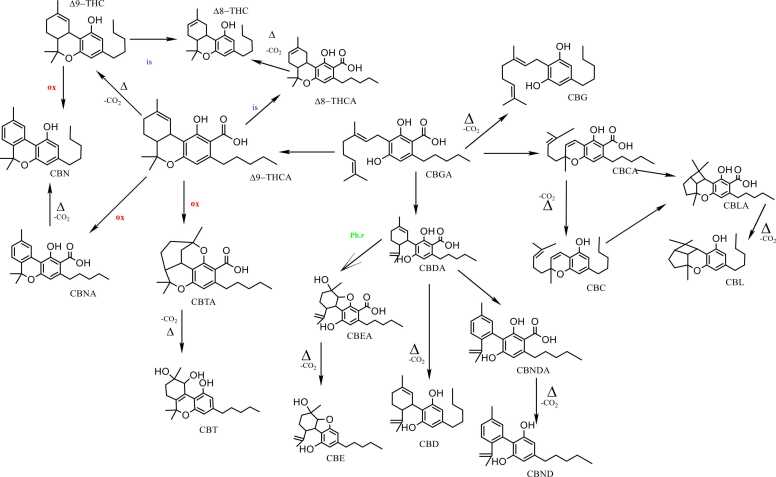
Cannabigerolic acid CBGA is the predominant cannabinoid acid present in unprocessed plant material. It is produced through the combination of olivetolic acid (OLA) with geranyl diphosphate (GPP) [104], [108], [20]. As the biosynthetic precursor for major cannabinoids, CBGA is cyclized to produce the acids THCA, CBDA and CBCA, through different reactions in which CBGA undergoes oxidation via flavin adenine dinucleotide (FAD)-dependent oxidases, specifically, Δ9-THCA synthase, CBDA synthase, and CBCA synthase [103]. After that, CBDA and THCA are cyclized to produce cannabinodiolic acid (CBNDA) and Cannabinolic acid (CBNA), respectively. Furthermore, other acids can be synthesized: cannabielsoic acid (CBEA) is the hydroxylated form of CBDA with one phenolic oxygen atom attached to the monoterpene unit, Cannabicyclolic acid (CBLA) is an extended version of CBCA, with two extra rings. Meanwhile, Cannabitriolic acid (CBTA) represents a hydroxylated variation of Δ9-THCA, marked by the presence of two additional hydroxyl groups at distinct positions [20], [51].
Despite the not intoxicant nature of the acids, they spontaneously decarboxylate upon heating on under alkaline conditions [44]. CBGA decarboxylate to cannabigerol CBG, Δ9-THCA (which consist 95 % of Δ9-THC in fresh plants), convert into its psychoactive precursor Δ9-THC, that produces CBN by oxidation, or ∆8-THC by isomerization. Also, CBDA (which consist 95 % of CBD in fresh biomass) and CBCA decarboxylate to CBD and CBC, respectively [51], [58]. Furthermore, CBD, CBC, and CBL coverts to cannabicyclolic CBL, cannabicyclolic acid (CBLA), and cannabigerol (CBG) [149], [42], [5].
Understanding the biosynthetic pathways of cannabinoids holds significant potential for developing specific therapeutic compounds with enhanced efficacy and tailored effects. This approach, known as synthetic biology or metabolic engineering, entails altering genetic and enzymatic processes in plants or microorganisms to optimize the production of desired compounds. Techniques such as molecular breeding, tissue culture, and genetic engineering can be employed to selectively breed cannabis varieties with targeted traits. Additionally, cannabis micropropagation allows for rapid multiplication while maintaining genetic integrity. Integrating gene editing methods to modify RNA and regulate gene expression within cannabis offers further tools to precisely control or modify biosynthetic pathways, thereby achieving desired cannabis characteristics [170]. However, the biosynthetic origin and genetic regulation of many potentially therapeutically relevant compounds is still unknown, limiting the possibilities of controlling biochemical pathways, as metabolic engineering in yeast and cell-free platforms are not yet compatible with commercial-scale production, while molecular pharming through the development of cell cultures is complicated by the complexity of cannabinoids at low molar concentrations [188]
3.1.3. The endocannabinoid system ECS and CB receptors
After the identification of Tetrahydrocannabinol (THC) and its isolation in 1964, Dr. Raphael Mechoulam discovered the human endocannabinoid system ECS during the late 80 s. an endogenous signaling system able to modulate and adjust diverse physiological processes. Carcieri et al., [156], [36]. To date, the major cannabinoid receptors identified are CB1 and CB2 (or CB1R and CB2R), categorized within the membrane protein family GPCR or G protein-coupled receptors, with unique pharmacological and signaling properties [156], [51]. As Cannabinergics (molecules –irrespective of its chemical or therapeutic activities- that modulates the ECS, including CB1 or CB2 receptor agonists or antagonists, compounds that serve as substrates or inhibitors for fatty-acid amide hydrolase, as well as the endocannabinoid transporters [135], cannabinoids can mimic endogenous ligands of the ECS. Other than CB1 and CB2, their physiological impacts are also mediated via alpha- and beta-adrenergic receptors, and the newly identified GPR55, GPR3 and GPR5 in the brain, also belonging to G protein-coupled receptors [99]. Moreover, cannabinoids can bind to non-cannabinoid receptors and other channels in the body.
CB1 is a central receptor mainly abundant in the brain, and spinal cord, moreover, it is found in low levels within, reproductive cells, the immune system, and several organs (kidney, liver…). This receptor regulates the liberation of various neurotransmitters including acetylcholine related to cognitive functions, norepinephrine involved in regulating heart rate, blood pressure…, the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), the excitatory neurotransmitter glutamate involved in cognitive processes, dopamine… [156].
CB2 is a peripheral receptor located in immune cells as it plays a role to modulate their migration and release cytokines, also it is present in gastrointestinal tract and in neuronal and non-neuronal brain cells at low levels [125], [135], [156].
THC was characterized as a compound with partial agonistic activity at CB1 and CB2 receptors, binding to both, the first one being responsible for mediating most of its psychoactive and analgesic effects. Moreover, THC’s interaction with CB1 inhibits ongoing neurotransmitter release [110], [149], [156], [178], [96]. While CBD exhibits negligible affinity for CB1 and CB2 receptors, resulting in minimal binding, it interacts with them as a negative allosteric modulator (reduces or inhibit their activity) [110], [178], [36], [96]. Also, CBD binds to GPR55 receptors in the cerebellum, and to the body’s ion channels and non-cannabinoid receptors [156].
Several studies have revealed that THC and CBD’s interaction with the endocannabinoid system receptors is crucial to the transmission of the nociceptive information (the sensory signals related to the detection and processing of pain or noxious stimuli in the body), giving feedback responses to modulate mood, pain, muscle relaxation, queasiness and more. Hacke et al., [112], [77]. Furthermore, THC’s immunomodulatory activity binds to the receptors of the immune system. These interactions with both the nervous and the immune systems affect various processes in the human body: neuroinflammation, bone remodeling, atherosclerosis, appetite, and pain management. Furthermore, it acts as an antiemetic, antikinetosic, and intraocular hypotensive agent (helps lower intraocular pressure (IOP) or fluid pressure inside the eye [149], [36].
Studies of cannabinoids led to the emergence of the “entourage effect” concept: the therapeutic potential of the whole plant is derived from the synergistic interplay of numerous cannabinoids (approximately more than 100), rather than solely relying on a single molecule. Addo et al., [1], [4]. The “entourage effect” was first observed in the binding affinity of endocannabinoid system ligand 2-arachidonoylglycerol (2-AG) for the endocannabinoid receptor 1 (CB1), as palmitoyl glycerol and linoleyl glycerol enhanced the binding affinity of 2-AG for CB1 [19]. This term was later adopted for the synergy between bioactive compounds, referring to the concept that plants have better therapeutic activity than the natural products isolated from them [92].
3.2. Terpenes
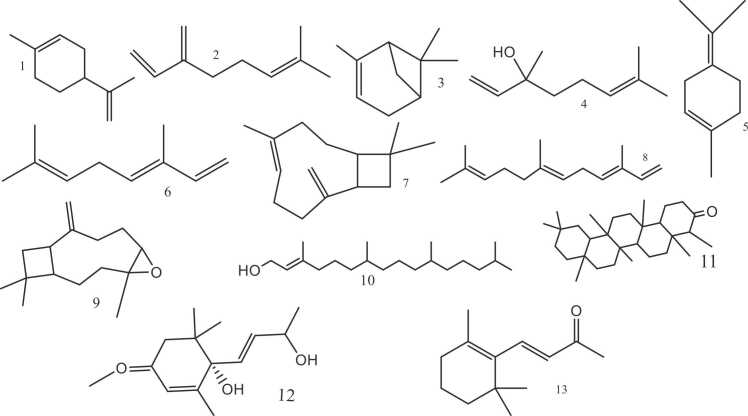
These molecules constitute the second largest class among compounds found in this plant with over 120 identified so far. They are aromatic, lipophilic molecules mainly found withing glandular trichomes of the inflorescences, and are responsible for their characteristic aroma. Isidore et al., [139], [83] These compounds are hydrocarbons derived from isoprene (C5H8) [94], they are primarily obtained from flowers, roots, leaves, trichomes, and essential oils [103]. Simonsen and Todd categorized terpenes as a separate category from cannabinoids for the first time in 1942. Sommano et al., [173]
Terpenoids also known as isoprenoids, are oxygen-contained terpenes that have various roles in plants, including functioning as phytohormones, membrane stabilizers, and participating in respiration, photosynthesis, signaling and protective mechanisms [64]. They can exist as linear, monocyclic, or polycyclic hydrocarbons possessing diverse functional groups like alcohols, ethers, aldehydes, ketones, and esters [51], [56]. These compounds were demonstrated to exhibit synergistic effects with cannabinoids. Pegoraro et al., [145].
Various categories of terpenes/terpenoids are isolated from cannabis:
- •
Monoterpenes (10 C): consisting of two isoprene units; englobing 61 compounds [150], [194], [94].They are volatile compounds that add to the unique, characteristic odor [194], [94]. The main compounds are limonene, β-myrcene, α-pinene, linalool, α-terpinolene and tranocimene. Their content in the plant increases in the late harvest time [83].
- •
Sesquiterpenes (15 C) of three isoprenes; containing 51 compounds [150], they are semi-volatile compounds, also contributing to the distinctive odor [194], [94]. The main compounds are E-caryophyllene, E-β-farnesene, caryophyllene oxide, and β-caryophyllene, and their content is the highest in the earlier harvest time [83].
- •
Diterpenes (20 C) of four isoprenes; exist as steroids, waxes, and resins. Currently, only 2 compounds are found in Cannabis sativa: Phytol and neophytadiene [150].
- •
Triterpenes (30 C) of six isoprenes; exist as steroids, waxes, and resins across different plant sections: stem, leaves, or roots (where the highest content has been recovered). The major compound; Friedelin was discovered in 1971 along with epifriedelin, which has been detected along with β-amyrin in smaller amounts [83].
- •
Miscellaneous Terpenes: four terpenes belonging to this sub-category, two were isolated form the plant’s stems and leaves: vomifoliol and dihydrovomifoliol while β-ionone and dihydroactinidiolide were identified in the plant’s oil [150].
Terpenes and terpenoids can be lost during the decarboxylation and the drying processes, due to the oxidative changes [58], [83].
3.3. Phenolic Compounds
Within cannabis, phenolic compounds are classified into three main categories: flavonoids, stilbenoids and lignans [147], [83], [85]. The first two are mainly identified in leaves, stalks, and inflorescence, whereas lignans are primarily found in seeds, roots, and fruits [147], [65]. During the growth of the plant, the content of these phenolic compounds decreases as they condensate with terpenes to form cannabinoids [106], [83].
3.3.1. Flavonoids
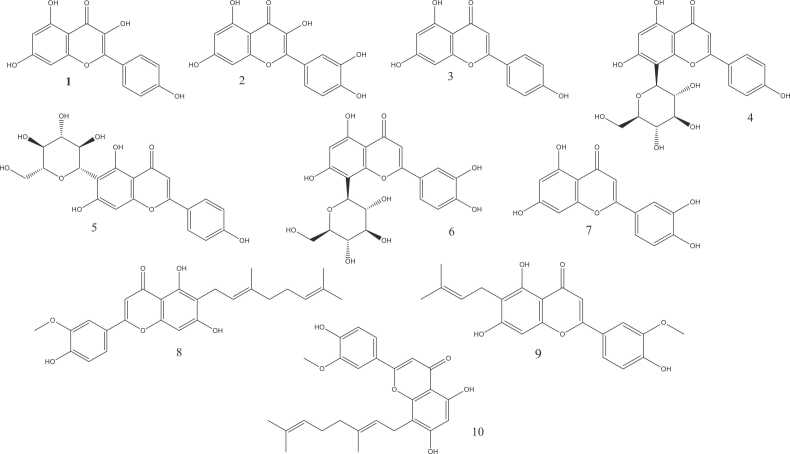
They are found in leaves, flowers, seeds, and pollen, and they constitute the biggest group within phenolic compounds. They are specialized metabolites considered as capable to enhance the plant’s diverse medicinal capabilities [103], [51]. More than 34 flavonoids were isolated from this plant, including kaempferol, quercetin, apigenin, vitexin, isovitexin, orientin, luteolin, and 3 flavones with prenyl or geranyl groups known as cannflavin A, B, and C. Bautista et al., [103], [161], [17], [56] Their concentrations within cannabis exhibits significant variation among species and among distinct plant components. Moreno et al., [130] Moreover, their production is impacted by environmental elements notably solar irradiance, thermal conditions, moisture, and precipitation [17], [35], [51], moreover, creating special circumstances including instances of biotic and abiotic stresses are necessary to produce some of these compounds.[1]
3.3.2. Stilbenes
Stilbenes are found in the inflorescences, stalk, verdures, and trichomes of the plant. They are mainly classified into 3 categories: [103], [150], [83]
Phenanthrenes or Dihydrophenanthrenes, containing 7 compounds.
Dihydrostilbenes: englobing 12 compounds, among them Canniprene that showed anti-inflammatory activity.
Spiro-indans: containing 16 compounds, of which Cannabispirone and cannabispirenone A, the main compounds of this category demonstrated potential effects against inflammation and cancer. Another compound; denbinobin showed pro-oxidative and antileukemic properties on human cancerous cells.
3.3.3. Lignans
Lignans derived from C. sativa fall into two primary categories: phenolic amides and lignanamides [83]. For the first group, five compounds were detected in trace amounts in cannabis: N-trans-coumaroyltyramine, N-trans-feruloyltyramine, N-trans-caffeoyltyramine, N-trans-caffeoyloctopamine and N-trans-coumaroyloctopamine. Isidore et al., [83] As for Lignanamides, more than 14 molecules were isolated from cannabis, among them: Cannabisin molecules A, B, C, D, E, F, G, M, N and O, 3,3′ -dimethyl-heliotropamide, grossamide, etc.
These compounds exhibit potent abilities to combat inflammation, oxidative stress, cancer, and high lipid levels as evidenced by cell culture, in vivo, and in vitro studies. Montero et al., [123] N-trans-caffeoyltyramine and cannabisin A have been reported to have powerful antioxidant capabilities, whereas grossamide and cannabisin F were documented for their ability to manage inflammation. Also, some lignanamides are considered promising prospective agents for multifaceted therapy against Alzheimer’s disease as they function as acetylcholinesterase inhibitors while also displaying antioxidant properties. Isidore et al., [123], [83]
4. Therapeutic proprieties of cannabis
Based on a survey involving 953 individuals across 31 country, this plant is known as a treatment for various symptoms and cases, particularly for pain management, anxiety, depression, and insomnia [125]. In Morocco, the therapeutic use of plant species is deeply rooted in culture and tradition, partly due to the challenges in supplying contemporary pharmaceuticals [38].
4.1. Cannabinoids
The impacts of phytocannabinoid generally include modulating (activating/ inhibiting) receptors -including GPCRs- and enzymes linked to ECS [25]. The pharmaceutic activity of cannabinoids is influenced by mode of administration, dose, chronic usage, etc.
Cannabis is well known for its therapeutic effects against inflammation and neuropathic and chronic pain that does not respond to corticosteroids, opioids, or nonsteroidal anti-inflammatory drugs (NSAIDs) [1], [36], [68] spasticity related to pain diseases including multiple sclerosis [161], [51], [68], facial and bodily spasms in Gilles de la Tourette Syndrome [161], [36]. It is widely reported to be beneficial in oncology for its antineoplastic effects, as the bioactive compounds inhibit tumor growth and contribute to the progression and restoration of homeostasis [1], [129], [161], [35], and to alleviate its symptoms and side-effects including pain, and chemotherapy-triggered emesis and queasiness. Carcieri et al., [125], [189], [36], [68]. Moreover, it represents a reasonable therapeutic option for neuroprotection, in neurological disorder such as epilepsy [105], [143], [174], [51], and neurodegenerative disorders affecting the structure and the overall neurological function, as a result of the progressive degeneration and dysfunction of the nervous system. Cannabis’s effects have been tested of some of these disorders: Multiple sclerosis (MS), Amyotrophic lateral sclerosis (ALS), Parkinson’s, Alzheimer’s, and Huntington’s disease [136], [51], [68].
In order to study the pain-management ability of cannabis, more than 41 trials have been conducted including at least 4550 patients with multiple sclerosis, chronic neuropathic pain, or patients undergoing chemotherapy for cancer treatment. For patients with MS, cannabis showed high efficacy (versus a placebo) as an analgesic of pain and spasticity, either muscle stiffness, spasms, cramps, tightness, or weakness. A small effect was reported among individuals experiencing persistent neuropathic pain, comparing to those receiving a placebo, as well as antiemetic effect against chemotherapy-induced nausea [6]. Lately in 2019, Cannabis was used in an epidemiological study conducted on fibromyalgia patients as this chronic disorder is characterized by widespread musculoskeletal pain, resulted in significant results reported by more than 80 % of the patients, as they responded to treatment and reported a decrease in pain intensity [160].
An in-vitro experiment using human colon cells was conducted on both cancerous and healthy cells cells confirmed the anti-cancer effect of cannabis extracts, as they selectively decreased the viability of these cells while shielding non-diseased intestinal cells from cytotoxic impacts [155].
Furthermore, this plant is documented to treat various diseases including glaucoma resistant to conventional therapies by reducing the intraocular pressure [167], [36], [7], depression, anxiety, insomnia, pulmonary disorders such as asthma, and cardiovascular disorders [136], [167], [36], [51], [68], they are also recognized in the therapy of HIV/AIDS patients, particularly to stimulate the appetite, against weight loss, cachexia and anorexia in individuals with cancer or AIDS, [189], [36], [68], in the management of schizophrenia [161], [48] as bone marrow stimulants [197], to control high blood pressure, and cholesterol levels, as well as managing dermatological disease and antiaging effects [175].
Cannabis was associated with a phenomenon of vision dimming, for the first time in the 11th century by Ibn Sina in the second volume of his renowned work “Canon of Medicine” [137], then in the 15th century in “Damascus” by al-Ghazzi. These proclamations were repeated in a modern investigation suggesting a reduction in the capacity for scotopic vision adjustment among Costa Rican cannabis chronic smokers. Moreover, an observational study focusing on nocturnal vision was conducted on 3 male cannabis smokers in Morocco, reported coherent enhancements in tests measuring dark adaptation and scotopic sensitivity after smoking cannabis. After 30 min dark adaptation, with strong dependability and consistency at intervals of 6 weeks [159].
Moreover, this plant possesses inherent antioxidant properties due to phenolic compounds and their ability to scavenge free radicals, such as syringic acid and sinapinic acid, which contributes to the antioxidant and anti-inflammatory activity [175], [18], [196], [2], [3], [95]. The antimicrobial efficacy of cannabis’s essential oil was tested against Candida and diverse pathogenic bacterial species, the study reported that it had moderate antimicrobial efficiency and exhibited interesting antioxidant potency [133]. In different experiment on edibles, hemp products (oil or flour) were used to test its antioxidant activity. Adding cannabis oil to the active material coating in trout fillets protected them from lipid peroxidation and bacterial growth during refrigeration storage, and adding cannabis flour in bread production resulted in increasing phenolic compounds and antioxidant activity of the bread [161]. Furthermore, synergistic interactions were observed between the EO and conventional antibiotics, enhancing their effectiveness against the tested microorganisms [133].
Using the whole plant or its compounds as a therapeutic solution to treat many conditions and symptoms still encounter some potential adverse effects including heightened heart rate, euphoria, hallucinations, anxiousness, perceptive alterations, impaired memory, and reduced coordination, and reflexes affecting reaction time [45]. Some research suggest that these side effects are primarily due to THC, and that using whole-plant extract have a better pharmaceutical and mood-altering while others claim that extracts from the entire cannabis plant yield enhanced pharmaceutical and mood-altering effects due to the ability of other compounds to mitigate these effects, and to the synergic action often referred to as “The entourage effects” [131].
The wrong uncontrolled use of cannabis can cause acute intoxication manifested in typical symptoms Anxiety, panic attacks, depression, allergic responses, emesis, gastrointestinal disturbances, dysphoria, tachycardia…, or withdrawal manifestations including hostility, anxiety, irritability, sleeplessness, perspiration, headache…(“International Conference on Harmonisation (ICH) [82]). To avoid intoxication or withdrawal symptoms of cannabis, following the safety guidelines outlined by the ICH International Counsil for Harmonisation, administering cannabis-derived product should start with low doses before increasing the quantity gradually after a satisfactory clinical evaluation period. Odieka et al., [139]
4.1.1. Tetrahydrocannabinol (THC)
The discovery of ECS steered a wide interest in THC, leading to various studies to explore its pharmacological potential. It has been approved to be useful in suppressing muscle’s spasticity and exercising anti-inflammatory effects powerful 20 times than aspirin, against arthritic conditions [125], [158], [165], and analgesic properties to treat pain related to epilepsy, multiple sclerosis, Tourette’s syndrome and spinal cord injury [115], [13], [4]. This cannabinoid has shown therapeutic activity against cardiovascular disorders [84], Parkinson’s, glaucoma, and autism [179], [5]. Moreover, it is administered to stimulate appetite and counteract nausea, particularly to treat nausea caused by cancer chemotherapy [4], [75]. At very low dozes, THC can protect the brain from cognitive deficits [4]. Also, THC is a neuroprotective antioxidant, exhibiting anti-pruritic properties [141], [158].
Regarding its cytotoxicity, THC was widely studied through clinical, in-vivo and in-vitro trials. Regarding breast cancer, lung cancer, melanoma and myeloma, in vitro studies reported its efficacity in inhibiting cellular growth, proliferation, chemotaxis, and invasion ([122], [23]; Preet et al., n.d.; [163], [177], [180]), also in inducing apoptosis, autophagy and cell cycle arrest by stimulating the CB2 receptor [180], [33]. in vivo studies proved THC’s ability to reduce or inhibit tumor expansion, proliferation, neovascularization and metastasis, while promoting apoptosis [21], [22], [23], [72], [148]. However, few in-vitro and in-vivo trials reported an increase in proliferation and tumor growth upon administering THC at minimal levels [118], [180], [195]. THC was also reported to induce apoptosis, and reduce tumor growth for pancreatic, prostate and colon cancer [180].Furthermore, in vitro studies concerning oral cancer proved that THC amplified the intracellular concentration of anticancer drugs in cells with multi-drug transporter activity, and educed invasion [180]. For liver cancer, particularly Hepatocellular Carcinoma, in-vitro studies shewed that THC reduced cellular vitality and proliferation, triggered autophagy and apoptosis, and reduced tumor growth [187], [98].
For brain cancer, in-vitro and in-vivo trials proved THC’s ability to suppress proliferation and cellular survival [107], [180], trigger apoptosis and autophagic processes, stimulate glioma cell growth and reduce tumor growth [162], [163], [180]. Clinical studies proved the regression of a kind of brain tumors; Pilocytic astrocytoma over three years of inhaling THC [180], the reduction of tumor cells in patients suffering from grade IV glioma, or GBM [76]. For leukemia, administering THC suppressed cell growth, demonstrated cytotoxic properties, and enhanced the sensitivity of leukemia cells to anticancer treatments while inducing apoptosis [102], [166], [180]. A clinical study on a person diagnosed with a late stage of acute lymphocytic leukemia resulted in remission after administering Cannabis sativa oil [87], [90].
However, this cannabinoid is reported to exhibits several side effects, besides inducing psychosis and sedation, THC can cause anxiety, cholinergic deficiencies, and immunosuppression [125], [83].
4.1.2. Cannabidiol (CBD)
The non-psychoactive CBD has several therapeutic effects, due to its ability to engages with various enzymes, receptors, and ion channels within ECS enhancing its expression, and affecting the immune system with diverse molecular targets and signaling pathways [5], [58]. As a result to its multitarget mechanism of action, CBD has been found to possess diverse effects. Aside from its antiemetic, anticonvulsant, antidiabetic, antispasmodic effects, and its capacity for neuroprotection, it exhibits notable antioxidant, analgesic, and anti-inflammatory activities that were evaluated in preclinical and clinical trials before it was reported as beneficial in treating neuroinflammatory disorders; Alzheimer’s, multiple sclerosis etc. Aizpurua-Olaizola et al., [125], [135], [149], [161], [4]. These studies also suggested that this cannabinoid can be useful to treat Parkinson’s disease [135], [5]; a dual-blind study involved 21 Parkinson’s disease patients proved that CBD, particularly at a high dose (300 mg/day) improved the patients’ life quality measures [39].
Clinical studies on human used CBD to treat peripheral neuropathic pain reported its effectiveness in alleviating intense pain, itchiness, and cold [192]. To prove pure CBS’s antiepileptic or anticonvulsive activity, a study on 132 patients diagnosed with treatment-resistant epilepsy proved CBD’s ability to reduce seizure’s frequency and severity [176]. Another clinical study associated CBD with antiepileptic treatment, on human Dravet syndrome for 96 weeks proved the efficiency of the suggested treatment in decreasing motor seizures and improving patient’s condition [50], [97].
This cannabinoid is reputed for its antischizophrenic, anti-sedating and anxiolytic effects as it was reported to modulate euphoric and anxiogenic effects of THC, fear-induced anxiety, and tobacco consumption [125], [154], [161], [4]. Moreover, CBD is reported to associate with schizophrenia and psychosis [5]; a clinical study using a double-blind parallel-group design focused on schizophrenia reported that CBD reduced psychotic symptoms, and as it didn’t depend on dopamine receptor antagonism, it was considered as an innovative treatment category for this condition, different from conventional antipsychotic medication [117].
In vitro studies on breast cancer, lung cancer, and Glioma and Neuroblastoma affecting the nervous system reported that CBD Induced apoptosis and autophagy [113], [169], [63], Inhibited proliferation, metastasis, migration, and invasion [116], [122], [152], [63]. It also increased sensitivity to anti-cancer agents: doxorubicin used against breast cancer and cisplatin used against lung cancer [122]. In vivo studies reported the same results found in in-vitro studies and reported that CBD decreased or inhibited tumor growth [113], [151], [152], [54].
Using CBD in in-vitro and in-vivo trials against prostate and colon cancers proved effective in inducing apoptosis, inhibiting cellular cycle, and reducing cellular proliferation, metastasis and tumor growth [12], similar results were obtained in in-vivo studies on mice [171] and in in-vitro studies [128], [180] to treat myeloma, melanoma, and leukemia.
Furthermore, cannabidiol is reported to have insecticidal, antibacterial, and antimicrobial effects, the last one was proved against Gram-positive bacteria, particularly drug-resistant strains of Staphylococcus aureus, and Staphylococcus epidermidis [109], [118], [125], [140], [161].
However, CBD’s use in human models was reported to cause some adverse effects such as diarrhea, fatigue, vomiting, somnolence… Namdar et al., [135]
4.1.3. Associating CBD and THC
The combination of phytocannabinoids enhances their activity, this statement has been initially made by Mechoulam and Ben-Shabat, demonstrating what specialists call nowadays, the “entourage effect” [120]. This synergy between phytocannabinoids was recently demonstrated at chemical levels and biological activity [134], [136]. Therefore, Δ9-tHC and CBD are often combined to improve their efficacy and THC’s tolerability profile [125]. Blázquez et al. reported that the association of the two cannabinoids in in-vitro studies increased sensitivity to radiation therapy in brain cancer [24]. In 2013, a long-term trials of a spray containing both THC and CBD on 43 cancer patients resulted in a sustained pain relief effect [88]. and in a clinical study in 2015, administering the two compounds in a 1:1 ratio increased autophagy and apoptosis, and decreased tumor growth in melanoma and myeloma [9]. More recently, in 2020, responses of lung cancer patients to THC and CBD were tracked, as they indicated that the association of these compounds inhibited cell proliferation and growth factor receptors [122]. The efficiency of a pharmaceutical-grade cannabis preparation containing 6.3 % THC and 8 % CBD was studied on 17 patients with burning mouth syndrome (BMS), for four weeks with follow-up visits for 6 months post-treatment cessation, resulted in statistically noteworthy alterations in anxiety and depression levels demonstrating a beneficial enhancement, along with important changes indicating a clinical remission of the oral symptoms. Gambino et al., [69].
4.1.4. Cannabigerol (CBG)
Cannabigerol (CBG) has proven through different studies its antibacterial, antifungal, antiproliferative, and bone-stimulant properties [31], [4], [5]. Furthermore, its therapeutic capacity extends to addressing multiple sclerosis, Parkinson’s, Huntington’s, and inflammatory bowel disorders by interacting with serotonin 1 A and adrenergic receptors [132], [27]. CBG was reported to have analgesic and anti erythemic effects, and it was described as an anti-depressant, and a mild anti-hypertensive agent [139], [31].
Overall, in-vitro trials proved CBG’s significant impacts on intracellular mechanisms, including inhibiting cell proliferation [14], [28], stimulating apoptosis and ROS reactive oxygen species’ production [28], modulating the activity of TRPV1 receptor involved in physiological processes such as pain and fever, and affecting the endocannabinoid system by inhibiting the uptake of the cannabinoid [14C] anandamide [101]. the activity of CBG against tumors was discovered through in-vivo studies, as a result of the antagonistic properties targeting TRPM8 receptors, which led to decreasing the tumor growth [28], making it useful to treat prostate tumor, hyperactive detrusor, and alleviating bladder pain. Russo, Marcu [158], also, it was described as highly efficacious against breast cancer, and demonstrated cytotoxicity at elevated dosages on human epithelioid carcinoma [14], [158].
Furthermore, its precursor, cannabigerolic acid (CBGA) was proved effective in treating cardiovascular diseases, metabolic disorders, and colon cancer [144].
4.1.5. Cannabichromene (CBC)
in vitro studies of CBC demonstrated its high potency in inhibiting viability and growth of prostate carcinoma, colorectal cancer, and breast cancer cells [101], [28]. Also, it can activate effectively caspase 3/7 enzymes involved in apoptosis and elevate intracellular Ca2+ levels [D87]. As an anti-inflammatory, CBC can bind to CB2 receptors, and engages with transient receptor potential (TRP) cation channels leading to the inhibition of ECS involved in inflammation and pain modulation [139], [158].
4.1.6. Cannabinol (CBN)
Research suggested that Cannabinol (CBN) has a potential effect on insomnia and sleep disorders [5]. and it can be applied to glaucoma cases that are resistant to regular therapy, due to its effectiveness in lowering intraocular pressure [139]. Furthermore, this nonintoxicating cannabinoid was reported to be used as a sedative, anti-convulsant, and anti-inflammatory, due to its interaction with CB2, and transient receptor potential (TRP) cation channels implicated in inflammatory process and pain management [158]. CBN has antibiotic activity against various bacterial strains including those resistant to treatments such as MRSA or methicillin-resistant Staphylococcus aureus [139], [158].
In vivo studies proved that CBC has cytotoxic effects at high concentrations in prostate cancer cell lines, it has antiproliferative effects against aggressive breast cancer cells [116]. Also, it inhibited multi-drug transporters responsible for conferring resistance to anti-cancer agents and promoted mitoxantrone’s accumulation [5].
4.1.7. Cannabielsoin CBE
Studies reported the ability of CBE to modulate the Wnt/β-catenin pathway, which plays a role in developmental processes and maintaining tissue equilibrium in order to control neuropathic pain [5].
4.1.8. Tetrahydrocannabivarin Δ9-THCV
This cannabinoid is an efficient anti-inflammatory, and anti-convulsant [139]. Moreover, Cascio et al. reported the ability of Δ9-THCV to enhance 5-HT1A or serotonin 1A receptor’s activity, involved in physiological and behavioral processes, suggesting its antipsychotic effects, and its ability to improve negative, cognitive and positive symptoms of schizophrenia [37].
Clinical studies on obese mice proved that Δ9-THCV causes weight loss, and decreases body fat, as it suppresses appetite by antagonizing CB1 receptors [158]. Another study conducted by Jadoon et al. evaluated its efficacy in suppressing fasting plasma glucose levels, giving Δ9-THCV the ability to control glycemic levels in patients with type 2 diabetes [86]. It was also used to suppress inflammation and heightened pain sensitivity stimulated in pre-clinical studies through the administration of carrageenan or formalin via CB1 and CB2 receptors, acting as an agonist and an antagonist depending on its concentration [158]. Regarding tumors, in vitro studies report that, at high concentrations, Δ9-THCV demonstrate cytotoxic effects in prostate cancer cell lines [180].
4.1.9. Tetrahydrocannabinolic acid (THCA-A)
THCA-A acts as a mild activator of CB1 and CB2 receptors comparing to THC. It is reported to possess anti-inflammatory, anti-neoplastic, antiemetic, Immunomodulatory, and neuroprotective properties [139], [158]. In vitro, it can increase cell survival in Parkinson’s disease, and reduces cancer cell’ lines viability [158].
4.1.10. Cannabidivarin CBDV
Cannabidivarin is mostly known for its anti-emetic effects besides anti-convulsant or anti-epileptic properties, particularly partial seizures onset [139], [158]. Moreover, in vitro studies reported CBDV to have variant dose-dependent impacts on prostate and colon carcinoma patients, as it reduces cellular viability [180].
4.2. Terpenes and terpenoids
Terpenes are responsible for the different flavors and aromas that specify each strains [173]. The synergy demonstrated at biological and chemical levels between phytocannabinoids was further proved between phytocannabinoids and terpenes [134], [136]. Russo documented their unique role in the entourage effect in medicinal qualities of cannabis [173], this interaction was reported to enhance the ability of cannabinoids to treat inflammation, addiction, caner, epilepsy, pain, fungal and bacterial infections. Moreover, terpenes possess analgesic and anxiolytic effects [173], [83], and can be more effective for stress relief, energy boost, maintain focus, elevating mood by aromatherapy, and treating anxiety [144], [173]. The effectiveness of these compounds was reported in treating various diseases; conditions related to nervous and gastrointestinal system due to their anti-oxidative, anti-inflammatory, gastroprotective, antidepressant, antiproliferative, antipyretic, anti-convulsive and neuroprotective activities [142], [144].
Terpenoids show anti-microbial, anti-bacterial, and anti-fungal activities [144], [83]. Using cannabis essential oil, dominated by monoterpenes and sesquiterpenes, Benelli et al. reported that they have a strong ability to control M. domestica and M. persicae, a moderate ability to control S. littoralis larvae and least toxic effects against C. quinquefasciatus larvae [3].
Terpenes in cannabis oil have various pharmacological effects:
- •
For Monoterpene:
β-myrcene enhances CBD and Δ9-THC’s pain-relieving action, and has exhibited anti-inflammatory, analgesic, muscle-relaxant, and anxiolytic properties and is employed as a sedative, flavor enhancer, solvent, or additive in lubricants [139], [51], [83]. Moreover, it provides a musky or hop-like aroma and serves as an antioxidant and anticarcinogen [28,66].
α-pinene and β-pinene: These compounds support memory function and alleviate cognitive impairment caused by THC intoxication, and they exhibit anti-inflammatory, antibacterial, and anti-microbial effects [139], [51], [60]. Moreover, the pine scent’s distinctive aroma is associated with its antiseptic properties. Sommano et al., [173].
Limonene: This Compound elevates serotonin and dopamine levels, promoting sedation, stress reduction, strong anxiolytic, and antidepressant properties. Dos Santos, Romão [139], [173], [51], [60]
Linalool: Its floral aroma can help alleviate anxiety when used in aromatherapy, and it acts like a local anesthetic and anticonvulsant. Dos Santos, Romão [173], [51], [60]
- •
For diterpene alcohol:
Nerolidol was investigated to explore its potential antimicrobial and anti-inflammatory properties, while Phytol has been investigated for its effects on reducing oxidation and inflammation. Dos Santos, Romão [51].
- •
For triterpenoid, and triterpenoid alcohol:
Friedelin was documented to exhibit anti-inflammatory, antipyretic and analgesic effects in mice and rats and have shown antiulcer, antidiabetic and cytotoxic activities [3]. Moreover, it is reported to be used against inflammation, tuberculosis and to reduce fever [139].
β-caryophyllene:
The most abundant sesquiterpenoid in cannabis, particularly following heat-induced decarboxylation, exhibits strong anti-inflammatory properties, along with potential anticancer, pain-relieving, antidepressant, gastroprotective, antifungal, antibacterial, antiproliferative, antioxidant and neuroprotective activities [51], [60], [83].
4.3. Flavonoids
Prenylflavonoids or cannflavins A (CFL-A), B (CFL-B) and C (CFL-C) are flavonoids with medicinal potentials, as they demonstrated several promising therapeutic properties, notably as radical scavengers, neuroprotectants, inflammation inhibitors, parasite and virus suppressors, anti-leishmanial and anti-tumor agents [57], [83].
Barrett and al. demonstrated the efficiency of cannflavin A and B in inhibiting prostaglandin E2 release; a local hormone that contribute to the processes of inflammation, pain and fever in human rheumatoid synovial cells, where the compounds proved their anti-inflammatory effectiveness surpassing that of aspirin by about 30 times [16]. Further pre-clinical translation and in-vivo trials demonstrated CFL-A and CFL-B’s anti-inflammatory activities via inhibiting prostaglandin and microsomal prostaglandin E synthase-1; a hormone-like substance and an enzyme, respectively, involved in physiological processes such as inflammation, fever, pain, reproductive, nervous and cardiovascular systems, gastric protection, immune response and kidney function [57].
Cannflavin A has also demonstrated its anti-oxidation, and antiparasitic activity while canflavin C (CFL-C) was demonstrated to have antiparasitic activity. Ahmed et al., [164], [3]
Research also focused on the anti-neoplastic properties of the isomer of cannflavin B; iso-cannflavin B in evaluating experimental models of breast and pancreatic cancer. In the first one, Iso B’s effects on estrogen receptors or on gonadotropin expression was studied, and it was proved that the compound promoted cell-cycle inhibition and autophagy [163]. Concerning pancreas cancer, in vivo and in vitro studies demonstrated a reduction in the size of both primary and metastatic tumors, leading to enhanced overall survival, therefore, IsoB demonstrated favorable results both as a standalone immunotherapy agent and when used in conjunction with radiotherapy [127].
5. Conclusions
This review provides a deeper understanding of the complex chemical profile of Cannabis sativa, highlighting major active compound families such as cannabinoids, terpenes, and phenolic compounds, including flavonoids, stilbenoids, and lignans. These compounds have garnered significant interest from researchers, leading to various studies, including in vivo and in vitro trials, to explore their chemical and biological capabilities. They have demonstrated considerable potential, exhibiting antioxidative and antibacterial properties, alongside a wide range of therapeutic effects that have proven effective in managing, alleviating, and mitigating various symptoms, while also elucidating the intricate biochemical processes that underpin these properties.
The phytocomplexity of Cannabis, combined with ongoing debates on its legalization, contributes to the limited data and information available about this plant. Current research aims to determine its complete profile—from chemical composition and biosynthesis to its therapeutic uses—along with optimal extraction techniques for each part of the plant, and methods for isolating and characterizing newfound chemicals. However, the plant’s diversity complicates this endeavor, as each phenotype is unique, possessing different levels of chemical compounds influenced by factors such as cultivation, harvest, and storage conditions. Despite the intense interest and ongoing research aimed at unlocking the full potential of cannabis, its existing and prospective applications are not yet fully realized. Many of its molecules promise extensive potential, with the capability to be applied in food, medical, nutraceutical, and beauty products.
CRediT authorship contribution statement
Mohamed REJDALI: Formal analysis, Data curation. Hassan AMHAMDI: Visualization, Supervision. Amin SALHI: Methodology, Investigation, Funding acquisition. Abedellah ELYOUSSFI: Formal analysis, Data curation. M’hamed AHARI: Writing – review & editing, Validation, Supervision, Project administration. Fatima-Zahrae LAABOUDI: Writing – original draft, Resources, Methodology, Investigation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof. L.H. Lash
Data availability
No data was used for the research described in the article.
References
- 1.Addo P.W., Desaulniers Brousseau V., Morello V., MacPherson S., Paris M., Lefsrud M. Cannabis chemistry, post-harvest processing methods and secondary metabolite profiling: a review. Ind. Crops Prod. 2021;170 doi: 10.1016/j.indcrop.2021.113743. [DOI] [Google Scholar]
- 2.Ahidar N., Labhar A., Benamari O., Ahari M., Salhi A., Elyoussfi A., Amhamdi H. Phenolic content and antioxidant activity of Cannabis sativa L. flowers from the ketama region in Northern Morocco. Ecol. Eng. Environ. Technol. 2024;25:209–215. doi: 10.12912/27197050/175125. [DOI] [Google Scholar]
- 3.Ahmed M., Ji M., Qin P., Gu Z., Liu Y., Sikandar A., Iqbal M.F., Javeed A. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019;17:6961–6979. doi: 10.15666/aeer/1703_69616979. [DOI] [Google Scholar]
- 4.Aizpurua-Olaizola O., Omar J., Navarro P., Olivares M., Etxebarria N., Usobiaga A. Identification and quantification of cannabinoids in Cannabis sativa L. plants by high performance liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2014;406:7549–7560. doi: 10.1007/s00216-014-8177-x. [DOI] [PubMed] [Google Scholar]
- 5.Al Ubeed H.M.S., Bhuyan D.J., Alsherbiny M.A., Basu A., Vuong Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules. 2022;27 doi: 10.3390/molecules27030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amato L., Minozzi S., Mitrova Z., Parmelli E., Saulle R., Cruciani F., Vecchi S., Davoli M. Revisione sistematica sull’efficacia terapeutica e la sicurezza della cannabis per i pazienti affetti da sclerosi multipla, dolore neuropatico cronico e pazienti oncologici che assumono chemioterapie. EP. 2017;41:1–15. doi: 10.19191/EP17.5-6.AD01.069. [DOI] [PubMed] [Google Scholar]
- 7.Ameur S., Haddou B., Derriche Z., Canselier J.P., Gourdon C. Cloud point extraction of Δ9-tetrahydrocannabinol from cannabis resin. Anal. Bioanal. Chem. 2013;405:3117–3123. doi: 10.1007/s00216-013-6743-2. [DOI] [PubMed] [Google Scholar]
- 8.Andre C.M., Hausman J.-F., Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong J.L., Hill D.S., McKee C.S., Hernandez-Tiedra S., Lorente M., Lopez-Valero I., Eleni Anagnostou M., Babatunde F., Corazzari M., Redfern C.P.F., Velasco G., Lovat P.E. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Invest. Dermatol. 2015;135:1629–1637. doi: 10.1038/jid.2015.45. [DOI] [PubMed] [Google Scholar]
- 10.Ashton C.H. Pharmacology and effects of cannabis: a brief review. Br. J. Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- 11.Audeval, C., 2003. Cannabis et thérapeutique: mise au point. Université de Limoges- Faculté de pharmacie.
- 12.Aviello G., Romano B., Borrelli F., Capasso R., Gallo L., Piscitelli F., Di Marzo V., Izzo A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012;90:925–934. doi: 10.1007/s00109-011-0856-x. [DOI] [PubMed] [Google Scholar]
- 13.Badrana F., Mokhtari A., Safini N., Soulaymani A., El Fahime E., El Amri H. Systematic review and meta-analysis for the biotechnological production of THC in Morocco. E3S Web Conf. 2021;319:02012. doi: 10.1051/e3sconf/202131902012. [DOI] [Google Scholar]
- 14.Baek S.H., Kim Y.O., Kwag J.S., Choi K.E., Jung W.Y., Han D.S. Boron trifluoride etherate on silica-A modified lewis acid reagent (VII). Antitumor activity of cannabigerol against human oral epitheloid carcinoma cells. Arch. Pharm. Res. 1998;21:353–356. doi: 10.1007/BF02975301. [DOI] [PubMed] [Google Scholar]
- 15.Balabanova, S., Parsche, F., Pirsig, W., n.d. First identification of drugs in Egyptian mummies. [DOI] [PubMed]
- 16.Barrett M.L., Scutt A.M., Evans F.J. Cannflavin A and B, prenylated flavones fromCannabis sativa L. Experientia. 1986;42:452–453. doi: 10.1007/BF02118655. [DOI] [PubMed] [Google Scholar]
- 17.Bautista J.L., Yu S., Tian L. Flavonoids in Cannabis sativa: biosynthesis, bioactivities, and biotechnology. ACS Omega. 2021;6:5119–5123. doi: 10.1021/acsomega.1c00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benamari O., Labhar A., Ahidar N., Salhi A., Ahari M., Elyoussfi A., Benyoub B., Amhamdi H. Investigation of the antioxidant and anti-inflammatory capacities of different extracts from Cistus ladanifer L. leaves in the ait ammart region (Northern Morocco) Ecol. Eng. Environ. Technol. 2024;25:178–184. doi: 10.12912/27197050/179421. [DOI] [Google Scholar]
- 19.Ben-Shabat S., Fride E., Sheskin T., Tamiri T., Rhee M.-H., Vogel Z., Bisogno T., De Petrocellis L., Di Marzo V., Mechoulam R. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998;353:23–31. doi: 10.1016/S0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- 20.Berman P., Futoran K., Lewitus G.M., Mukha D., Benami M., Shlomi T., Meiri D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-32651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasco-Benito S., Moreno E., Seijo-Vila M., Tundidor I., Andradas C., Caffarel M.M., Caro-Villalobos M., Urigüen L., Diez-Alarcia R., Moreno-Bueno G., Hernández L., Manso L., Homar-Ruano P., McCormick P.J., Bibic L., Bernadó-Morales C., Arribas J., Canals M., Casadó V., Canela E.I., Guzmán M., Pérez-Gómez E., Sánchez C. Therapeutic targeting of HER2–CB 2 R heteromers in HER2-positive breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2019;116:3863–3872. doi: 10.1073/pnas.1815034116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blasco-Benito S., Seijo-Vila M., Caro-Villalobos M., Tundidor I., Andradas C., García-Taboada E., Wade J., Smith S., Guzmán M., Pérez-Gómez E., Gordon M., Sánchez C. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018;157:285–293. doi: 10.1016/j.bcp.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Blázquez C., Carracedo A., Barrado L., José Real P., Luis Fernández-Luna J., Velasco G., Malumbres M., Guzmán M., Blázquez C., Carracedo A., Barrado L., José Real P., Luis Fernández-Luna J., Velasco G., Malumbres M., Guzmán M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20:2633–2635. doi: 10.1096/fj.06-6638fje. [DOI] [PubMed] [Google Scholar]
- 24.Blázquez C., Salazar M., Carracedo A., Lorente M., Egia A., González-Feria L., Haro A., Velasco G., Guzmán M. Cannabinoids Inhibit Glioma Cell Invasion by Down-regulating Matrix Metalloproteinase-2 expression. Cancer Res. 2008;68:1945–1952. doi: 10.1158/0008-5472.CAN-07-5176. [DOI] [PubMed] [Google Scholar]
- 25.Blebea N.M., Hancu G., Vlad R.A., Pricopie A. Applications of capillary electrophoresis for the determination of cannabinoids in different matrices. Molecules. 2023;28:638. doi: 10.3390/molecules28020638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonini S.A., Premoli M., Tambaro S., Kumar A., Maccarinelli G., Memo M., Mastinu A. Cannabis sativa: a comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018;227:300–315. doi: 10.1016/j.jep.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Borrelli F., Fasolino I., Romano B., Capasso R., Maiello F., Coppola D., Orlando P., Battista G., Pagano E., Di Marzo V., Izzo A.A. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013;85:1306–1316. doi: 10.1016/j.bcp.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Borrelli F., Pagano E., Romano B., Panzera S., Maiello F., Coppola D., De Petrocellis L., Buono L., Orlando P., Izzo A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis. 2014;35:2787–2797. doi: 10.1093/carcin/bgu205. [DOI] [PubMed] [Google Scholar]
- 29.Botineau, M., 2010. Botanique systématique et appliquée des plantes à fleurs. Éd. Tec & doc-Lavoisier, Paris.
- 30.Bowen J.K., Chaparro J.M., McCorkle A.M., Palumbo E., Prenni J.E. The impact of extraction protocol on the chemical profile of cannabis extracts from a single cultivar. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-01378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brierley D.I., Samuels J., Duncan M., Whalley B.J., Williams C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology. 2016;233:3603–3613. doi: 10.1007/s00213-016-4397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunetti P., Pichini S., Pacifici R., Busardò F.P., Del Rio A. Herbal preparations of medical cannabis: a vademecum for prescribing doctors. Medicina. 2020;56:237. doi: 10.3390/medicina56050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caffarel M.M., Sarrió D., Palacios J., Guzmán M., Sánchez C. Δ9-Tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res. 2006;66:6615–6621. doi: 10.1158/0008-5472.CAN-05-4566. [DOI] [PubMed] [Google Scholar]
- 34.Cai C., Yu W., Wang C., Liu L., Li F., Tan Z. Green extraction of cannabidiol from industrial hemp (Cannabis sativa L.) using deep eutectic solvents coupled with further enrichment and recovery by macroporous resin. J. Mol. Liq. 2019;287 doi: 10.1016/j.molliq.2019.110957. [DOI] [Google Scholar]
- 35.Calzolari D., Magagnini G., Lucini L., Grassi G., Appendino G.B., Amaducci S. High added-value compounds from Cannabis threshing residues. Ind. Crops Prod. 2017;108:558–563. doi: 10.1016/j.indcrop.2017.06.063. [DOI] [Google Scholar]
- 36.Carcieri C., Tomasello C., Simiele M., De Nicolò A., Avataneo V., Canzoneri L., Cusato J., Di Perri G., D’Avolio A. Cannabinoids concentration variability in cannabis olive oil galenic preparations. J. Pharm. Pharmacol. 2018;70:143–149. doi: 10.1111/jphp.12845. [DOI] [PubMed] [Google Scholar]
- 37.Cascio M.G., Zamberletti E., Marini P., Parolaro D., Pertwee R.G. The phytocannabinoid, Δ 9 -tetrahydrocannabivarin, can act through 5-HT 1 A receptors to produce antipsychotic effects: THCV, 5-HT 1 A and schizophrenia. Br. J. Pharm. 2015;172:1305–1318. doi: 10.1111/bph.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaachouay N., Douira A., Zidane L. Herbal medicine used in the treatment of human diseases in the Rif, Northern Morocco. Arab J. Sci. Eng. 2022;47:131–153. doi: 10.1007/s13369-021-05501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chagas M.H.N., Zuardi A.W., Tumas V., Pena-Pereira M.A., Sobreira E.T., Bergamaschi M.M., Dos Santos A.C., Teixeira A.L., Hallak J.E., Crippa J.A.S. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J. Psychopharmacol. 2014;28:1088–1098. doi: 10.1177/0269881114550355. [DOI] [PubMed] [Google Scholar]
- 40.2017, Suman Chandra, H. Lata, M.A. ElSohly (Eds.), Cannabis sativa L. – Botany and Biotechnology., Springer International Publishing, Cham10.1007/978-3-319-54564-6.
- 41.Chandra Suman, Lata H., ElSohly M.A., Walker L.A., Potter D. Cannabis cultivation: Methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017;70:302–312. doi: 10.1016/j.yebeh.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 42.Chang C.-W., Yen C.-C., Wu M.-T., Hsu M.-C., Wu T.-Y. Microwave-assisted extraction of cannabinoids in hemp nut using response surface methodology: optimization and comparative study. Molecules. 2017;22 doi: 10.3390/molecules22111894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christodoulou M.C., Christou A., Stavrou I.J., Kapnissi-Christodoulou C.P. Evaluation of different extraction procedures for the quantification of seven cannabinoids in cannabis-based edibles by the use of LC-MS. J. Food Compos. Anal. 2023;115 doi: 10.1016/j.jfca.2022.104915. [DOI] [Google Scholar]
- 44.Citti C., Braghiroli D., Vandelli M.A., Cannazza G. Pharmaceutical and biomedical analysis of cannabinoids: a critical review. J. Pharm. Biomed. Anal. 2018;147:565–579. doi: 10.1016/j.jpba.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Clark P.A., Capuzzi K., Fick C. Medical marijuana: medical necessity versus political. Med Sci. Monit. 2011 doi: 10.12659/MSM.882116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke R.C., Merlin M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. Crit. Rev. Plant Sci. 2016;35:293–327. doi: 10.1080/07352689.2016.1267498. [DOI] [Google Scholar]
- 47.A.R. De PinhoSocial and medical aspects of the use of cannabis in Brazil Chicago, Illinois , MOUTON PUBLISHERS• THE HAGUE , Cannabis and Culture , 1975, , 293–302, 10.1515/9783110812060.
- 48.Deiana S. Medical use of cannabis. Cannabidiol: a new light for schizophrenia?: Cannabidiol: a new light for schizophrenia. Drug Test. Anal. 2013;5:46–51. doi: 10.1002/dta.1425. [DOI] [PubMed] [Google Scholar]
- 49.Deidda R., Avohou H.T., Baronti R., Davolio P.L., Pasquini B., Del Bubba M., Hubert C., Hubert P., Orlandini S., Furlanetto S. Analytical quality by design: development and control strategy for a LC method to evaluate the cannabinoids content in cannabis olive oil extracts. J. Pharm. Biomed. Anal. 2019;166:326–335. doi: 10.1016/j.jpba.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Devinsky O., Cross J.H., Laux L., Marsh E., Miller I., Nabbout R., Scheffer I.E., Thiele E.A., Wright S. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N. Engl. J. Med. 2017;376:2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 51.Dos Santos N.A., Romão W. Cannabis – a state of the art about the millenary plant: Part I. Forensic Chem. 2023;32 doi: 10.1016/j.forc.2023.100470. [DOI] [Google Scholar]
- 52.DU TOIT, B.M., 1975. Dagga: the history and ethnographic setting of Cannabis sativa in southern Africa, in: Cannabis and Culture. Univ. of Florida Etats-Unis. United States.
- 53.Duvall C.S. A brief agricultural history of cannabis in Africa, from prehistory to canna-colony. echogeo. 2019 doi: 10.4000/echogeo.17599. [DOI] [Google Scholar]
- 54.Elbaz M., Nasser M.W., Ravi J., Wani N.A., Ahirwar D.K., Zhao H., Oghumu S., Satoskar A.R., Shilo K., Carson W.E., Ganju R.K. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015;9:906–919. doi: 10.1016/j.molonc.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Sohaimy S.A., Androsova N.V., Toshev A.D., El Enshasy H.A. Nutritional Quality, Chemical, and Functional Characteristics of Hemp (Cannabis sativa ssp. sativa) Protein Isolate. Plants. 2022;11 doi: 10.3390/plants11212825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.2007, M.A. ElSohly (Ed.), Marijuana and the cannabinoids, Forensic science and medicine, Humana Press, Totowa, N.J.
- 57.Erridge S., Mangal N., Salazar O., Pacchetti B., Sodergren M.H. Cannflavins – From plant to patient: a scoping review. Fitoterapia. 2020;146 doi: 10.1016/j.fitote.2020.104712. [DOI] [PubMed] [Google Scholar]
- 58.Fallahi S., Bobak L., Opalinski S. Hemp in animal diets-cannabidiol. Animals. 2022;12:2541. doi: 10.3390/ani12192541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farag, S., n.d. Chapter 1 – The Cannabis Plant: Botanical Aspects.
- 60.Fernández N., Lanosa D.A., Janezic N.S., Quiroga P.N. Development and validation of a GC-MS method for quantification of terpenes in cannabis oil (Cannabis sativa L.). Application to commercially available preparations in Argentina. J. Appl. Res. Med. Aromat. Plants. 2023;35 doi: 10.1016/j.jarmap.2023.100466. [DOI] [Google Scholar]
- 61.Fernández S., Carreras T., Castro R., Perelmuter K., Giorgi V., Vila A., Rosales A., Pazos M., Moyna G., Carrera Ignacio, Bollati-Fogolín M., García-Carnelli C., Carrera Inés, Vieitez I. A comparative study of supercritical fluid and ethanol extracts of cannabis inflorescences: chemical profile and biological activity. J. Supercrit. Fluids. 2022;179 doi: 10.1016/j.supflu.2021.105385. [DOI] [Google Scholar]
- 62.Finley S.J., Javan G.T., Green R.L. Bridging disciplines: applications of forensic science and industrial hemp. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.760374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fisher T., Golan H., Schiby G., PriChen S., Smoum R., Moshe I., Peshes-Yaloz N., Castiel A., Waldman D., Gallily R., Mechoulam R., Toren A. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. 2016;23:15–22. doi: 10.3747/co.23.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flores-Sanchez I.J., Verpoorte R. Secondary metabolism in cannabis. Phytochem Rev. 2008;7:615–639. doi: 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- 65.Flores-Sanchez I.J., Verpoorte R. PKS activities and biosynthesis of cannabinoids and flavonoids in cannabis sativa L. plants. Plant Cell Physiol. 2008;49:1767–1782. doi: 10.1093/pcp/pcn150. [DOI] [PubMed] [Google Scholar]
- 66.Fraguas-Sánchez A.I., Torres-Suárez A.I. Medical use of cannabinoids. Drugs. 2018;78:1665–1703. doi: 10.1007/s40265-018-0996-1. [DOI] [PubMed] [Google Scholar]
- 67.Friedman D., Sirven J.I. Historical perspective on the medical use of cannabis for epilepsy: ancient times to the 1980s. Epilepsy Behav. 2017;70:298–301. doi: 10.1016/j.yebeh.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 68.Gallo-Molina A.C., Castro-Vargas H.I., Garzón-Méndez W.F., Martínez Ramírez J.A., Rivera Monroy Z.J., King J.W., Parada-Alfonso F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids. 2019;146:208–216. doi: 10.1016/j.supflu.2019.01.020. [DOI] [Google Scholar]
- 69.Gambino A., Cabras M., Panagiotakos E., Calvo F., Macciotta A., Cafaro A., Suria M., El Haddad G., Broccoletti R., Arduino P.G. Evaluating the suitability and potential efficiency of Cannabis sativa oil for patients with primary burning mouth syndrome: A prospective, open-label, single-arm pilot study. Pain. Med. 2021;22:142–151. doi: 10.1093/pm/pnaa318. [DOI] [PubMed] [Google Scholar]
- 70.Gaoni Y., Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 71.Giordano C., Maleci L., Agati G., Petruccelli R. Ficus carica L. leaf anatomy: trichomes and solid inclusions. Ann. Appl. Biol. 2020;176:47–54. doi: 10.1111/aab.12557. [DOI] [Google Scholar]
- 72.Glodde N., Jakobs M., Bald T., Tüting T., Gaffal E. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35–40. doi: 10.1016/j.lfs.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 73.Gonçalves E.C.D., Baldasso G.M., Bicca M.A., Paes R.S., Capasso R., Dutra R.C. Terpenoids, cannabimimetic ligands, beyond the cannabis plant. Molecules. 2020;25:1567. doi: 10.3390/molecules25071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grof C.P.L. Cannabis, from plant to pill. Br. J. Clin. Pharmacol. 2018;84:2463–2467. doi: 10.1111/bcp.13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guzmán M. Cannabinoids: potential anticancer agents. Nat. Rev. Cancer. 2003;3:745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 76.Guzmán M., Duarte M.J., Blázquez C., Ravina J., Rosa M.C., Galve-Roperh I., Sánchez C., Velasco G., González-Feria L. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer. 2006;95:197–203. doi: 10.1038/sj.bjc.6603236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hacke A.C.M., Lima D., De Costa F., Deshmukh K., Li N., Chow A.M., Marques J.A., Pereira R.P., Kerman K. Probing the antioxidant activity of Δ 9 -tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst. 2019;144:4952–4961. doi: 10.1039/C9AN00890J. [DOI] [PubMed] [Google Scholar]
- 78.Haddou S., Hassania Loukili E., Hbika A., Chahine A., Hammouti B. Phytochemical study using HPLC-UV/GC–MS of different of Cannabis sativa L seeds extracts from Morocco. Mater. Today.: Proc. 2023;72:3896–3903. doi: 10.1016/j.matpr.2022.10.215. [DOI] [Google Scholar]
- 79.Hanuš L.O., Meyer S.M., Muñoz E., Taglialatela-Scafati O., Appendino G. Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 2016;33:1357–1392. doi: 10.1039/C6NP00074F. [DOI] [PubMed] [Google Scholar]
- 80.Happyana N., Agnolet S., Muntendam R., Van Dam A., Schneider B., Kayser O. Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry. 2013;87:51–59. doi: 10.1016/j.phytochem.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 81.How Z.T., Gamal El-Din M. A critical review on the detection, occurrence, fate, toxicity, and removal of cannabinoids in the water system and the environment. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.International Conference on Harmonisation (ICH)., n.d. All Guidelines. URL 〈https://www.ich.org/page/ich-guidelines〉. [DOI] [PubMed]
- 83.Isidore E., Karim H., Ioannou I. Extraction of phenolic compounds and terpenes from Cannabis sativa L. by-products: from conventional to intensified processes. Antioxidants. 2021;10:942. doi: 10.3390/antiox10060942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Izzo L., Castaldo L., Narváez A., Graziani G., Gaspari A., Rodríguez-Carrasco Y., Ritieni A. Analysis of phenolic compounds in commercial Cannabis sativa L. inflorescences using UHPLC-Q-orbitrap HRMS. Molecules. 2020;25:631. doi: 10.3390/molecules25030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jadoon K.A., Ratcliffe S.H., Barrett D.A., Thomas E.L., Stott C., Bell J.D., O’Sullivan S.E., Tan G.D. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39:1777–1786. doi: 10.2337/dc16-0650. [DOI] [PubMed] [Google Scholar]
- 87.Jia W., Hegde V.L., Singh N.P., Sisco D., Grant S., Nagarkatti M., Nagarkatti P.S. Δ9-Tetrahydrocannabinol-Induced Apoptosis in Jurkat Leukemia T Cells Is Regulated by Translocation of Bad to Mitochondria. Mol. Cancer Res. 2006;4:549–562. doi: 10.1158/1541-7786.MCR-05-0193. [DOI] [PubMed] [Google Scholar]
- 88.Johnson J.R., Lossignol D., Burnell-Nugent M., Fallon M.T. An Open-Label Extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J. Pain. Symptom Manag. 2013;46:207–218. doi: 10.1016/j.jpainsymman.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 89.Johnson, R., 2018. Hemp as an Agricultural Commodity. Congressional Research Service, CRS Report.
- 90.Kampa-Schittenhelm K.M., Salitzky O., Akmut F., Illing B., Kanz L., Salih H.R., Schittenhelm M.M. Dronabinol has preferential antileukemic activity in acute lymphoblastic and myeloid leukemia with lymphoid differentiation patterns. BMC Cancer. 2016;16:25. doi: 10.1186/s12885-015-2029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa Cham , Springer International Publishing , Progress in the Chemistry of Organic Natural Products (Eds.), A.D. Kinghorn, H. Falk, S. Gibbons, J. Kobayashi , in: 2017, A.D. KinghornH. FalkS. Gibbons, J. Kobayashiin: (Eds.), , , 10.1007/978-3-319-45541-9.
- 92.Koltai H., Namdar D. Cannabis phytomolecule “entourage”: from domestication to medical use. Trends Plant Sci. 2020;25:976–984. doi: 10.1016/j.tplants.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Koturbash I., MacKay D. Cannabidiol and other cannabinoids: from toxicology and pharmacology to the development of a regulatory pathway. J. Diet. Suppl. 2020;17:487–492. doi: 10.1080/19390211.2020.1796886. [DOI] [PubMed] [Google Scholar]
- 94.Krill C., Rochfort S., Spangenberg G. A high-throughput method for the comprehensive analysis of terpenes and terpenoids in medicinal cannabis biomass. Metabolites. 2020;10:1–14. doi: 10.3390/metabo10070276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Labhar A., Benamari O., El-Mernissi Y., Salhi A., Ahari M., El barkany S., Amhamdi H. Phytochemical, anti-inflammatory and antioxidant activities of Pistacia lentiscus L. Leaves from Ajdir, Al Hoceima Province, Morocco. Ecol. Eng. Environ. Technol. 2023;24:172–177. doi: 10.12912/27197050/169935. [DOI] [Google Scholar]
- 96.Laprairie R.B., Bagher A.M., Kelly M.E.M., Denovan-Wright E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor: negative allosteric modulation of CB 1 by cannabidiol. Br. J. Pharmacol. 2015;172:4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laux L.C., Bebin E.M., Checketts D., Chez M., Flamini R., Marsh E.D., Miller I., Nichol K., Park Y., Segal E., Seltzer L., Szaflarski J.P., Thiele E.A., Weinstock A. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res. 2019;154:13–20. doi: 10.1016/j.eplepsyres.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 98.Leelawat S., Leelawat K., Narong S., Matangkasombut O. The Dual Effects of Δ 9 -Tetrahydrocannabinol on Cholangiocarcinoma Cells: anti-Invasion Activity at Low Concentration and Apoptosis Induction at High Concentration. Cancer Invest. 2010;28:357–363. doi: 10.3109/07357900903405934. [DOI] [PubMed] [Google Scholar]
- 99.Lewis M.M., Yang Y., Wasilewski E., Clarke H.A., Kotra L.P. Chemical PRofiling of Medical Cannabis Extracts. ACS Omega. 2017;2:6091–6103. doi: 10.1021/acsomega.7b00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li H.-L. An archaeological and historical account of Cannabis in China. Econ. Bot. 1974;28:437–448. [Google Scholar]
- 101.Ligresti A., Moriello A.S., Starowicz K., Matias I., Pisanti S., De Petrocellis L., Laezza C., Portella G., Bifulco M., Di Marzo V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharm. Exp. Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 102.Liu W.M., Scott K.A., Shamash J., Joel S., Powles T.B. Enhancing the in vitro cytotoxic activity of Δ 9 -tetrahydrocannabinol in leukemic cells through a combinatorial approach. Leuk. Lymphoma. 2008;49:1800–1809. doi: 10.1080/10428190802239188. [DOI] [PubMed] [Google Scholar]
- 103.Liu Y., Liu H.-Y., Li S.-H., Ma W., Wu D.-T., Li H.-B., Xiao A.-P., Liu L.-L., Zhu F., Gan R.-Y. Cannabis sativa bioactive compounds and their extraction, separation, purification, and identification technologies: an updated review. TrAC Trends Anal. Chem. 2022;149 doi: 10.1016/j.trac.2022.116554. [DOI] [Google Scholar]
- 104.Luca S.V., Braumann L., Gerigk M., Frank O., Minceva M. Separation of minor cannabinoids from hemp extract with trapping multiple dual mode liquid-liquid chromatography. J. Chromatogr. A. 2021;1658 doi: 10.1016/j.chroma.2021.462608. [DOI] [PubMed] [Google Scholar]
- 105.Maa E., Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55:783–786. doi: 10.1111/epi.12610. [DOI] [PubMed] [Google Scholar]
- 106.Mahlberg P.G., Kim E.S. Accumulation of cannabinoids in glandular trichomes of Cannabis (Cannabaceae) J. Ind. Hemp. 2004;9:15–36. doi: 10.1300/J237v09n01_04. [DOI] [Google Scholar]
- 107.Marcu J.P., Christian R.T., Lau D., Zielinski A.J., Horowitz M.P., Lee J., Pakdel A., Allison J., Limbad C., Moore D.H., Yount G.L., Desprez P.-Y., McAllister S.D. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and Survival. Mol. Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mariotti K.D.C., Marcelo M.C.A., Ortiz R.S., Borille B.T., Dos Reis M., Fett M.S., Ferrão M.F., Limberger R.P. Seized cannabis seeds cultivated in greenhouse: a chemical study by gas chromatography–mass spectrometry and chemometric analysis. Sci. Justice. 2016;56:35–41. doi: 10.1016/j.scijus.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Martinenghi L.D., Jønsson R., Lund T., Jenssen H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules. 2020;10:900. doi: 10.3390/biom10060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martínez-Pinilla E., Varani K., Reyes-Resina I., Angelats E., Vincenzi F., Ferreiro-Vera C., Oyarzabal J., Canela E.I., Lanciego J.L., Nadal X., Navarro G., Borea P.A., Franco R. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front. Pharmacol. 2017;8:744. doi: 10.3389/fphar.2017.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marzo V.D., Bifulco M., Petrocellis L.D. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 112.Marzorati S., Friscione D., Picchi E., Verotta L. Cannabidiol from inflorescences of Cannabis sativa L.: Green extraction and purification processes. Ind. Crops Prod. 2020;155 doi: 10.1016/j.indcrop.2020.112816. [DOI] [Google Scholar]
- 113.Massi P., Vaccani A., Ceruti S., Colombo A., Abbracchio M.P., Parolaro D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J. Pharm. Exp. Ther. 2004;308:838–845. doi: 10.1124/jpet.103.061002. [DOI] [PubMed] [Google Scholar]
- 114.Mastellone G., Marengo A., Sgorbini B., Scaglia F., Capetti F., Gai F., Peiretti P.G., Rubiolo P., Cagliero C. Characterization and biological activity of fiber-type Cannabis sativa L. aerial parts at different growth stages. Plants. 2022;11 doi: 10.3390/plants11030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maurya N., Velmurugan B.K. Therapeutic applications of cannabinoids. Chem. -Biol. Interact. 2018;293:77–88. doi: 10.1016/j.cbi.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 116.McAllister S.D., Christian R.T., Horowitz M.P., Garcia A., Desprez P.-Y. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 2007;6:2921–2927. doi: 10.1158/1535-7163.MCT-07-0371. [DOI] [PubMed] [Google Scholar]
- 117.McGuire P., Robson P., Cubala W.J., Vasile D., Morrison P.D., Barron R., Taylor A., Wright S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. AJP. 2018;175:225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 118.McKallip R.J., Nagarkatti M., Nagarkatti P.S. Δ-9-Tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. 2005;174:3281–3289. doi: 10.4049/jimmunol.174.6.3281. [DOI] [PubMed] [Google Scholar]
- 119.Mechoulam R. Academic Press; 1973. Marijuana: Chemistry, Pharmacology, Metabolism and Clinical Effects. [Google Scholar]
- 120.Mechoulam R., Ben-Shabat S. From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: the ongoing story of cannabis. Nat. Prod. Rep. 1999;16:131–143. doi: 10.1039/a703973e. [DOI] [PubMed] [Google Scholar]
- 121.Mikuriya T.H. Marijuana in medicine: past, present and future. Calif. Med. 1969;110:34–40. [PMC free article] [PubMed] [Google Scholar]
- 122.Milian L., Mata M., Alcacer J., Oliver M., Sancho-Tello M., Martín De Llano J.J., Camps C., Galbis J., Carretero J., Carda C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0228909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Montero L., Ballesteros-Vivas D., Gonzalez-Barrios A.F., Sánchez-Camargo A.D.P. Hemp seeds: nutritional value, associated bioactivities and the potential food applications in the Colombian context. Front. Nutr. 2023;9 doi: 10.3389/fnut.2022.1039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Monton C., Chankana N., Leelawat S., Suksaeree J., Songsak T. Optimization of supercritical carbon dioxide fluid extraction of seized cannabis and self-emulsifying drug delivery system for enhancing the dissolution of cannabis extract. J. Supercrit. Fluids. 2022;179 doi: 10.1016/j.supflu.2021.105423. [DOI] [Google Scholar]
- 125.Monton C., Madaka F., Settharaksa S., Wunnakup T., Suksaeree J., Songsak T. Optimal condition of cannabis maceration to obtain the high cannabidiol and Δ9-tetrahydrocannabinol content. An. Acad. Bras. Cienc. 2019;91 doi: 10.1590/0001-3765201920190676. [DOI] [PubMed] [Google Scholar]
- 126.Moreau de Tours, J.-J., 1845. Du Hachisch et de l’Alienation Mentale: Etudes Psychologiques. Paris.
- 127.Moreau M., Ibeh U., Decosmo K., Bih N., Yasmin-Karim S., Toyang N., Lowe H., Ngwa W. Flavonoid derivative of cannabis demonstrates therapeutic potential in preclinical models of metastatic pancreatic cancer. Front. Oncol. 2019;9:660. doi: 10.3389/fonc.2019.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morelli M.B., Offidani M., Alesiani F., Discepoli G., Liberati S., Olivieri A., Santoni M., Santoni G., Leoni P., Nabissi M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2: Cannabidiol Induces Cytotoxicity in Myeloma Cells. Int. J. Cancer. 2014;134:2534–2546. doi: 10.1002/ijc.28591. [DOI] [PubMed] [Google Scholar]
- 129.Moreno E., Cavic M., Krivokuca A., Casadó V., Canela E. The endocannabinoid system as a target in cancer diseases: are we there yet? Front. Pharmacol. 2019;10:339. doi: 10.3389/fphar.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moreno T., Montanes F., Tallon S.J., Fenton T., King J.W. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: an overview of different processing options. J. Supercrit. Fluids. 2020;161 doi: 10.1016/j.supflu.2020.104850. [DOI] [Google Scholar]
- 131.Moreno-Sanz G., Vera C.F., Sánchez-Carnerero C., Roura X.N., de Medina Baena V.S. Biological Activity of Cannabis sativa L. extracts critically depends on solvent polarity and decarboxylation. Separations. 2020;7:1–16. doi: 10.3390/separations7040056. [DOI] [Google Scholar]
- 132.Nachnani R., Raup-Konsavage W.M., Vrana K.E. The pharmacological case for cannabigerol. J. Pharm. Exp. Ther. 2021;376:204–212. doi: 10.1124/jpet.120.000340. [DOI] [PubMed] [Google Scholar]
- 133.Nafis A., Kasrati A., Jamali C.A., Mezrioui N., Setzer W., Abbad A., Hassani L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019;137:396–400. doi: 10.1016/j.indcrop.2019.05.032. [DOI] [Google Scholar]
- 134.Nallathambi R., Mazuz M., Ion A., Selvaraj G., Weininger S., Fridlender M., Nasser A., Sagee O., Kumari P., Nemichenizer D., Mendelovitz M., Firstein N., Hanin O., Konikoff F., Kapulnik Y., Naftali T., Koltai H. Anti-Inflammatory activity in colon models is derived from Δ9-tetrahydrocannabinolic acid that interacts with additional compounds in Cannabis extracts. Cannabis Cannabinoid Res. 2017;2:167–182. doi: 10.1089/can.2017.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Namdar D., Anis O., Poulin P., Koltai H. Chronological review and rational and future prospects of cannabis-based drug development. Molecules. 2020;25:4821. doi: 10.3390/molecules25204821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Namdar D., Voet H., Ajjampura V., Nadarajan S., Mayzlish-Gati E., Mazuz M., Shalev N., Koltai H. Terpenoids and phytocannabinoids co-produced in cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules. 2019;24:3031. doi: 10.3390/molecules24173031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nasser M., Tibi A., Savage-Smith E. Ibn Sina’s Canon of Medicine: 11th century rules for assessing the effects of drugs. J. R. Soc. Med. 2009;102:78–80. doi: 10.1258/jrsm.2008.08k040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nsuala B.N., Enslin G., Viljoen A. Wild cannabis”: a review of the traditional use and phytochemistry of Leonotis leonurus. J. Ethnopharmacol. 2015;174:520–539. doi: 10.1016/j.jep.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 139.Odieka A.E., Obuzor G.U., Oyedeji O.O., Gondwe M., Hosu Y.S., Oyedeji A.O. The medicinal natural products of Cannabis sativa Linn.: a review. Molecules. 2022;27 doi: 10.3390/molecules27051689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ostapczuk K., Apori S.O., Estrada G., Tian F. Hemp growth factors and extraction methods effect on antimicrobial activity of hemp seed oil: a systematic review. Separations. 2021;8:183. doi: 10.3390/separations8100183. [DOI] [Google Scholar]
- 141.Pacher P., Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system. Prog. Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Palmieri S., Pellegrini M., Ricci A., Compagnone D., Lo Sterzo C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: a comparison study of maceration, soxhlet, UAE and RSLDE techniques. Foods. 2020;9 doi: 10.3390/foods9091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pamplona F.A., Da Silva L.R., Coan A.C. Potential clinical benefits of cbd-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: observational data meta-analysis. Front. Neurol. 2018;9:759. doi: 10.3389/fneur.2018.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pattnaik F., Nanda S., Mohanty S., Dalai A.K., Kumar V., Ponnusamy S.K., Naik S. Cannabis: chemistry, extraction and therapeutic applications. Chemosphere. 2022;289 doi: 10.1016/j.chemosphere.2021.133012. [DOI] [PubMed] [Google Scholar]
- 145.Pegoraro C.N., Nutter D., Thevenon M., Ramirez C.L. Chemical profiles of Cannabis sativa medicinal oil using different extraction and concentration methods. Nat. Prod. Res. 2021;35:2249–2252. doi: 10.1080/14786419.2019.1663515. [DOI] [PubMed] [Google Scholar]
- 146.Pisanti S., Bifulco M. Medical Cannabis: A plurimillennial history of an evergreen. J. Cell. Physiol. 2019;234:8342–8351. doi: 10.1002/jcp.27725. [DOI] [PubMed] [Google Scholar]
- 147.Pollastro F., Minassi A., Fresu L.G. Cannabis phenolics and their bioactivities. CMC. 2018;25:1160–1185. doi: 10.2174/0929867324666170810164636. [DOI] [PubMed] [Google Scholar]
- 148.Preet, A., Ganju, R., Groopman, J., n.d. D9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. [DOI] [PubMed]
- 149.Protti M., Brighenti V., Battaglia M.R., Anceschi L., Pellati F., Mercolini L. Cannabinoids from Cannabis sativa L.: a new tool based on HPLC-DAD-MS/MS for a rational use in medicinal chemistry. ACS Med. Chem. Lett. 2019;10:539–544. doi: 10.1021/acsmedchemlett.8b00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Radwan M.M., Chandra S., Gul S., ElSohly M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules. 2021;26:2774. doi: 10.3390/molecules26092774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ramer R., Heinemann K., Merkord J., Rohde H., Salamon A., Linnebacher M., Hinz B. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther. 2013;12:69–82. doi: 10.1158/1535-7163.MCT-12-0335. [DOI] [PubMed] [Google Scholar]
- 152.Ramer R., Merkord J., Rohde H., Hinz B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010;79:955–966. doi: 10.1016/j.bcp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 153.Ren M., Tang Z., Wu X., Spengler R., Jiang H., Yang Y., Boivin N. The origins of cannabis smoking: chemical residue evidence from the first millennium BCE in the Pamirs. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aaw1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rong C., Lee Y., Carmona N.E., Cha D.S., Ragguett R.-M., Rosenblat J.D., Mansur R.B., Ho R.C., McIntyre R.S. Cannabidiol in medical marijuana: research vistas and potential opportunities. Pharmacol. Res. 2017;121:213–218. doi: 10.1016/j.phrs.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 155.Rožanc J., Kotnik P., Milojević M., Gradišnik L., Hrnčič M.K., Knez Ž., Maver U. Different Cannabis sativa extraction methods result in different biological activities against a colon cancer cell line and healthy colon cells. Plants. 2021;10:1–16. doi: 10.3390/plants10030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rupasinghe H.P.V., Davis A., Kumar S.K., Murray B., Zheljazkov V.D. Industrial Hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules. 2020;25:4078. doi: 10.3390/molecules25184078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Russo E.B., Jiang H.-E., Li X., Sutton A., Carboni A., Del Bianco F., Mandolino G., Potter D.J., Zhao Y.-X., Bera S., Zhang Y.-B., Lü E.-G., Ferguson D.K., Hueber F., Zhao L.-C., Liu C.-J., Wang Y.-F., Li C.-S. Phytochemical and genetic analyses of ancient cannabis from Central Asia. J. Exp. Bot. 2008;59:4171–4182. doi: 10.1093/jxb/ern260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.E.B. Russo, J. MarcuCannabis Pharmacology: The Usual Suspects and a Few Promising Leads Elsevier , Advances in Pharmacology , 2017, , 67–134, 10.1016/bs.apha.2017.03.004. [DOI] [PubMed]
- 159.Russo E.B., Merzouki A., Mesa J.M., Frey K.A., Bach P.J. Cannabis improves night vision: a case study of dark adaptometry and scotopic sensitivity in kif smokers of the Rif mountains of northern Morocco. J. Ethnopharmacol. 2004;93:99–104. doi: 10.1016/j.jep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 160.Sagy I., Bar-Lev Schleider L., Abu-Shakra M., Novack V. Safety and efficacy of medical cannabis in fibromyalgia. JCM. 2019;8:807. doi: 10.3390/jcm8060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sainz Martinez A., Lanaridi O., Stagel K., Halbwirth H., Schnürch M., Bica-Schröder K. Extraction techniques for bioactive compounds of cannabis. Nat. Prod. Rep. 2023;40:676–717. doi: 10.1039/D2NP00059H. [DOI] [PubMed] [Google Scholar]
- 162.Salazar M., Carracedo A., Salanueva Í.J., Hernández-Tiedra S., Egia A., Lorente M., Vázquez P., Torres S., Iovanna J.L., Guzmán M., Boya P., Velasco G. TRB3 links ER stress to autophagy in cannabinoid antitumoral action. Autophagy. 2009;5:1048–1049. doi: 10.4161/auto.5.7.9508. [DOI] [PubMed] [Google Scholar]
- 163.Salazar M., Carracedo A., Salanueva Í.J., Hernández-Tiedra S., Lorente M., Egia A., Vázquez P., Blázquez C., Torres S., García S., Nowak J., Fimia G.M., Piacentini M., Cecconi F., Pandolfi P.P., González-Feria L., Iovanna J.L., Guzmán M., Boya P., Velasco G. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Salem M.M., Capers J., Rito S., Werbovetz K.A. Antiparasitic activity of C -Geranyl Flavonoids from Mimulus bigelovii: C -GERANYL FLAVONOIDS FROM Mimulus bigelovii. Phytother. Res. 2011;25:1246–1249. doi: 10.1002/ptr.3404. [DOI] [PubMed] [Google Scholar]
- 165.Schley M., Legler A., Skopp G., Schmelz M., Konrad C., Rukwied R. Delta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr. Med. Res. Opin. 2006;22:1269–1276. doi: 10.1185/030079906X112651. [DOI] [PubMed] [Google Scholar]
- 166.Scott K.A., Dalgleish A.G., Liu W.M. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int. J. Oncol. 2017;51:369–377. doi: 10.3892/ijo.2017.4022. [DOI] [PubMed] [Google Scholar]
- 167.Seamon M.J., Fass J.A., Maniscalco-Feichtl M., Abu-Shraie N.A. Medical marijuana and the developing role of the pharmacist. Am. J. Health-Syst. Pharm. 2007;64:1037–1044. doi: 10.2146/ajhp060471. [DOI] [PubMed] [Google Scholar]
- 168.Shevyrin V.A., Morzherin Yu.Yu. Cannabinoids: structures, effects, and classification. Russ. Chem. Bull. 2015;64:1249–1266. doi: 10.1007/s11172-015-1008-1. [DOI] [Google Scholar]
- 169.Shrivastava A., Kuzontkoski P.M., Groopman J.E., Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011;10:1161–1172. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- 170.Shujat S., Robinson G.I., Norouzkhani F., Kovalchuk I. Using advanced biotechnological techniques to improve cannabis cultivars. Biocatal. Agric. Biotechnol. 2024 doi: 10.1016/j.bcab.2024.103250. [DOI] [Google Scholar]
- 171.Simmerman E., Qin X., Yu J.C., Baban B. Cannabinoids as a potential new and novel treatment for melanoma: a pilot study in a murine model. J. Surg. Res. 2019;235:210–215. doi: 10.1016/j.jss.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 172.Small E., Cronquist A. A practical and natural taxonomy for cannabis. TAXON. 1976;25:405–435. doi: 10.2307/1220524. [DOI] [Google Scholar]
- 173.Sommano S.R., Chittasupho C., Ruksiriwanich W., Jantrawut P. The Cannabis Terpenes. Molecules. 2020;25:5792. doi: 10.3390/molecules25245792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stockings E., Zagic D., Campbell G., Weier M., Hall W.D., Nielsen S., Herkes G.K., Farrell M., Degenhardt L. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J. Neurol. Neurosurg. Psychiatry. 2018;89:741–753. doi: 10.1136/jnnp-2017-317168. [DOI] [PubMed] [Google Scholar]
- 175.Subratti A., Lalgee L.J., Jalsa N.K. Liquified dimethyl ether (DME): a green solvent for the extraction of hemp (Cannabis sativa L.) seed oil. Sustain. Chem. Pharm. 2019;12 doi: 10.1016/j.scp.2019.100144. [DOI] [Google Scholar]
- 176.Szaflarski J.P., Bebin E.M., Cutter G., DeWolfe J., Dure L.S., Gaston T.E., Kankirawatana P., Liu Y., Singh R., Standaert D.G., Thomas A.E., Ver Hoef L.W. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018;87:131–136. doi: 10.1016/j.yebeh.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 177.Takeda S., Yoshida K., Nishimura H., Harada M., Okajima S., Miyoshi H., Okamoto Y., Amamoto T., Watanabe K., Omiecinski C.J., Aramaki H. Δ 9 -Tetrahydrocannabinol disrupts estrogen-signaling through up-regulation of estrogen receptor β (ERβ) Chem. Res. Toxicol. 2013;26:1073–1079. doi: 10.1021/tx4000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tham M., Yilmaz O., Alaverdashvili M., Kelly M.E.M., Denovan-Wright E.M., Laprairie R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors: CBD and CBD-DMH at the cannabinoid receptors. Br. J. Pharmacol. 2019;176:1455–1469. doi: 10.1111/bph.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tomida I. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004;88:708–713. doi: 10.1136/bjo.2003.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tomko A.M., Whynot E.G., Ellis L.D., Dupré D.J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers. 2020;12:1985. doi: 10.3390/cancers12071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Touw M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs. 1981;13:23–34. doi: 10.1080/02791072.1981.10471447. [DOI] [PubMed] [Google Scholar]
- 182.Tzimas, P.S., n.d. Effective determination of the principal non-psychoactive cannabinoids in fiber-type Cannabis sativa L. by UPLC-PDA following a comprehensive design and optimization of extraction methodology. Analytica Chimica Acta. [DOI] [PubMed]
- 183.Ueda, N., n.d. Chapter 8 – Metabolic Enzymes for Endocannabinoids and Endocannabinoid-Like Mediators.
- 184.United Nations Office on Drugs and Crime, 2022. World Drug Report 2022 (Research and Trend Analysis Branch). United Nations publication.
- 185.US Food and Drug Administration, Case Med. FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy. URL 〈https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms〉.
- 186.Valizadehderakhshan M., Shahbazi A., Kazem-Rostami M., Todd M.S., Bhowmik A., Wang L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture. 2021;11:384. doi: 10.3390/agriculture11050384. [DOI] [Google Scholar]
- 187.Vara D., Salazar M., Olea-Herrero N., Guzmán M., Velasco G., Díaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18:1099–1111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Welling M.T., Deseo M.A., Bacic A., Doblin M.S. Biosynthetic origins of unusual cannabimimetic phytocannabinoids in Cannabis sativa L: a review. Phytochemistry. 2022;201 doi: 10.1016/j.phytochem.2022.113282. [DOI] [PubMed] [Google Scholar]
- 189.Whiting P.F., Wolff R.F., Deshpande S., Di Nisio M., Duffy S., Hernandez A.V., Keurentjes J.C., Lang S., Misso K., Ryder S., Schmidlkofer S., Westwood M., Kleijnen J. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 190.Wright R. EverBlu Capital Reaserch; 2017. Cannabis Industry Report. [Google Scholar]
- 191.Wulf, N., Marienstras, E., 2005. Révoltes et révolutions en Amérique, Clefs Concours. Atlande.
- 192.Xu D.H., Cullen B.D., Tang M., Fang Y. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. CPB. 2020;21:390–402. doi: 10.2174/1389201020666191202111534. [DOI] [PubMed] [Google Scholar]
- 193.Žampachová L., Aturki Z., Mariani F., Bednář P. A rapid nano-liquid chromatographic method for the analysis of cannabinoids in cannabis sativa l. extracts. Molecules. 2021;26:1825. doi: 10.3390/molecules26071825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zheljazkov V.D., Maggi F. Valorization of CBD-hemp through distillation to provide essential oil and improved cannabinoids profile. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-99335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu L.X., Sharma S., Stolina M., Gardner B., Roth M.D., Tashkin D.P., Dubinett S.M. Δ-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J. Immunol. 2000;165:373–380. doi: 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]
- 196.Zimniewska M., Rozańska W., Gryszczynska A., Romanowska B., Kicinska-Jakubowska A. Antioxidant potential of hemp and flax fibers depending on their chemical composition. Molecules. 2018;23 doi: 10.3390/molecules23081993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zivovinovic S., Alder R., Allenspach M.D., Steuer C. Determination of cannabinoids in Cannabis sativa L. samples for recreational, medical, and forensic purposes by reversed-phase liquid chromatography-ultraviolet detection. J. Anal. Sci. Technol. 2018;9 doi: 10.1186/s40543-018-0159-8. [DOI] [Google Scholar]
- 198.Zuardi A.W. History of cannabis as a medicine: a review História da cannabis como medicamento: uma revisão. Rev. Bras. Psiquiatr. 2005 [Google Scholar]
- 199.Zulfiqar F., Ross S.A., Slade D., Ahmed S.A., Radwan M.M., Ali Z., Khan I.A., ElSohly M.A. Cannabisol, a novel Δ9-THC dimer possessing a unique methylene bridge, isolated from Cannabis sativa. Tetrahedron Lett. 2012;53:3560–3562. doi: 10.1016/j.tetlet.2012.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.