Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Sep 10;23:15347354241267979. doi: 10.1177/15347354241267979
Abstract
Multiple myeloma is a hematological cancer caused by the uncontrolled proliferation of abnormal plasma cells in the bone marrow, leading to excessive immunoglobulin production. Our study aimed to examine the anticancer properties of BRF1A, a cannabinoid (CBD)-enriched product, on 2 myeloma cell lines: U266 and ARH-7. We treated U266 and ARH-77 myeloma cells with varying doses of BRF1A and measured the production of IgE and IgG antibodies using ELISA. Cell viability was assessed using trypan blue and CCK-8 assays. We measured the expression of genes related to the production of IgE and IgG antibodies, IgEH, and IgGH. We determined its effect on the expression of telomerase and its phosphorylated form as an indicator of telomere stabilization. Furthermore, we determined its effect on other cancer-related targets such as NF-ĸB, c-Myc, and TP53 in U266 cells using reverse transcription polymerase chain reaction (RT-PCR) and western blotting. BRF1A reduced myeloma cell IgE and IgG production in a time and dose-dependent manner. It also suppressed the expression of p-IκBα, p-NFκB (p65), and total NFκB protein, as well as XBP1u and XBP1s. It increased the gene and protein expression of telomere and hTERT and significantly increased cancer suppressor TP53 gene and p53 protein expression. Additionally, BRF1A decreased the c-Myc gene and protein expression. Our study has shown that a CBD-enriched product can reduce the growth of myeloma cells by suppressing the critical functions of IgE- and IgG-producing cells. This study could help bridge the gap in understanding how cannabinoid-containing products affect cancer, aging, telomere, and cancer-suppressor gene activity.
Introduction
As per the American Society of Clinical Oncology, people residing in the United States have a lifetime risk of approximately 0.97% of developing multiple myeloma.1It is expected that up to 35 780 new cases will be diagnosed in 2024, with 12 540 deaths expected from the disease. Targeted therapies such as proteasome inhibitors, histone deacetylase inhibitors, and monoclonal antibodies have shown promising results in treating myeloma.2These therapeutics target specific genes or proteins in the cancer cell or tissue environment.3However, since not all tumors have the same target molecules, there is an ongoing search for new targets and treatments.3
Telomeres are repetitive nucleotide sequences—TTAGG—located at the end of chromosomes and are important for chromosome replication.4Telomeric DNA comprises 500 to 3000 repeats of the nucleotide sequence TTAGG, and during each cycle of mitosis, it is estimated that about 50 to 100 base pairs of telomeric DNA are lost.5Therefore, with each cell division, there is a shortening of telomeric DNA until the cell reaches early crisis.6In early crisis, P53-dependent apoptosis is triggered due to the shortened dysfunctional telomeres, which is recognized as DNA damage.6Shortened dysfunctional telomeres lead to genomic instability, which is a hallmark of cancer.6Telomerase is a nuclear enzyme that increases the length of telomeres. It is an enzyme complex composed of telomerase reverse transcriptase (TERT), telomerase RNA (TERC), and dyskerin. TERT uses the TERC as a template to add telomeric repeats to the ends of chromosomes.7
Studies have shown that in cancer cells, there is an increase in telomerase expression, which causes the lengthening of telomeres leading to cell immortalization.8On the other hand, other studies have also shown that an overexpression of tumor suppressor p53 along with telomere lengthening has been beneficial in increasing resistance to cancer.9Studies have also shown that cannabinoids such as tetrahydrocannabinol (THC) and cannabidiol (CBD) have anti-cancer effects. They induce cancer cell death and decrease cancer cell proliferation and metastases.10,11 CBD has been shown to have anti-inflammatory effects by decreasing pro-inflammatory cytokines through regulating transcription factor NF-κB.12,13 The NF-κB is important in controlling cancer cell growth, differentiation, and survival. Dysregulation of these targets or pathways can contribute to cancer development by preventing apoptosis and promoting cell proliferation. There are limited studies on the anticancer effect of cannabinoids on myeloma cells, telomere, or cancer suppressor gene regulation. BRF1-A is a product that is enriched in cannabinoids. It is derived from a specific Cannabis sativa chemotype known for its high cannabidiol (CBD) content. Our study proposed that BRF1A has an anti-myeloma effect on IgE and IgG-producing myeloma cells. We hypothesized that this effect is achieved by suppressing myeloma cell function, which decreases IgE and IgG production. Furthermore, we believe that BRF1A, a cannabinoid-containing product, regulates genes crucial for cancer suppression as shown in review studies conducted by Velasco et al on the potential anticancer mechanisms of cannabinoids, as well as by Chakravarti et al on the therapeutic potential of cannabinoids as anticancer agents.10,11 Our research is novel because it demonstrated that BRF1A could mediate an anticancer effect by suppressing myeloma cell function, upregulating telomere and telomerase while increasing cancer suppressor genes, and decreasing oncogenes in myeloma cells.
Materials and Methods
Plant Material and Extraction, Compound Preparation, Cannabinoid Content Analysis
BRF1-A is a product that contains enriched cannabinoids and was provided by the Breslin Research Foundation located in Taos, NM. The product was derived from a Cannabis sativa chemotype that was cultivated in southern Colorado to have a high cannabidiol (CBD) content, which is known as “3× Spectrum.” The dried flowers, seeds, and leaves of this plant were extracted using CO2 under standardized parameters at room temperature to produce a crude extract. The crude extract was then refined through a winterization process, which resulted in a refined CO2 extract suspended to 2.2× its weight of 95% ethanol by mixing 2.08 mL of the above-described winterized CO2 extract and 95% ethanol into 132 mL of certified organic extra virgin olive oil. This mixture was filtered through a 0.2-µm sterile nylon filter. The ethanol was then separated from the winterized CO2 extract using rotary evaporation, followed by further solvent removal in a vacuum oven, resulting in an amber-colored oil of high viscosity. The filtered solution was added to a fused quartz glass breaker and imprinted using the QBITS Technology, which yielded BRF1A. BRF1-A was analyzed by LC-MS at Marigold Botanicals in Taos, NM, to determine its cannabinoid content and terpene profile. The results of the analysis showed that this extract contained 32.91% cannabidiolic acid CBDa, 28.08% CBD, 1.52% Delta 9-Tetrahydrocannabinol (Delta 9 THC), and 0% Delta 9-Tetrahydrocannabinolic acid (THCA-A). The terpene composition was found to be 1.393%, with the predominant terpenes being alpha-bisabolol (0.627%), beta-caryophyllene (0.505%), alpha-humulene (0.165%), and beta-myrcene (0.096%). Based on the data obtained through the analysis of the concentrated CO2 extract, the estimated cannabinoid content in the BRF1A product was found to be 0.44 mg of combined cannabinoids and terpenes per 132 mL of extra virgin olive oil. This equates to approximately 0.0033 mg combined cannabinoids and terpenes per 1 mL of BRF1A. Using the molar mass of CBD (314.47 g/mol), the concentration of cannabinoids in BRF1A was estimated to be 10.65 µM.
IgE, IgG Myeloma Cell Culture and IgE, IgG Level Measurement Via ELISA
The cell line referred to as human U266 cells (RRID:CVCL_0566) are myeloma cells that produce IgE. These cells were purchased from the American Type Culture Collections (Rockville, MD, USA) and were derived from the B cell lymphocyte of a 53-year-old patient with myeloma. In addition to the U266 cells, the ARH-77 cell line (RRID:CVCL_1072) was also used in the experiment. These cells were also purchased from the American Type Culture Collection (Rockville, MD, USA).14U266 and ARH-77 cells were cultured as previously described by Yang et al15in a media composed of RPMI 1640 medium supplemented, 10% FBS, 1 mM sodium pyruvate, 1 × 10−5 M β-ME and 0.5% penicillin-streptomycin and incubated at 37°C under 5% CO2. U266 at 1 × 106 cells/mL were incubated with BRF1A at 0.25 µM, 0.5 µM, 1.0 µM and 0.1% DMSO for the untreated control in a 24-well plate for 1, 3, and 5 days. Similarly, ARH77 cells at 1 × 106 cells/mL were incubated with BRF1A at 1.0 µM and 0.1% DMSO for the untreated control in a 24-well plate for 1, 3, and 5 days. The levels of immunoglobulin E and G were determined according to previously published methods by Yang et al15by harvesting the supernatant obtained from the culture and using immunoglobulin E and G ELISA kit purchased from Mabtech, Inc. (Cincinnati, OH, USA).
Human ARH-77 and U266 Cell Viability Via Trypan Blue Exclusion Assay
The cell viability of Human U266 (RRID: CVCL_0566) and ARH-77 (RRID: CVCL_1072) was determined by using trypan blue exclusion assay as previously described.16A total of 10 µL from the cell culture was mixed with 10 µL of trypan blue dye purchased from Sigma Aldrich (Burlington, MA, USA). The mixture was loaded on a hemocytometer and counted. The percentage of live cells in the total number of cells was calculated to determine cell viability.
Human U266 Cell Proliferation Via CCK-8 Assay
CCK8 assay is a rapid and simple method to measure living cell activity. Cell Counting Kit-8 was utilized to assess cell cytotoxicity following the manufacturer’s guidelines (Dojindo, Rockville, MD, USA) and previously published methods.15,17–19 U266 cells were cultured as mentioned earlier, then incubated with BRF1A at concentrations of 0.25 µM, 0.5 µM, 1.0 µM, and 0.1% DMSO (untreated control) in a 24-well plate for 1, 3, and 5 days. Similarly, ARH77 cells were cultured and incubated with BRF1A at concentrations of 1.0 µM and 0.1% DMSO (untreated control) in a 24-well plate for 1, 3, and 5 days. Then, we transferred 100 μL of cell suspension into a 96-well plate. Next, 10 μL of CCK8 solution was added to each well of the plate and incubated for 1 hour. Finally, the optical density was measured using a microplate reader at 450 nm.
Real-Time Polymerase Chain Reaction
The quantitative real-time polymerase chain reaction was used to measure the effect of BRF1A on the gene expression of myeloma cells based on previous studies by Rafat et al20and Yang et al.15We cultured U266 and ARH-77 cells as previously described in the cell culture methods. Human U266 cells at a concentration of 1.0 × 106 cells/mL were cultured with BRF1A at a concentration of 1 µM and incubated at different time points of 24 and 48 hours. After incubation, the cells were harvested and washed with PBS by centrifuging at 1000 rpm for 10 minutes. We isolated RNA from the harvested cells using the QIAGEN RNeasy kit purchased from Redwood City, CA. Transfer Buffer RLT into Eppendorf tubes containing cell pellets, vortex for 2 minutes, and centrifuge for 3 minutes. Extract DNA by transferring lysate to Allprep DNA spin columns and spinning at 50 000 rpm for 30 seconds. Add 70% ethanol to the flow-through and mix gently. Extract RNA by transferring 700 μL of sample to the RNeasy spin column, washing with Buffer RW1 and Buffer RPE, and spinning at maximum speed. Add 60 μL of RNA-free water to the spin column membrane and centrifuge for 1 minute at 10 000 rpm. We quantified the RNA using the NanoDrop™ 2000/2000c Spectrophotometer. Next, we generated cDNA from the isolated RNA using the reverse transcription RevertAid RT Reverse Transcription Kit (Thermo Fisher, Waltham, MA, USA) according to the instructions provided by the manufacturer. Thin-walled and reaction tubes were chilled on ice, as was nuclease-free water. RNA (up to 1 µg) and the cDNA primer were mixed in nuclease-free water on ice to a final volume of 5 µL per RT reaction. The tube was then placed into a preheated 70°C heat block for 5 minutes and immediately chilled on ice for 5 minutes. Then, each tube was spun to collect all the condensate. The reverse transcription reaction mix was prepared with ImProm-II™ 5X Reaction Buffer, MgCl2, dNTP, Recombinant RNasin® Ribonuclease Inhibitor, ImProm-II™ Reverse Transcriptase, and nuclease-free water, bringing the final volume to 15 μL. A total of 15 µL of the reverse transcription reaction mix was added to each reaction tube containing the 5 µL RNA and primer mix, resulting in a final volume of 20 µL. The RT reaction was completed using PCR at 25°C for 5 minutes, 42°C for 60 minutes, and 70°C for 15 minutes. The RT-PCR amplification was conducted using the Maxima™ SYBR Green qPCR Master Mix (2×) kit (ThermoFisher, Waltham, MA, USA) with epsilon germline primers or GAPDH primers. We measured the mRNA expression of our target genes immunoglobulin E heavy chain (IgEH), immunoglobulin G heavy chain (IgGH), Tel (Telomere), hTERT (human telomerase reverse transcriptase), tumor protein 53 (TP53), cellular myelocytomatosis (cMyc) and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) using the Maxima SYBR Green qPCR Master Mix kit obtained from Fisher Scientific, Pittsburgh, Pennsylvania. The primer sequences of the targets used in the study were purchased from Integrated DNA Technologies (Coralville, IA, USA) and are listed in Supplemental Table 2.
Western Blotting
Rafat et al20utilized western blotting to investigate telomerase inhibition in primary AML and KG-1a cells. Similarly, we assessed protein expression of the targets regulated by BRF1A; Western blot analysis was performed. Human U266 cells (3.0 × 106 cells/mL) were cultured with BRF1A at 1.0 µM for 48 hours. The cells were harvested and supernatant removed by centrifugation at 3000 rpm for 5 minutes. The cells’ suspension was washed with PBS at 3000 rpm for 5 minutes. Protein extraction was done through cell lysis, using RIPA lysis buffer, according to the manufacturer’s instructions (Santa Cruz Biotechnology, Santa Cruz, CA, USA). To extract the protein, we added 100 to 200 µL of RIPA lysis buffer and vortex vigorously while incubating on ice for about 1 hour. Then, we centrifuged the mixture at 14 000 rpm for 20 minutes at 4°C. The isolated protein was used for standard western blot analysis. We ran the gel using electrophoresis with settings at 90 V for 15 min followed by 100 to 110 V for the remaining time. After running the gel, we transferred the proteins to a PVDF membrane. We incubated the membrane overnight with primary antibodies of p-IκBα, p-NFκB, NFκB, XBP-1s, XBP-1u, hTERT, p-TERT, P53, c-Myc, β-actin, and GAPDH. After incubating the membrane overnight with the primary antibodies, we washed the membrane with PBST the next day and incubated the membrane with secondary antibodies for 2 hours. The following primary antibodies used in the study: p-IκBα, p-NFκB, NFκB, XBP-1u, XBP-1s, P53, c-Myc, β-actin, and GAPDH. These antibodies were ordered from Cell Signaling (Danvers, MA, USA) and were used at a 1:1000 dilution. The primary antibody of XBP-1u was purchased from Proteintech (Rosemont, IL, USA) and was used at dilutions ranging from 1:500 to 1:1000. The primary antibody of p-TERT was purchased from Invitrogen (Waltham, MA, USA) and was used at 1:500. The primary antibody of hTERT was purchased from Bioss Antibodies (Woburn, MA, USA) and used at a 1:500 dilution. The secondary antibodies used in the study were ordered from Cell Signaling (Danvers, MA, USA) and were used at a 1:10 000 dilution.
Statistics
We used the GraphPad Prism software (version 9; GraphPad Software, Inc., San Diego, CA, USA) for statistical analyses. To compare pairwise differences, we performed 1-way ANOVA (analysis of variance) followed by Bonferroni correction. A P-value of ≤.05 was considered statistically significant.
Results
BRF1A Inhibits the Excessive Production of IgE U266 Cells in a Dose- and Time-Dependent Manner
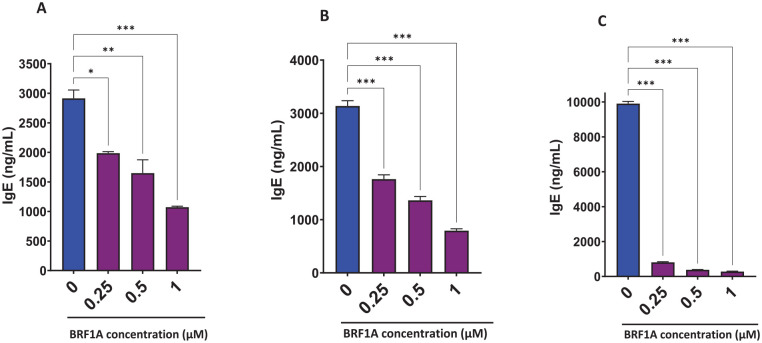
The key pathologic function of myeloma cells is over production of immunoglobulins leading to tissue damage.21To evaluate the suppressive effect of BRF1A on myeloma cell function, we cultured BRF1A with the IgE-producing myeloma cell line, U266, which is aggressive and resistant to anti-cancer drugs.22BRF1A was added to U266 culture at various concentrations of 0.25 µM, 0.5 µM, 1.0 µM, and 0.1% DMSO at multiple time points of 1, 3, and 5 days to establish the dose-response and time course of anti-myeloma suppressive effects. Our results showed that the production of IgE was significantly reduced after 1 day of culture in the group that received BRF1A at concentrations of 0.25, 0.5, and 1.0 µM, compared to the untreated culture with 0.1% DMSO (Figure 1A; P < .05 to P < .001). The reduction percentages observed were 23% to 36%, 27% to 61%, and 59% to 64%, respectively. We also found that IgE production in the day 3 and 5 cultures was significantly decreased across the different concentrations of BRF1A compared to the untreated culture at 0.1% DMSO. The IgE reduction percentages in the day 3 culture group were 37% to 49%, 52% to 61%, and 71% to 77% (Figure 1B, P < .001). In the day 5 culture group, we observed a further decrease in IgE reduction percentages of 91% to 92%, 95% to 96%, and 96% to 97% (Figure 1C, P < .001). These results suggest that BRF1A effectively suppressed myeloma cell function in a dose- and time-dependent manner, as evidenced by its significant reduction of IgE production.
Figure 1.
Data represent triplicate experiments and are expressed as mean ± SD. *P < .05; **P < .01; ***P < .001 versus control (0.1% DMSO).
Dose and Time-Dependent Effect of BRF1A on Myeloma Cell
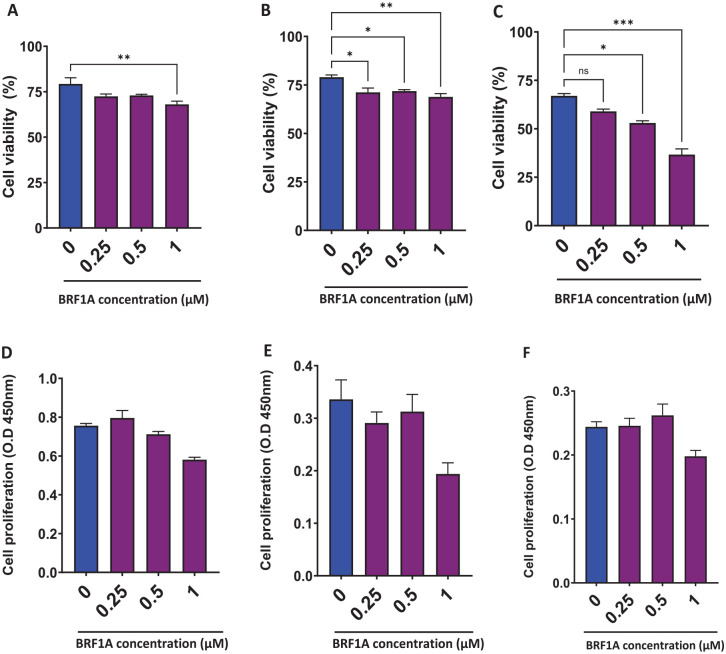
BRF1A decreased IgE production in IgE myeloma cells; therefore, we evaluated the effect of BRF1A on cell viability. The cell viability was evaluated at various concentrations of 0.25, 0.5, and 1.0 µM. The cell viability of myeloma cells treated with 0.5 µM concentration of BRF1A ranged from 72% to 75%, at 0.5 µM it ranged from 72% to 74%, and at 1.0 µM it ranged from 65% to 71.1% compared to the untreated culture group, which showed a viability between 75% and 86% (Figure 2A, P < .05). Similarly, on day 3, the cell viability for the culture group treated with 0.25 µM of BRF1A was between 68.2% and 75.5%, for 0.5 µM it was 70.9% to 73.3%, and for 1.0 µM it was 66.8% to 72.2% (Figure 2B, P < .05; P < .01), while the untreated group had viability between 75% and 81%. By day 5, cell viability was further decreased across all treatment groups compared to the untreated culture, with 0.25 µM of BRF1A between 57% and 61%; for 0.5 µM it was 51% to 55%, and for 1.0 µM it was 31% to 41% (Figure 2C, P < .05; P < .001). These findings indicate the potential of BRF1A as an antimyeloma agent. Furthermore, the results suggest that BRF1A exerts its antimyeloma effect within 24 hours and this effect increases with time.
Figure 2.
Data represent triplicate experiments and are expressed as mean ± SD. *P < .05; **P < .01; ***P < .001 versus control (0.1% DMSO). OD, optical density.
We next used the colorimetric assay CCK-8, which evaluates the cytotoxic effects of anticancer drugs. The results were similar to the cell viability results across all concentrations of 0.25, 0.5, and 1.0 µM as compared to untreated controls (Supplemental Figure 3A-D). This indicates that BRF1A inhibits myeloma cell function in a non- or less-cytotoxic manner.
BRF1A Inhibits Excessive IgG Production in ARH77 Cells Without Cytotoxicity
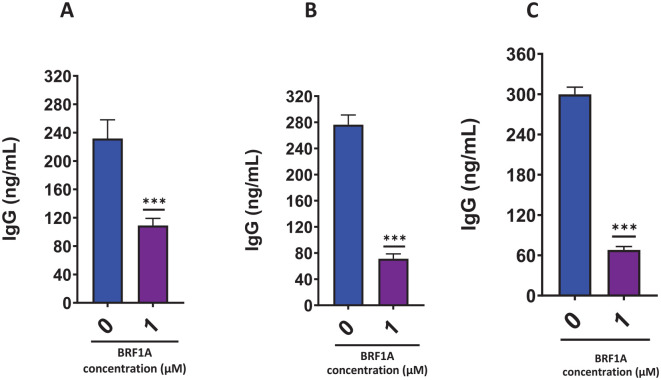
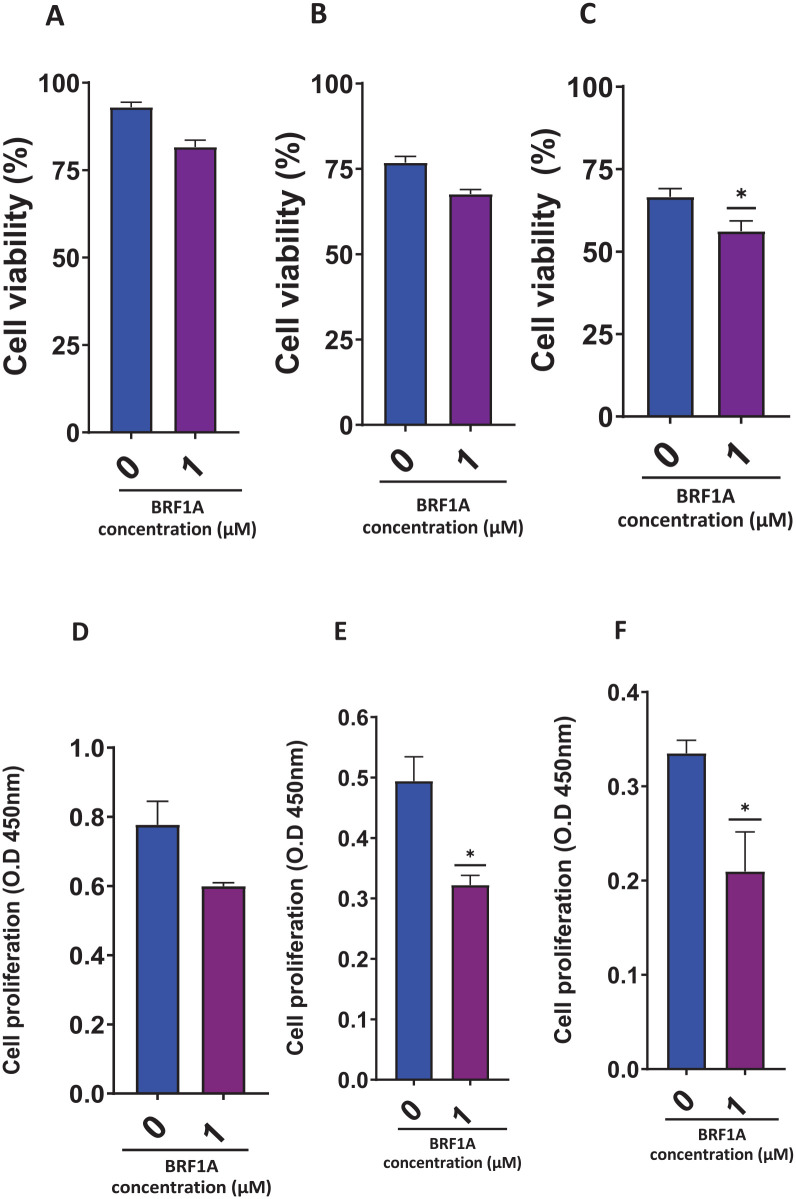
BRF1A suppressed IgE-producing myeloma function; therefore, we wanted to see if it has a similar suppressive effect in IgG-producing myeloma ARH-77 cells. We tested this by using a concentration of 1.0 µM of BRF1A, which had previously been shown to inhibit IgE production. We cultured the ARH-77 cells with this concentration of BRF1A for 1, 3, and 5 days. Our findings showed that compared to untreated cultures, there was a significant decrease in IgG production on days 1, 3, and 5 (as seen in Figure 3A-C, P < .001). Within 24 hours, the IgG concentration of the cells cultured with BRF1A was decreased to 79 to 144 ng/mL, compared to the untreated culture group where the IgG concentration ranged between 185 and 299 ng/mL (as shown in Figure 1A, P < .05 to P < .001). A subsequent decrease in IgG concentration was observed upon reaching days 3 and 5 of the culture period. In the treatment group, the IgG concentration ranged between 46 and 99 ng/mL on day 3, while the untreated culture group had IgG concentrations between 244 and 316 ng/mL. Similarly, on day 5, the treatment group showed IgG concentrations of 44 to 94 ng/mL, while the untreated group exhibited 278 to 329 ng/mL concentrations. We also observed no significant cytotoxicity in the cells at day 1 and 3 time points (as seen in Figure 4A-C).
Figure 3.
Data represent triplicate experiments and are expressed as mean ± SD. *P < .05; **P < .01; ***P < .001 versus control (0.1% DMSO).
Figure 4.
Data represent triplicate experiments and are expressed as mean ± SD. *P < .05 versus control (0.1% DMSO).
Additionally, using CCK-8, we found that BRF1A had a similar effect on IgG-producing myeloma cells as it did on IgE-producing myeloma cells (as seen in Figure 4D-F). Therefore, our results suggest that BRF1A can suppress both IgE- and IgG-producing myeloma cells. This suggests that its suppressive effects are not specific to IgE-producing myeloma cells, thereby highlighting the potential of BRF1A as a therapeutic agent for myeloma.
BRF1A Inhibited the Expression of Immunoglobulin E and G Heavy Chain Genes in Myeloma Cells
We observed a significant decrease in IgE and IgG production when BRF1A was cultured with myeloma cells at a concentration of 1.0 µM on days 1 and 3 of the culture, with minimal change in cell viability. This led us to investigate whether BRF1A regulates the gene expression of IgEH, IgGH which are biomarkers of IgE and IgG myeloma cell function. We cultured BRF1A with myeloma cells at a concentration of 1.0 µM for 24 hours and isolated RNA to measure the mRNA expression of IgEH. Our results revealed a significant decrease in IgEH (P < .001) in the 24-hour culture time point (Figure 5A). We next determined if BRF1A also decreased the gene expression of immunoglobulin G heavy chain (IgGH) in ARH-77 cells, we cultured BRF1A at a similar concentration and time point as U266 cells and isolated RNA to measure mRNA expression. Our results indicate that BRF1A significantly decreased the mRNA expression of IgGH (Figure 5B, P < .001). These results demonstrate that BRF1A suppresses IgG and IgE antibody production in IgG and IgE myeloma cells, respectively, by suppressing the IgEH and IgGH genes. Since BRF-1A similarly inhibited U266 and ARH-77 antibody production and antibody heavy chain expression, and because U266 cells are aggressive and resistant to anti-cancer drugs,22we further assessed the mechanism focusing on U266 cells in the following studies.
Figure 5.
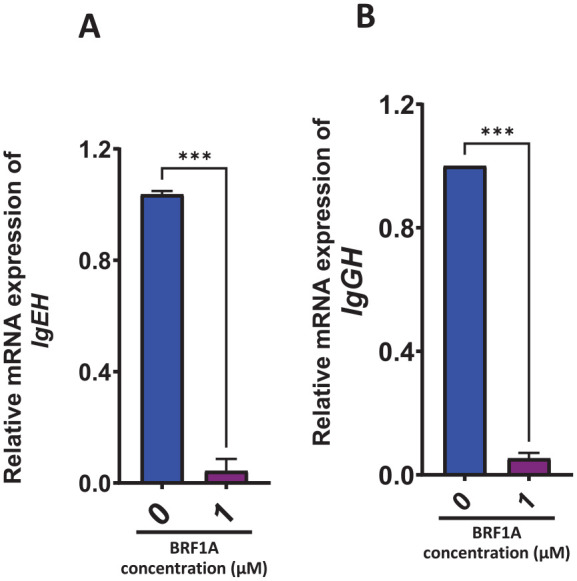
Data represent triplicate experiments and are expressed as mean ± SD. ***P < .001 versus control (0.1% DMSO).
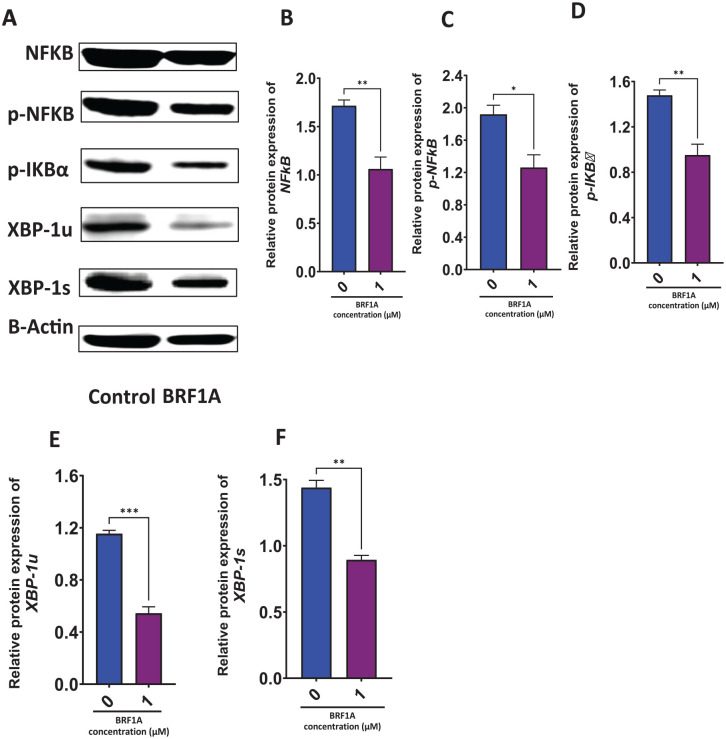
BRF1A Suppressed Protein Expression Critical for Myeloma Cell Function and Survival
The transcription factor, NF-ĸB, plays an important role in the production of IgE in plasma B cells. In human B cells, activation of transcription factors of the NF-ĸB family results in the degradation of IκBα and IκBβ. This leads to the eventual release of NF-κB—p50 and NF-κB—p65, which translocate to the nucleus to trigger the transcription of target genes.23Decreased levels of p-IĸBα indicate that NF-κB is inhibited. XBP-1 is a survival transcription factor for plasma cells, which helps them function under stress. During endoplasmic reticulum (ER) stress, XBP1s translocate into the nucleus and activate target genes encoding ER chaperones and ER-associated degradation (ERAD) components. This, in turn, enhances the capacity of protein folding and degradation, to maintain ER homeostasis.24Therefore, we determined the effect of BRF1A on proteins NFκB, p-NFκB, p-IκBα, XBP-1u, and XBP-1s in U266 cells. We cultured BRF1A at a concentration of 1.0 µM with 3.0 × 106 cells/mL U266 cells for 48 hours to measure protein expression. Our results show that BRF1A significantly inhibited the protein expression of NFκB (P < .01), p-NFκB (P < .05), p-IκBα (P < .01), XBP-1u (P < .05), XBP-1s (P < .01; see Figure 6A-E).
Figure 6.
Data represent triplicate experiments and are expressed as mean ± SD. *P < .05; **P < .01, ***P < .001 versus control (0.1% DMSO).
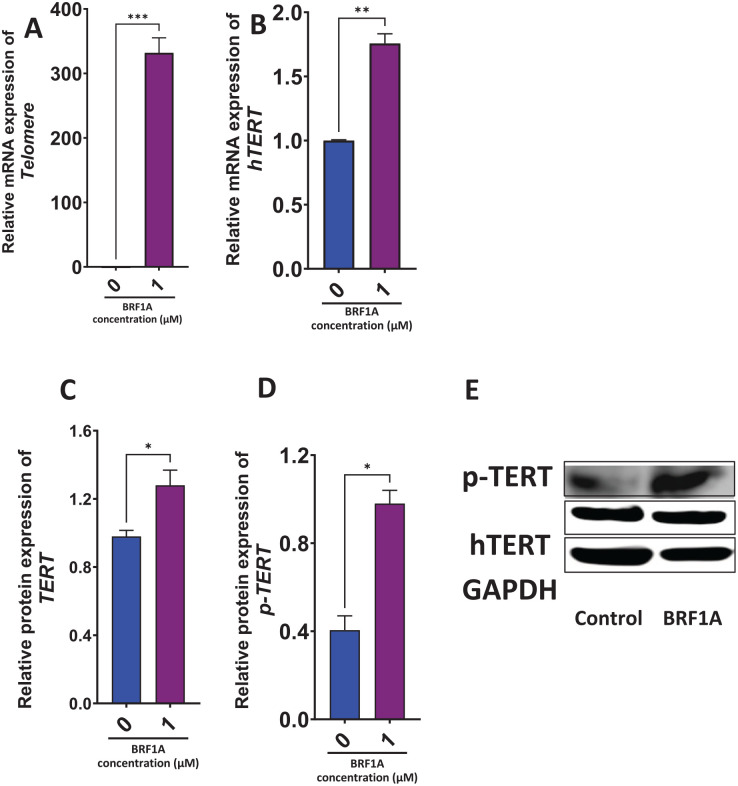
BRF1A Increased the Gene Expression of Telomerase in Myeloma Cells
Next, we determined if BRF1A has any effect on telomerase gene expression given its relevance in cancer progression. The human telomerase is a ribonucleoprotein enzyme made up of at least 2 components, a catalytic subunit known as telomerase reverse transcriptase (hTERT), and a template RNA component called hTR.25This ribonucleoprotein (RNP) enzyme slows down telomere attrition by adding repetitive sequences to chromosome ends, thus maintaining telomere length. To determine the effect of BRF1A on the gene expression of telomerase, we cultured BRF1A at 1.0 µM with 3.0 × 106 cells/mL myeloma cells for 48 hours. Our results revealed that BRF1A increased the expression of hTERT gene (P < .01) after 48 hours (Figure 7A and B).
Figure 7.
Data represent triplicate experiments and are expressed as mean ± SD. *P < .05; **P < .01; ***P < .001 versus control (0.1% DMSO).
BRF1A Increased the Protein Expression of hTERT, p-hTERT in Myeloma Cells
The gene expression of telomerase was shown to be increased by product BRF1A. To determine if this regulation is posttranslational, we measured the protein expression of hTERT and p-TERT in U266 cells after treating them with BRF1A at a concentration of 1.0 µM for 48 hours. BRF1A treatment, to determine if the regulation of telomere and telomerase is posttranslational. Our results demonstrated a significant increase in the protein expression of hTERT (P < .05) and p-TERT (P < .05) (Figure 7C and D).
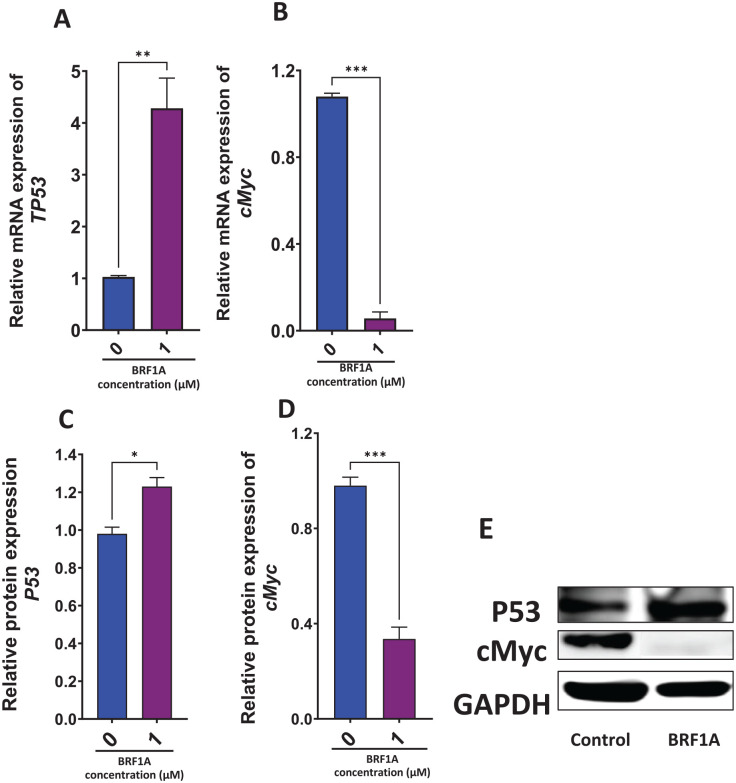
BRF1A Increased the Gene Expression of Tumor Suppressor Gene and Decreased Oncogenes in Myeloma Cells
Next, we determined if BRF1A has any effect on tumor suppressor TP53 gene and oncogene c-Myc. Myc regulates different types of cell functions, therefore it is a target for most anticancer drugs.26Most cancers cause a shutdown in the P53 pathway, therefore increasing its expression will lead to a P53 dependent apoptosis.27We cultured BRF1A at a concentration of 1.0 µM with 3.0 × 106 cells/mL myeloma cells for 48 hours and measured the gene expression of c-Myc and TP53. In 48 hours, BRF1A significantly increased the gene expression of TP53 (P < .01), and significantly decreased the expression of c-Myc (P < .001; Figure 8A and B).
Figure 8.
Data represent triplicate experiments and are expressed as mean ± SD. *P < .05, **P < .01; ***P < .001 versus control (0.1% DMSO).
Figure 9.
BRF1A Increased TP53 and Decreased c-Myc Protein Expression
BRF1A increased the gene expression of TP53 and suppressed the gene expression of c-Myc. Therefore, we next measured the effect of BRF1A on the protein expression of p53 and c-Myc. We cultured BRF1A at a concentration of 1.0 µM with 3.0 × 106 cells/mL myeloma cells for 48 hours and measured the protein expression of p53 and c-Myc. Our results demonstrated that BRF1A significantly increased the protein expression of p53 (P < .05) and decreased the protein expression of c-Myc (P < .001) (Figure 8C and D). These findings suggest that BRF1A could be a potential therapeutic agent for myeloma treatment.
Discussion
Multiple studies have been conducted on CBD’s effects on different types of cancer cells. These studies have shown that CBD has anti-cancer properties, which makes it a promising therapy for cancer treatment. Researchers such as Sarfaraz et al and Katona et al have reviewed the data and confirmed the positive effects of cannabinoids on cancer cells.28–30 Numerous studies have also shown the proapoptotic and antiproliferative effects of CBD. Ramet et al demonstrated the proapoptotic properties of CBD in lung cancer cell lines and primary cell lines taken from cancer patients.31Morelli et al demonstrated that CBD by itself reduces cell proliferation in multiple myeloma.12Furthermore, Nabissi et al demonstrated that the THC-CBD combination stimulates autophagic-dependent cell death in multiple myeloma cell lines.32In this study, we have demonstrated the efficacy of BRF1A, a CBD-enriched product, in inhibiting the function of rare but aggressive IgE and common IgG myelomas, similar to other studies. The main pathological mechanism of IgE and IgG myeloma is the overproduction of immunoglobulin E and G antibodies. Studies by Ying et al indicate that measuring serum IgG1 levels could be a potential indicator for evaluating the therapeutic effect in patients with multiple myeloma. Our study showed that within 24 hours, a CBD-enriched product called BRF1A can inhibit the production of both IgE and IgG while also decreasing cell viability. This effect continues to be effective as the duration of the culture increases. This study is the first to report such an effect in multiple myeloma.
Numerous studies have shown that there is a strong correlation between hypermutation and overexpression of immunoglobulin E and immunoglobulin G heavy chain genes in patients with multiple myeloma. Medina et al demonstrated this correlation by highlighting hypermutation in the immunoglobulin G heavy chain gene in patients with multiple myeloma.33González et al showed the role of immunoglobulin gene rearrangement in the pathogenesis of multiple myeloma.34Similarly, we determined the effect of BRF1A on immunoglobulin E and G heavy chain genes in IgE and IgG-producing myeloma, respectively. Our results revealed that BRF1A significantly suppressed the expression of both genes on both IgE and IgG myeloma cells U266 and ARH77 cells. Our study is the first to show that a cannabinoid-containing product affects IgE and IgG genes in myeloma.
Aksenova et al reviewed various studies on the impact of genomic instability on multiple myeloma. They showed that the development and progression of the myeloma were associated with the targets and pathways, such as XBP-1, NFκB, telomere, hTERT, c-Myc, and P53, among others.35Similarly, we determined if BRF1A affects the protein expression of critical targets that are important for IgE myeloma cell function, such as NFκB, p-NFκB, p-IκBα, XBP-1u, XBP-1s. Our data demonstrated that BRF1A inhibited the protein expression of these targets. NF-ĸB plays a pivotal role in activating the ɛ germline center, which is necessary for class switch recombination in IgE heavy chain transcript production. The IgE heavy chain is essential for the formation of the immunoglobulin E antibody, without which the antibody cannot be produced. XBP1 is critical in coordinating plasma cell differentiation and survival. It functions in regulating unfolded protein response in endoplasmic reticulum stress in antibody-producing plasma cells.36–38 Tang et al showed that XBP1 is highly expressed in myeloma cells as they excessively produce antibody XBP1.39Here, our study demonstrates that BRF1A inhibits critical targets that are vital for IgE myeloma cell function and survival. This suggests the potential of BRF1A as a significant factor in modulating myeloma cell activity and warrants further exploration in the field of myeloma research.
Cong et al reviewed numerous studies on the role of telomeres and telomerase in the development and prognosis of many types of cancers.40Telomerase is a ribonucleoprotein enzyme that adds nucleotide repeats to the end of telomeric DNA, thereby maintaining the stability of telomere and preventing telomere shortening.40Telomerase is made of 2 components, a catalytic subunit, (hTERT) human telomerase reverse transcriptase and (hTR) human template RNA component.40Telomerase is regulated on multiple levels, including transcriptionally, mRNA splicing, maturation, and modifications of hTR and hTERT, transport, and subcellular localization of each component, assembly of holoenzyme to an active ribonucleoprotein, accessibility and proper function on its telomeric substrates.40In this study, we assessed the telomerase activity by measuring the expression of the hTERT subunit. Our findings revealed that telomerase was upregulated transcriptionally at the gene level and post-transcriptionally at the protein level. This was surprising since most anticancer drugs that have an effect on telomere lengthening usually have a suppressive effect, in which they prevent the telomere lengthening that is associated with cancer progression. However, our findings are more consistent with the study by Cong,40which demonstrated that stage III MM patients frequently had shorter telomere lengths compared to stage I, who frequently have longer telomere lengths. Additionally, the MM patients with shorter telomere lengths were non-responders for induction therapy, while those with longer telomere lengths expressed more frequent complete responses for therapy. These findings of Aref et al41are similar to ours in which BRF1A increased telomere gene expression.
Telomere deficiency has been linked to a lower incidence of cancer. However, a study conducted by González-Suárez et al42demonstrated that the undesirable effect of constitutive overexpression of telomerase, which results in a slightly increased cancer incidence, can be counterbalanced by increasing the levels of tumor suppressors TP53. The study found that mice with increased expression of cancer suppressor genes lived longer than the control group.42BRF1A increased telomerase expression while also increasing the gene expression of cancer suppressor gene TP53 in U266 cells. This BRF1A-related upregulation of TP53 expression was also observed in the ARH77 myeloma cell line (Supplemental Figure S1). Increasing tumor suppressor genes counterbalances the undesirable effects of telomerase overexpression. In their studies, Muller et al found that TP53 mutations occur in 50% of all human cancers.43TP53 alterations, mutations, and deletions increase in the late stages of multiple myeloma. According to Drach et al,44defects in TP53 are associated with resistance to treatment. Similarly, we showed that the product BRF1A increases the gene expression of TP53 in both IgE- and IgG-producing myeloma cells. This implies that BRF1A’s antimyeloma effect is by enhancing the expression of the TP53 gene.
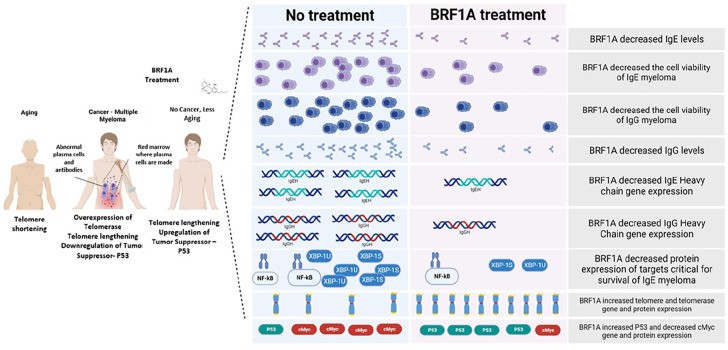
In conclusion, we have shown that the cannabinoid-containing product BRF1A has an anticancer effect in IgE and IgG-producing myeloma cells. Its anticancer effect is achieved by shutting down the critical functions of the cell that are necessary for its antibody hyper-production while simultaneously increasing the activity of telomerase and cancer suppressor genes. This product shows great potential as a therapeutic option for targeted cancer therapy, but further studies are needed to confirm its effectiveness.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354241267979 for Inhibition of Myeloma Cell Function by Cannabinoid-Enriched Product Associated With Regulation of Telomere and TP53 by Ibrahim Musa, Nan Yang, Joseph Breslin, Orion Paulden, Jan Geliebter, Raj Tiwari and Xiu-Min Li in Integrative Cancer Therapies
Acknowledgments
We thank Henry Ehrlich for reading this manuscript.
Footnotes
Author Contributions: X.-M.L. designed the experiment and revised the manuscript. I.M., conducted the research work, data analysis and wrote the manuscript. I.M., X.-M.L., R.T., J.G., and J.B. revised the manuscript. All authors read the final version.
Data Availability Statement: The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: X.-M.L. received research support to her institution from the National Institutes of Health (NIH)/National Center for Complementary and Alternative Medicine (NCCAM) #1P01 AT002644725-01 “Center for Chinese Herbal Therapy (CHT) for Asthma,” and grant #1R01AT001495-01A1 and 2R01 AT001495-05A2, NIH/NIAID R43AI148039, Food Allergy Research and Education (FARE), Winston Wolkoff Integrative Medicine Fund for Allergies and Wellness, the Parker Foundation and Henan University of Chinese Medicine; received consultancy fees from FARE and Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Bayer Global Health LLC; received royalties from UpToDate; share US patent US7820175B2 (FAHF-2), US10500169B2 (XPP), US10406191B2 (S. Flavescens), US10028985B2 (WL); US11351157B2 (nanoBBR): take compensation from her practice at Center for Integrative Health and Acupuncture PC; US Times Technology Inc. is managed by her related party; is a member of General Nutraceutical Technology LLC. J.B. is director of Breslin Research Foundation and provided funding and BRF1A for the research related to the current manuscript. Other authors declare that there are no potential conflicts of interest related to the current study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by Fidelity Charitable DAS Fund and Breslin Research Foundation fund to X.-M.L. at New York Medical College.
ORCID iD: Ibrahim Musa  https://orcid.org/0000-0001-9309-9430
https://orcid.org/0000-0001-9309-9430
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Key statistics about multiple myeloma. Accessed April, 2024. https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html
- 2. Cejalvo MJ, de la Rubia J. Which therapies will move to the front line for multiple myeloma? Expert Rev Hematol. 2017;10:383-392. [DOI] [PubMed] [Google Scholar]
- 3. Rajkumar SV. Multiple myeloma: every year a new standard? Hematol Oncol. 2019;37 Suppl 1(Suppl 1):62-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montpetit AJ, Alhareeri AA, Montpetit M, et al. Telomere length: a review of methods for measurement. Nurs Res. 2014;63:289-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayflick L. [The cellular basis of biological aging (author’s transl)]. Verh Dtsch Ges Pathol. 1975;59:52-66. [PubMed] [Google Scholar]
- 6. Kong CM, Lee XW, Wang X. Telomere shortening in human diseases. FEBS J. 2013;280:3180-3193. [DOI] [PubMed] [Google Scholar]
- 7. Cao Y, Bryan TM, Reddel RR. Increased copy number of the TERT and TERC telomerase subunit genes in cancer cells. Cancer Sci. 2008;99:1092-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu L, Zhang C, Zhu G, et al. Telomerase expression and telomere length in breast cancer and their associations with adjuvant treatment and disease outcome. Breast Cancer Res. 2011;13:R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donate LE, Blasco MA. Telomeres in cancer and ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Velasco G, Sánchez C, Guzmán M. Anticancer mechanisms of cannabinoids. Curr Oncol. 2016;23:S23-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chakravarti B, Ravi J, Ganju RK. Cannabinoids as therapeutic agents in cancer: current status and future implications. Oncotarget. 2014;5:5852-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morelli MB, Offidani M, Alesiani F, et al. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int J Cancer. 2014;134:2534-2546. [DOI] [PubMed] [Google Scholar]
- 13. Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z. Cannabinoids delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem. 2010;285:1616-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drewinko B, Mars W, Stragand JJ, et al. ARH-77, an established human IgG-producing myeloma cell line. II. Growth kinetics, clonogenic capacity, chalone production, xenogeneic transplantations, and response to melphalan. Cancer. 1984;54:1893-1903. [DOI] [PubMed] [Google Scholar]
- 15. Yang N, Musa I, Maskey AR, et al. Formononetin isolated from Sophorae flavescentis inhibits B cell-IgE production by regulating ER-stress transcription factor XBP-1. Front Allergy. 2022;3:1056203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang N, Patil S, Zhuge J, et al. Glycyrrhiza uralensis flavonoids present in anti-asthma formula, ASHMI™, inhibit memory Th2 responses in vitro and in vivo. Phytother Res. 2013;27:1381-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lou J, Chu G, Zhou G, et al. Comparison between two kinds of cigarette smoke condensates (CSCs) of the cytogenotoxicity and protein expression in a human B-cell lymphoblastoid cell line using CCK-8 assay, comet assay and protein microarray. Mutat Res. 2010;697:55-59. [DOI] [PubMed] [Google Scholar]
- 18. Li K, Yu XH, Maskey AR, et al. Cytochrome P450 3A4 suppression by epimedium and active compound kaempferol leads to synergistic anti-inflammatory effect with corticosteroid. Front Pharmacol. 2022;13:1042756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kagawa K, Nakano A, Miki H, et al. Inhibition of TACE activity enhances the susceptibility of myeloma cells to TRAIL. PLoS One. 2012;7:e31594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rafat A, Dizaji Asl K, Mazloumi Z, et al. Telomerase inhibition on acute myeloid leukemia stem cell induced apoptosis with both intrinsic and extrinsic pathways. Life Sci. 2022;295:120402. [DOI] [PubMed] [Google Scholar]
- 21. Salmon SE, McIntyre OR, Ogawa M. IgE myeloma: total body tumor cell number and synthesis of IgE and DNA. Blood. 1971;37:696-705. [PubMed] [Google Scholar]
- 22. Gougelet A, Mansuy A, Blay JY, Alberti L, Vermot-Desroches C. Lymphoma and myeloma cell resistance to cytotoxic agents and ionizing radiations is not affected by exposure to anti-IL-6 antibody. PLoS One. 2009;4:e8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rolling C, Treton D, Pellegrini S, Galanaud P, Richard Y. IL4 and IL13 receptors share the gamma c chain and activate STAT6, STAT3 and STAT5 proteins in normal human B cells. FEBS Lett. 1996;393:53-56. [DOI] [PubMed] [Google Scholar]
- 24. Adolph TE, Niederreiter L, Blumberg RS, Kaser A. Endoplasmic reticulum stress and inflammation. Dig Dis. 2012;30:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Armbruster BN, Banik SS, Guo C, Smith AC, Counter CM. N-terminal domains of the human telomerase catalytic subunit required for enzyme activity in vivo. Mol Cell Biol. 2001;21:7775-7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jovanović KK, Roche-Lestienne C, Ghobrial IM, Facon T, Quesnel B, Manier S. Targeting MYC in multiple myeloma. Leukemia. 2018;32:1295-1306. [DOI] [PubMed] [Google Scholar]
- 27. Herrero AB, Rojas EA, Misiewicz-Krzeminska I, Krzeminski P, Gutiérrez NC. Molecular mechanisms of p53 deregulation in cancer: an overview in multiple myeloma. Int J Mol Sci. 2016;17:2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008;68:339-342. [DOI] [PubMed] [Google Scholar]
- 29. Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923-930. [DOI] [PubMed] [Google Scholar]
- 30. Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramer R, Heinemann K, Merkord J, et al. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol Cancer Ther. 2013;12:69-82. [DOI] [PubMed] [Google Scholar]
- 32. Nabissi M, Morelli MB, Offidani M, et al. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget. 2016;7:77543-77557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medina A, Jiménez C, Sarasquete ME, et al. Molecular profiling of immunoglobulin heavy-chain gene rearrangements unveils new potential prognostic markers for multiple myeloma patients. Blood Cancer J. 2020;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. González D, van der Burg M, García-Sanz R, et al. Immunoglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood. 2007;110:3112-3121. [DOI] [PubMed] [Google Scholar]
- 35. Aksenova AY, Zhuk AS, Lada AG, et al. Genome instability in multiple myeloma: facts and factors. Cancers (Basel). 2021;13:5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100:9946-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davenport EL, Moore HE, Dunlop AS, et al. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110:2641-2649. [DOI] [PubMed] [Google Scholar]
- 38. Davenport EL, Morgan GJ, Davies FE. Untangling the unfolded protein response. Cell Cycle. 2008;7:865-869. [DOI] [PubMed] [Google Scholar]
- 39. Tang TF, Chan YT, Cheong HC, et al. Regulatory network of BLIMP1, IRF4, and XBP1 triad in plasmacytic differentiation and multiple myeloma pathogenesis. Cell Immunol. 2022;380:104594. [DOI] [PubMed] [Google Scholar]
- 40. Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407-425 (Table of contents). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aref S, Al Saeed A, El Menshawy N, Abdalla D, El Ashery M. Prognostic relevance of telomere length and telomerase reverse transcriptase variant (rs2242652) on the multiple myeloma patients. J Clin Lab Anal. 2020;34:e23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. González-Suárez E, Geserick C, Flores JM, Blasco MA. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene. 2005;24:2256-2270. [DOI] [PubMed] [Google Scholar]
- 43. Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2-8. [DOI] [PubMed] [Google Scholar]
- 44. Drach J, Ackermann J, Fritz E, et al. Presence of a p53 gene deletion in patients with multiple myeloma predicts for short survival after conventional-dose chemotherapy. Blood. 1998;92:802-809. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354241267979 for Inhibition of Myeloma Cell Function by Cannabinoid-Enriched Product Associated With Regulation of Telomere and TP53 by Ibrahim Musa, Nan Yang, Joseph Breslin, Orion Paulden, Jan Geliebter, Raj Tiwari and Xiu-Min Li in Integrative Cancer Therapies