- Oxford Journals
- Life Sciences
- Plant and Cell Physiology
- Volume 49, Issue 12
- Pp. 1767-1782.
PKS Activities and Biosynthesis of Cannabinoids and Flavonoids in Cannabis sativaL. Plants
+Author Affiliations
Pharmacognosy Department/Metabolomics, Institute of Biology, Gorlaeus Laboratories, PO Box 9502, 2300 RA Leiden University, Leiden, The Netherlands
- *Corresponding author: E-mail, verpoort@chem.leidenuniv.nl; Fax, +31-71-52-74 511.
- Received July 2, 2008.
- Accepted October 7, 2008.
Abstract
Polyketide synthase (PKS) enzymatic activities were analyzed in crude protein extracts from cannabis plant tissues. Chalcone synthase (CHS, EC 2.3.1.74), stilbene synthase (STS, EC 2.3.1.95), phlorisovalerophenone synthase (VPS, EC 2.3.1.156), isobutyrophenone synthase (BUS) and olivetol synthase activities were detected during the development and growth of glandular trichomes on bracts. Cannabinoid biosynthesis and accumulation take place in these glandular trichomes. In the biosynthesis of the first precursor of cannabinoids, olivetolic acid, a PKS could be involved; however, no activity for an olivetolic acid-forming PKS was detected. Content analyses of cannabinoids and flavonoids, two secondary metabolites present in this plant, from plant tissues revealed differences in their distribution, suggesting a diverse regulatory control for these biosynthetic fluxes in the plant.
Key words: Cannabinoids, Cannabis sativa, Flavonoids, Glandular trichomes, Olivetol synthase, Polyketide synthase
 Introduction
Introduction
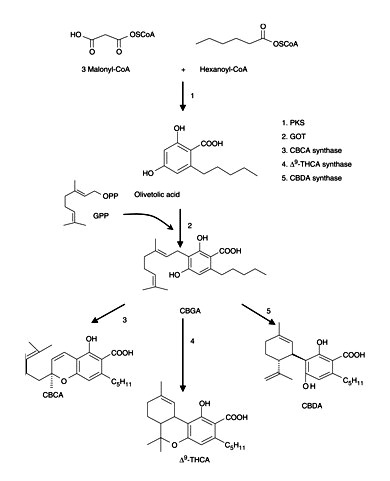
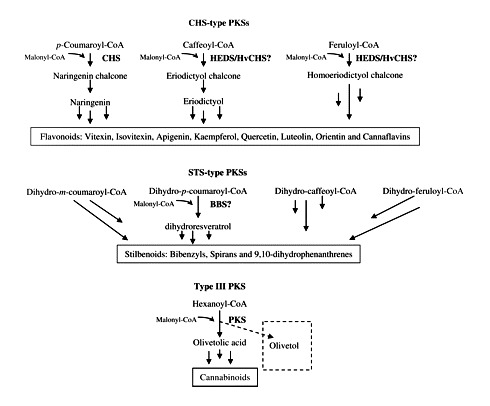
The PKS enzymes are a group of condensing enzymes that catalyze the initial key reactions in the biosynthesis of a myriad of secondary metabolites (Schröder 1997). In plants, several PKS enzymes have been found which participate in the biosynthesis of compounds from secondary metabolism. CHS, stilbene synthase (STS), VPS, BUS, bibenzyl synthase (BBS), homoeriodictyol/eriodictyol synthase (HEDS or HvCHS) and stilbene carboxylate synthase (STCS) are some examples of the type III group of PKS enzymes as they have been classified (Austin and Noel 2003, Eckermann et al. 2003, Klingauf et al. 2005). Type III PKS enzymes use a variety of thioesters of coenzyme A as substrates from aliphatic-CoA to aromatic-CoA, from small (acetyl-CoA) to bulky (p-coumaroyl-CoA) or from polar (malonyl-CoA) to non-polar (isovaleryl-CoA). For example, CHS (Kreuzaler and Hahlbrock 1972) and STS (Rupprich and Kindl 1978) condense one molecule of p-coumaroyl-CoA with three molecules of malonyl-CoA, forming naringenin-chalcone and resveratrol, respectively. VPS (Paniego et al. 1999) and biphenyl synthase (Liu et al. 2007) use isovaleryl-CoA and benzoyl-CoA, respectively, as starter substrates instead of p-coumaroyl-CoA.
The obvious goal for engineering of zero-cannabinoid plants is thus knocking out the first step of the biosynthesis catalyzed by a type III PKS enzyme. Because often several PKS genes are found in plants, we studied the occurrence of different type III PKS enzyme activities in the plant. Here, we report the PKS activities found in different tissues of cannabis plants and show a correlation between the production of polyketide-derived secondary metabolites and the activity of these PKS enzymes in the plant.
Results and Discussion
Activities of PKS enzymes present in plant tissues from Cannabis sativa
For positive control of PKS activity, CHS from Pinus sylvestris, STS from Arachis hypogaea and VPS from Humulus lupulus were used (Supplementary Table S1 online). The activities of these enzymes were similar to those previously reported for STS (58.6 pKat mg–1 protein) from peanut cell cultures (Schoppner and Kindl 1984), CHS (30 pKat mg–1 protein) from Phaseolus vulgaris cell cultures (Whitehead and Dixon 1983) and VPS (35.76 pKat mg–1 protein) from hop (Okada et al. 2000), respectively. Negative control assays consisted of a standard reaction mixture adding 50 μl of water as starter and extender substrate. The final pH for CHS and benzalacetone synthase (BAS) assays was 8.0, which is optimum for naringenin (Schröder et al. 1979, Whitehead and Dixon 1983) and benzalacetone (Abe et al. 2001, Abe et al. 2007) formation, while for the rest of the PKS assays it was maintained at 7.0. Due to limited availability of substrates and standards, for detection of STS-type activity in cannabis protein extracts we decided to perform the assay using the starter substrate p-coumaroyl-CoA for resveratrol formation as a general indicator from STS activities. For detection of CHS-type activities, the assay was carried out with p-coumaroyl-CoA as starter substrate, and naringenin-chalcone formation was an indicator of CHS-type activity. For detection of VPS and BUS activities, the assays were achieved with the starter substrates isovaleryl-CoA and isobutyryl-CoA, respectively.
The protein extracts were from different plant tissues of four varieties of C. sativa plants, two drug (Skunk and Fourway) and two fiber (Kompolti and Fasamo) types. For the analysis of the assays of PKS enzyme activities by HPLC, we started with the eluent system reported by Robert et al. (2001), which was slightly modified, as described in Materials and Methods (method 1). Narigenin [retention time (Rt) 33.55 min] and resveratrol (Rt 26.36 min) had a good separation in this solvent system; however, the retention times of olivetol, PiVP and PiBP (Supplementary Table S2) were longer than that of naringenin. Four elution gradients were tested in order to reduce the retention times of these standards, and method 5 was used subsequently for the analysis by HPLC and liquid chromatography–mass spectrometry (LC-MS).
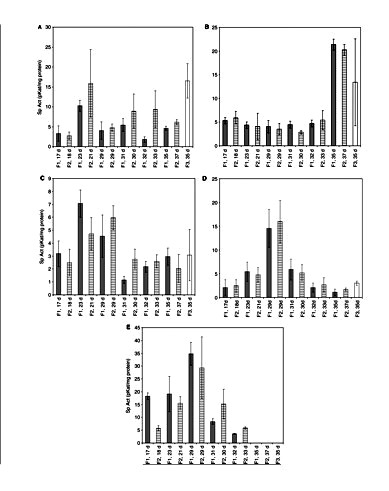
In Fig. 2, the activities found in the different plant parts of four varieties are presented. The products formed from the different incubations were confirmed by means of LC-MS (Supplementary Fig. S1). If we consider the plant parts first, it is clear that each plant part has another spectrum of activity. Olivetol synthase activity is found in leaves and bracts, i.e. correlates with the occurrence of cannabinoids in the plant. In roots it is the CHS activity that is most important. Interestingly, roots also have STS activity, but not olivetol synthase activity, though mechanistically they concern the same reaction. Apparently, there is an enzyme in roots that only accepts coumaroyl-CoA as substrate, whereas in leaves both olivetol synthase and CHS enzyme activities are present. The roots also have STS activity, but not olivetol synthase, again showing a clear preference for the coumaroyl-CoA as substrate for the PKS enzymes present. STS activity is also found in fruits and seedlings, where no olivetol synthase activity is found. BUS and VPS activity show a similar pattern, except for male flowers where high activity is found for VPS, but not for BUS. BUS and VPS are found in the same plant tissues as olivetol synthase, but also in seedlings where no olivetol synthase activity is present. CHS activity is found in all plant tissues.
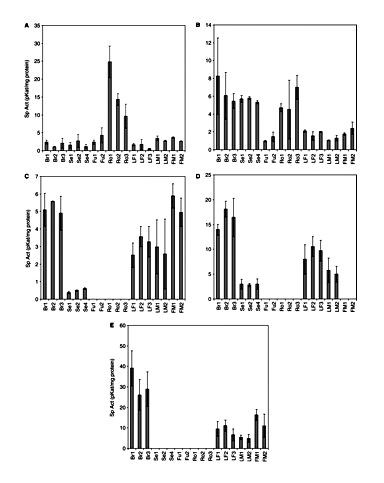
PKS activities in several crude extracts from different cannabis tissues. (A) CHS; (B) STS; (C) VPS; (D) BUS; (E) olivetol synthase. Br, bracts; Se, seedlings; Fu, fruits; Ro, roots; LF, female leaf; LM, male leaf; FM, male flower. 1, Skunk variety; 2, Fourway variety; 3, Kompolti variety; 4, Fasamo variety. Bracts of 29-day-old flowers. Values are expressed as means of three replicates with standard deviations.
Comparing leaves for gender, no significant difference was found in PKS activities (P < 0.05). In terms of specific activity, olivetol synthase is the highest of all measured activities (in the bracts ∼25–40 pKat mg–1protein). BUS activity is generally somewhat higher than the other activities in bracts and leaves. A comparison between fiber-type and drug-type plants does not show any major differences in activities. Apparently, every plant tissue has a specific set of enzymes that is capable of producing different compounds. The bracts and leaves showed all activities, though in different ratios. The compounds found so far in the plant are flavonoids, stilbenoids and cannabinoids (Flores-Sanchez and Verpoorte 2008), but the PKS enzymes present might be capable of producing various other alkyl-substituted phloroglucinol and resorcinol derivatives.
The PKS activities were also followed through the development of the glandular trichomes on the female flowers (Fig. 3). The enzymes showed different patterns: the STS activity was more or less stable, reaching the highest level at days 35 for the ‘Skunk and Kompolti’ varieties and 37 for the ‘Fourway’ variety; whereas the other activities showed maximum activity at earlier stages of plant development. In particular, olivetol synthase, BUS and VPS showed the highest activity at 21–29 d. In the case of CHS activity, the peak is not as pronounced (significant, P < 0.05). Both varieties (Skunk and Fourway) show similar patterns of the PKS enzyme activities with time, as well as for the variety Kompolti on day 35. No activity for an olivetolic acid-forming PKS was detected during the time course of the growth and development of glandular trichomes on female flowers. However, HPLC and LC-MS analyses confirmed formation of olivetol (Rt 18.21 ± 0.24 min and m/z 181.2 [M + H]+; Supplementary Fig. S1) using hexanoyl-CoA as starter substrate.
Raharjo et al. (2004a) suggested that olivetol was formed by a PKS, and Kozubek and Tyman (1999) proposed that alkylresorcinols, such as olivetol, are formed from biosynthesized alkylresorcinolic acids by enzymatic decarboxylation or via modified fatty acid-synthesizing enzymes, where the olivetolic acid carboxylic group would be expected also to be attached either to ACP (acyl carrier protein) or to CoA. Thus, in the release of the molecule from the protein compartment in which it was attached or elongated, simultaneous decarboxylation of olivetolic acid may occur, otherwise the olivetolic acid would be the final product. PKS isolation and identification of genes forming alkylresorcinolic acids (Gaucher and Shepherd 1968, Gaisser et al. 1997, Funa et al. 2007) and stilbene carboxylic acids [Eckermann et al., 2003; Schröder Group (http://www.biologie.uni-freiburg.de/data/bio2/schroeder/stilbenecarboxylates.html)] have been reported. Conversion of tetraketides (free acids or lactones) synthesized in vivo by stilbene carboxylic acid synthases (Schröder Group) or by chemical synthesis (Money et al. 1967) into the carboxylic acids at a suitable pH (mildly acidic or basic conditions) has also been suggested. Raharjo et al. (2004a) did not observe any effect on the formation of olivetol by either the incubation time of the PKS assays or the mildly acidic conditions used. Enzymatic decarboxylation in vitro and in vivo, and purification of carboxylic acid decarboxylases has been reported from liverworts (Pryce 1972, Pryce and Linton 1974), lichens (Mosbach and Ehrensvard 1966) and microorganisms (Pettersson 1965, Huang et al. 1994, Dhar et al. 2007, Stratford et al. 2007). We did not observe formation of olivetol by an enzymatic or chemical decarboxylation from olivetolic acid (Supplementary Table S3). Although the recovery for the standards orcinolic acid and 2,4-dihydroxy-benzoic acid was >95%, no orcinol or resorcinol (1,3-dihydroxy-benzene) was detected; methyl-olivetolate was used as a negative control of decarboxylation. Purification of this olivetol-forming PKS is required in order to characterize it and analyze the mechanism of reaction. In addition, no activity was detected with benzoyl-CoA at pH 7.0, 7.5 or 8.0, and no BAS activity was found. Small amounts of derailment by-products were detected from the PKS assays.
Cannabinoid profiling by HPLC
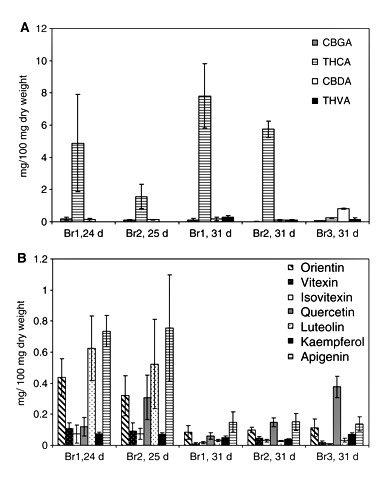
Fig. 4 shows the variations in the cannabinoid content with respect to tissues and the varieties (chemotypes) of the plants analyzed. In chemotype I or drug-type cannabis plants, an eight times higher concentration of Δ9-THCA was detected in female flowers than male flowers and there was no significant difference between the two varieties analyzed (P < 0.05). No significant differences were found in the Δ9-THCA contents in male flowers, fruits and male or female leaves in the chemotype I plants analyzed (P < 0.05). Previous studies confirmed that there is no significant difference in the cannabinoid content in leaves of the two genders from the same variety (Holley et al. 1975, Kushima et al. 1980). As we expected, in chemotype III or fiber-type plants the Δ9-THCA content in flowers (Kompolti) was less (7-fold) than the content in drug-type plants (Skunk and Fourway varieties). The Δ9-THCA contents in female leaves and male flowers were four times less than the contents in the drug-type cannabis plants analyzed. Δ9-THVA was only detected in male and female flowers, and fruits. The concentration of this cannabinoid in female flowers was more than seven and three times higher than the contents in fruits and male flowers, respectively. No significant differences were found among the three varieties of the plants analyzed (P < 0.05). The CBGA contents in female flowers and male and female leaves were not significantly different. The content of this cannabinoid in fruits was six times less than in female flowers. The CBGA concentration detected in male flowers was not significantly different from that of fruits, and no significant difference was observed in the three varieties analyzed (P < 0.05). CBDA was identified in flowers and leaves. In chemotype I plants, the CBDA content from female flowers was 2.6 times higher than in male flowers. The CBDA contents from leaves were not significantly different from those of male flowers, and no significant differences were observed in the two drug-type plants; but in male leaves from the variety Fourway the CBDA content was significantly different (P < 0.05). On the other hand, the CBDA content in the fiber-type plants was significantly different from the CBDA content in drug-type plants (P < 0.05). The CBDA content in female leaves from the variety Kompolti was six times higher than the contents from the varieties Fourway and Skunk, while the contents in male and female flowers from Kompolti plants were three times higher than the CBDA contents from Fourway and Skunk plants.
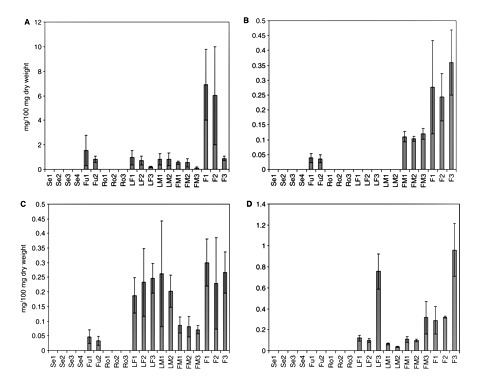
Cannabinoid content in different cannabis plant tissues. (A) Δ9-THCA; (B) Δ9-THVA; (C) CBGA; (D) CBDA. Br, bracts; Se, seedlings; Fu, fruits; Ro, roots; LF, female leaf; LM, male leaf; FM, male flower; F, female flower. 1, Skunk variety; 2, Fourway variety; 3, Kompolti variety; 4, Fasamo variety. Female flowers were from 35-day-old female flowers. Values are expressed as means of three replicates with standard deviations.
The increment on the concentration of cannabinoids corresponds to the development and growth of the glandular trichomes on the bracts (Table 1 and Fig. 5A). No significant differences were found in the CBGA contents from the three varieties of cannabis plants analyzed (Skunk, Fourway and Kompolti; P < 0.05). Although the cannabinoid content in the individual gland trichomes can vary with age, type and location (Turner et al. 1977, Turner et al. 1978), a correlation exists between glandular density and cannabinoid content at each stage of bract development (Turner et al. 1981). As CBGA is the precursor of Δ9-THCA and CBDA, its concentration decreased as it was observed during the time course of glandular trichome development. The Δ9-THCA content increased at day 31 and the contents of this cannabinoid were not significantly different in the two drug-type plants (Fourway and Skunk). On the other hand, Δ9-THVA accumulation started only after day 24 for Skunk and day 25 for Fourway. In the fiber-type plant Kompolti, the CBDA content at day 31 was seven times higher than the CBDA contents in the drug-type plants. Natural (plant decarboxylation) or artificial degradation (oxidation, isomerization, UV light) of cannabinoids occurred to a lesser extent in our plant material (Table 1). Neither cannabinoid acids nor their decarboxylated forms were found in seedlings and roots in the four varieties of cannabis plant analyzed (Skunk, Fourway, Kompolti and Fasamo). In male flowers, minimal amounts of cannabinoids have been detected (Potter 2004) due to the presence of glandular trichomes on the underside of the anther lobes; these are called antherial capitate-sessile glands (Mahlberg et al. 1984).
| Tissue | Cannabinoidsa (acid forms) | Δ9-THC | CBG | CBD | CBN | Total |
|---|---|---|---|---|---|---|
| Bracts: | ||||||
| Br1, 24 d | 5.19 | 0.42 ± 0.03 | – | – | – | 5.61 |
| Br1, 31 d | 8.40 | 0.12 ± 0.03 | – | – | 0.08 ± 0.002 | 8.60 |
| Br2, 25 d | 1.80 | – | – | – | – | 1.80 |
| Br2, 31 d | 5.97 | 0.08 ± 0.02 | – | – | 0.05 ± 0.041 | 6.10 |
| Br3, 31 d | 1.26 | 0.13 ± 0.01 | 0.16 ± 0.011 | – | – | 1.55 |
| Fruits: | ||||||
| Fu1 | 1.63 | 0.04 ± 0.02 | – | – | – | 1.67 |
| Fu2 | 0.87 | 0.10 ± 0.03 | – | – | – | 0.97 |
| Leaves: | ||||||
| Female | ||||||
| LF1 | 1.26 | 0.43 ± 0.31 | – | – | 0.06 ± 0.01 | 1.75 |
| LF2 | 1.04 | 0.36 ± 0.06 | – | – | – | 1.40 |
| LF3 | 1.21 | 0.10 ± 0.09 | 0.23 ± 0.02 | 0.12 ± 0.001 | – | 1.67 |
| Male | ||||||
| LM1 | 1.13 | – | – | – | – | 1.13 |
| LM2 | 1.07 | 0.37 ± 0.04 | – | – | 0.06 ± 0.010 | 1.50 |
| Flowers: | ||||||
| Female | ||||||
| F1 | 7.78 | 0.15 ± 0.006 | – | – | 0.09 ± 0.005 | 8.02 |
| F2 | 6.79 | 0.39 ± 0.004 | – | – | – | 7.18 |
| F3 | 2.48 | 0.10 ± 0.03 | – | – | – | 2.58 |
| Male | ||||||
| FM1 | 0.86 | – | – | – | – | 0.86 |
| FM2 | 0.84 | – | – | – | – | 0.84 |
| FM3 | 0.63 | – | – | – | – | 0.63 |
Flavonoid profiling by HPLC
As standards for most flavonoid glycosides are not commercially available, we proceeded to hydrolyze the samples in order to analyze the aglycones. Apigenin, luteolin, apigenin-7-O-Glc and luteolin-7-O-Glc were used as internal standards. The recovery percentage of aglycones from standards was >90% (Supplementary Table S4). Typical profiles corresponding to a standard mixture of the selected flavones and flavonols with our samples are shown in Supplementary Fig. S2, and analyses by LC-MS confirmed the identity of the aglycones (Supplementary Fig. S3).
Flavonoid content varied from one plant tissue to another (Fig. 6). No flavonoids were detected in roots. Orientin content in flowers and leaves did not differ significantly in different genders and varieties of the cannabis plant. The orientin contents in seedlings and fruits were 14 times less than the contents in leaves, and they were not significantly different for the chemotypes of the cannabis plants analyzed in this study. Vitexin contents in fruits were similar and the lowest of all plant tissues. Higher vitexin contents were detected in seedlings, and no significant differences were observed in the three varieties. Similarly, no significant differences in the contents of vitexin and isovitexin were found in the three varieties of cannabis plants analyzed (P < 0.05). The lowest isovitexin and quercetin contents were detected in fruits, and the highest amounts of quercetin in male flowers. No significant differences were observed in the content of this aglycone in the other tissues by gender and variety (P < 0.05). However, the quercetin content in male flowers from fiber-type plants (Kompolti) was significantly different from that of the drug-type plants (P < 0.05). The luteolin content in male flowers was significantly different from the content in leaves, but no significant difference was found among the three varieties of the male flowers or leaves (Skunk, Fourway and Kompolti; P < 0.05). The kaempferol distribution also did not show any significant difference for the three varieties. The contents of this aglycone in seedlings and female flowers were 17 times higher than the contents in fruits. In leaves, the apigenin contents were not significantly different by gender but the contents in flowers were significantly different by gender (P < 0.05). The lowest contents of this aglycone were detected in fruits. Luteolin and vitexin contents are similar to results reported by Vanhoenacker et al. (2002), but apigenin and orientin contents are higher in our samples. Though Raharjo (2004) only reported apigenin and luteolin in leaves and flowers of C. sativa Fourway plants, the contents were different from our results, probably because of differences in plant tissue age. In contrast to the cannabinoid accumulation during the growth and development of glandular trichomes, the flavonoid content decreased (Fig. 5B and Table 2). The isovitexin content was not significantly different during the growth and development of glandular trichomes on the bracts (P < 0.05). No significant differences were found in the flavonoid contents between the varieties Skunk and Fourway at days 24 and 25, respectively. However, the contents of the aglycones vitexin and quercetin were significantly different at day 31 (P < 0.05). The kaempferol content in bracts of the variety Kompolti (fiber-type) was significantly different compared with the varieties Skunk and Fourway (drug-type) at day 31 (P < 0.05). The isovitexin content in bracts from fiber-type (Kompolti) was 2.5 times less than the content in the bracts from drug-type plants, whereas the quercetin contents in bracts from fiber-type (Kompolti) plants were six times and twice higher than the contents in bracts from the varieties Skunk and Fourway (drug-type), respectively. Some studies suggest that flavonoid distribution could have a chemotaxonomic value in cannabis plants (Clark and Bohm 1979, Vanhoenacker et al. 2002).
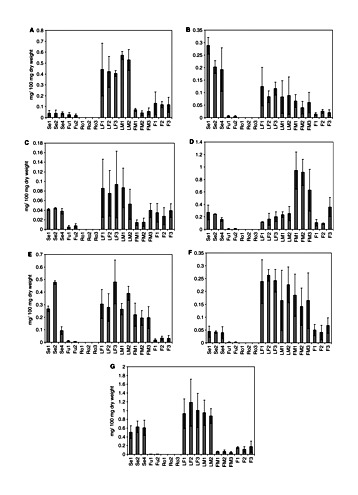
Flavonoid content in different cannabis plant tissues. (A) Orientin; (B) vitexin; (C) isovitexin; (D) quercetin; (E) luteolin; (F) kaempferol; (G) apigenin. Se, seedlings; Fu, fruits; Ro, roots; LF, female leaf; LM, male leaf; FM, male flower; F, female flower. 1, Skunk variety; 2, Fourway variety; 3, Kompolti variety; 4, Fasamo variety. 35-day-old female flowers. Values are expressed as means of three replicates with standard deviations.
| Tissue | Flavonoid total content (mg 100 mg–1 DW) |
|---|---|
| Bracts: | |
| Br1, 24 d | 2.18 |
| Br1, 31 d | 0.40 |
| Br2, 25 d | 2.14 |
| Br2, 31 d | 0.54 |
| Br3, 31 d | 0.74 |
| Fruits: | |
| Fu1 | 0.06 |
| Fu2 | 0.05 |
| Seedlings | |
| Se1 | 1.46 |
| Se2 | 1.67 |
| Se4 | 1.17 |
| Leaves: | |
| Female | |
| LF1 | 2.24 |
| LF2 | 2.37 |
| LF3 | 2.54 |
| Male | |
| LM1 | 2.36 |
| LM2 | 2.42 |
| Flowers: | |
| Female | |
| F1 | 1.56 |
| F2 | 1.41 |
| F3 | 1.19 |
| Male | |
| FM1 | 0.51 |
| FM2 | 0.46 |
| FM3 | 0.81 |
PKS activities and secondary metabolites in C. sativa
In plant tissues from C. sativa, in vitro PKS activities of CHS, STS, BUS and VPS, as well as activity for an olivetol-forming PKS were detected. Content analyses of cannabinoids and flavonoids, two secondary metabolites present in this plant (Flores-Sanchez and Verpoorte, 2008), revealed differences in their distribution, suggesting a diverse regulatory control in these biosynthetic fluxes in the plant. Apigenin, luteolin and kaempferol are widespread compounds in plants (Valant-Vetschera and Wollenweber 2006). Quercetin and kaempferol have a role in fertility of male flowers (Vogt et al. 1995, Napoli et al. 1999), and higher levels of these two flavonols in cannabis male flowers (Fig. 6) support this role. Moreover, protection from UV-B (280–315 nm) by flavone or flavonol glycosides has been reported (Lois and Buchanan 1994, Rozema et al. 2002) and their occurrence in aerial cannabis tissues should be vital. Furthermore, roles as growth regulators have been suggested (Ylstra et al. 1994, Gould and Lister 2006). Quercetin, apigenin and kaempferol can modulate auxin-mediated processes (Jacobs and Rubery 1988), and this role should not be excluded in cannabis. It has been reported that luteolin and apigenin derivatives acted as feeding deterrents to Lepidoptera larvae (Erhard et al. 2007). On the other hand, it is known that cannabinoids are cytotoxic compounds (Rothschild et al. 1977, Roy and Dutta 2003, Sirikantaramas et al. 2005) and they can act as plant defense compounds against predators such as insects. Moreover, a regulatory role in cell death has been suggested as cannabinoids have the ability to induce cell death through mitochondrial permeability transition (Morimoto et al. 2007). The accumulation of cannabinoids in bracts during the growth and development of glandular trichomes from flowers (Fig. 5A) could be related to floral protection and, consequently, during seed maturation, the cannabinoid content may decrease. Lower contents of cannabinoids were detected in fruits (seed and cup-like bracteole) than in female flowers (Table 1). Moreover, no significant differences were found between the cannabinoid and flavonoid contents in fruits, and they were not significantly different between the varieties Fourway and Skunk (P < 0.05). It seems that cannabinoid accumulation is correlated with maximum activities for an olivetol-forming PKS (Figs 3, 5A) and the CHS activity preceded the accumulation of flavonoids at day 24 (Figs 3, 5B). A significant STS-type activity was detected at day 35 (Fig. 3). Although significant enzymatic activities for VPS and BUS were also detected in crude protein extracts, no acylphloroglucinols have been identified in cannabis so far (Flores-Sanchez and Verpoorte 2008). Acylphloroglucinols and activities of VPS and BUS have been detected in Humulus lupulus (Paniego et al. 1999) and Hypericum perforatum (Hoelzl and Petersen 2003, Klingauf et al. 2005). It is known that PKS enzymes can use a broad range of substrates efficiently (Springob et al. 2000, Samappito et al. 2003, Novak et al. 2006) and probably the cannabis PKS enzymes also have this notorious in vitro substrate promiscuity. Zuurbier et al. (1988) showed that CHS and STS can have VPS- and BUS-type activities, and the VPS and BUS activities identified in this study could be from CHS or olivetol-forming PKS, or even from STS. Although significant CHS and STS activities were detected in crude protein extracts from roots (Fig. 2), no flavonoids were identified in these tissues (Fig. 6). There are no reports about isolation or detection of flavonoids and stilbenoids in roots (Flores-Sanchez and Verpoorte 2008) and this seems to contradict the CHS- and STS-type activities detected in roots. Low expression of the CHS-type PKS gene in roots and the absence of flavonoids in this plant tissue were previously reported (Raharjo 2004, Raharjo et al. 2004b). Stilbenoids have been isolated from cannabis leaves and resin (Flores-Sanchez and Verpoorte 2008), but they could not be identified in the methanol : water fractions from leaves and bracts by LC-MS analysis; this could be due to the low STS-type activity (Fig. 3). Gehlert and Kindl (1991) found a relationship between induced formation by wounding of stilbenes and the PKS BBS in orchids. Stilbenoid functions in plants include constitutive and inducible defense mechanisms (Chiron et al. 2001, Jeandet et al. 2002), plant growth inhibitors and dormancy factors (Gorham 1980).
It is known that induction of enzymatic activity in early steps of a biosynthetic pathway precedes the accumulation of final products (Fig. 7). The cannabinoid content in female flowers from drug-type plants (Skunk and Fourway varieties) was five times higher than the flavonoid content, but in female flowers from fiber-type plants (Kompolti variety) it was only twice the amount (Tables 1, 2). During the development of the glandular trichomes on the flowers, the activity of the olivetol-forming PKS at day 29 was seven times higher than the CHS activity (Fig. 3) and it was correlated with the cannabinoid accumulation (Table 1). Although, STS activity detected during the time course was low, it increased at the end, being five and 18 higher than the CHS and olivetol-forming PKS activities, respectively. This STS activity can be associated with precursor formation in stilbenoid biosynthesis. The profiling shown here suggests the presence of three PKS activities, one CHS type, one STS type and another for olivetol biosynthesis. Using the present approach, we believe that we have clear evidence for the presence of different PKS profiles in the different tissues; which are further supported by the phytochemical analyses of these tissues. The role of olivetol synthase in the plant needs further clarification. Its activity coincides with the occurrence of the cannabinoids. On the other hand, no activity of olivetolic acid synthase has been found in cannabis so far. This raises the question about whether the in planta activity of this enzyme could be different from its in vitro activity. Further studies are required to identify the substrate specificities of these individual PKS enzymes in cannabis plants. Purification and characterization of the PKS enzymes will be necessary to determine their catalytic potential and regulation, which may lead to the identification of their role in the plant.
Materials and Methods
Plant material
Seeds of C. sativa, drug-type varieties Skunk and Fourway (The Sensi Seed Bank, Amsterdam, The Netherlands), and fiber-type varieties Kompolti and Fasamo (Dr. D. Watson, HortaPharm, Amsterdam, The Netherlands), were germinated and 9-day-old seedlings were planted in 11 LC pots with soil (substrate 45 L, Holland Potgrond, Van der Knaap Group, Kwintsheul, The Netherlands) and maintained under a light intensity of 1,930 lux, at 26°C and 60 ± 7% relative humidity (RH). After 3 weeks, the small plants were transplanted into 10 liter pots for continued growth until flowering. To initiate flowering, 2-month-old plants were transferred to a photoperiod chamber (12 h light, 27°C and 37 ± 11% RH). Five day-old seedlings, young leaves from 13-week-old plants, female flowers and bracts at different stages of development, and male flowers from 4-month-old plants were harvested. Three-month-old male plants were used for pollination of female plants. The fruits were harvested 18 d after pollination. Roots from 4-month-old female plants were harvested and washed with cold water to remove residual soil. All vegetabl material was weighed and stored at –80°C.
Chemicals
Benzoyl-CoA, hexanoyl-CoA, isobutyryl-CoA, isovaleryl-CoA, malonyl-CoA, resveratrol, naringenin and 2,4-dihydroxy-benzoic acid were obtained from Sigma (St Louis, MO, USA). Olivetol was acquired from Aldrich Chem (Milwaukee, WI, USA) and 4-hydroxybenzyledeneacetone (pHBA) from Alfa Aesar (Karlsruhe, Germany). Orcinolic acid (orsellinic acid) was from AApin Chemicals Ltd (Abingdon, UK) and resorcinol (1,3-dihydroxy-benzene) from Merck Schuchardt (München, Germany). p-Coumaroyl-CoA was synthesized according to Stöckigt and Zenk (1975), and phlorisovalerophenone (PiVP) and phlorisobutyrophenone (PiBP) were previously synthesized in our laboratory (Fung et al. 1994). Olivetolic acid was obtained from hydrolysis of methyl olivetolate (Horper and Marner 1996), and methyl olivetolate was a gift from Professor Dr. J. Tappey (Virginia Military Institute, USA). The cannabinoids Δ9-THCA, CBGA, Δ9-THC, Δ8-THC, CBG, CBD and CBN were isolated from plant materials previously in our laboratory (Hazekamp et al. 2004). Δ9-THVA was identified based on its relative retention time and UV spectra (Hazekamp et al. 2005), and its quantification was relative to Δ9-THCA. The flavonoids kaempferol, orientin and luteolin were purchased from Extrasynthese (Genay, France), and vitexin, isovitexin and apigenin from Sigma-Aldrich (Buchs, Switzerland). Quercetin, apigenin-7-O-Glc and luteolin-7-O-Glc were from our standard collection. All chemical products and mineral salts were of analytical grade.
Protein extracts
Frozen plant material was homogenized in a mortar with nitrogen liquid, the powder was thawed in polyvinylpolypyrrolidone (PVPP) and extraction buffer [0.1 M potassium phosphate buffer, pH 7, 0.5 M sucrose, 3 mM EDTA, 10 mM dithiothreitol (DTT) and 0.1 mM leupeptin], squeezed through Miracloth and centrifuged at 14,000 r.p.m. for 20 min. Per each gram of fresh weight, 0.1 g of PVPP and 2 ml of extraction buffer were used. The crude protein extracts were desalted using Sephadex G-25 M (PD-10) columns and eluted with the same extraction buffer without addition of leupeptin. All steps were performed at 4°C.
PKS assays
PKS activity was measured by the conversion of starter CoA esters and malonyl-CoA into reaction products.
The standard reaction mixture, in a final volume of 500 μl, contained 50 mM K-Pi buffer (pH 7), 20 μM starter-CoA, 40 μM malonyl-CoA, 0.5 M sucrose and 1 mM DTT. The reaction was initiated by addition of 250 μl of crude desalted extracts (100–440 μg of protein) and was incubated for 90 min at 30°C. Reactions were stopped by addition of 20 μl of 4 N HCl, extracted twice with 800 μl of ethyl acetate and centrifuged for 2 min. The combined organic phases were evaporated in a vacuum centrifuge and the residue was kept at 4°C. Samples were resuspended in 100 and 40 μl of methanol for analysis by HPLC and LC-MS, respectively.
VPS was isolated previously in our laboratory (Paniego et al. 1999), and CHS and STS were a gift from Professor Dr. J. Schröder (Freiburg University, Germany).
Protein determination
Protein concentration was measured as described by Peterson (1977) with bovine serum albumin as standard.
HPLC analysis
The system consisted of a Waters 626 pump, a Waters 600S controller, a Waters 2996 photodiode array detector and a Waters 717 plus autosampler (Waters, Milford, MA, USA), equipped with a reversed-phase C18 column (250 mm×4.6 mm, Inertsil ODS-3, GL Sciences, Tokyo, Japan). A 80 μl aliquot of sample was injected; the gradient solvent system consisted of methanol and water, both containing 0.1% trifluoroacetic acid (TFA). Method (1) 0–40 min, 20–80% methanol; 40–43 min, 80% methanol; 43–48 min, 80–20% methanol; 40–50 min, 20% methanol. Method (2) 0–30 min, 40–60% methanol; 30–33 min, 60% methanol; 35–38 min, 60–40% methanol 38–40 min, 40% methanol. Method (3) 0–40 min, 40–60% methanol; 40–43 min, 60% methanol; 43–44 min, 40–60% methanol; 44–45 min, 40% methanol. Method (4) 0–40 min, 50–100% methanol; 40–43 min, 100% methanol; 43–44 min, 100–50% methanol; 44–45 min, 50% methanol. Method (5) 0–20 min, 50–80% methanol; 20–30 min, 80% methanol; 30–35 min, 80–50% methanol; 35–40 min, 50% methanol. The flow rate was 1 ml min–1 at 25°C; olivetol, methyl olivetolate, olivetolic acid, PiVP, PiBP, naringenin and resveratrol were detected at 280 nm, orcinolic acid at 260 nm, orcinol at 273 nm and 2,4-dihydroxy-benzoic acid at 256 nm. pHBA was detected at 320 nm. Calibration curves with the respective standards were made.
LC-MS analysis
For the confirmation of the identity of enzymatic products, 20 μl of samples were analyzed in an Agilent 1100 Series LC/MS system (Agilent Technologies, Palo Alto, CA, USA) with positive/negative atmospheric pressure chemical ionization (APCI), using elution system method 5 with a flow rate of 0.5 ml min–1. The optimum APCI conditions included a N2 nebulizer pressure of 45 p.s.i., a vaporizer temperature of 400°C, an N2 drying gas temperature of 350°C at 10 liters min–1, a capillary voltage of 4,000 V, a corona current of 4 μA and a fragmentor voltage of 100 V. A reversed-phase C18 column (150 mm×4.6 mm, 5 μm, Zorbax Eclipse XDB-C18, Agilent) was used.
Extraction of compounds
Extraction was carried out as described by Choi et al. (2004) with slight modifications. To 0.1 g of lyophilized and ground plant material was added 4 ml of MeOH : H2O (1 : 1, v/v) and 4 ml of CHCl3, vortexed for 30 s and sonicated for 10 min. The mixtures were centrifuged in the cold at 3,000 r.p.m. for 20 min. The MeOH : H2O and CHCl3 fractions were separated and evaporated. The extraction was performed twice. The extracts were resuspended on 1 ml of MeOH : H2O (1 : 1) and CHCl3, respectively, for the subsequent cannabinoid and flavonoid analyses.
Cannabinoid analysis by HPLC
The column used was a Grace Vydac (WR Grace, Columbia, MD, USA) C18 (250 mm×4.6 mm, Mass Spec 5 μm) with a Waters Bondapak C18 guard column (2 mm×20 mm, 50 μm). The solvent system and the operational conditions were the same as previously reported by Hazekamp et al. (2004). For preparation of samples, 100 μl of the CHCl3 fraction from extraction was evaporated using N2 gas. The samples were dissolved in 1 ml of EtOH and 20 μl was injected in the HPLC system. Cannabinoids were detected at 228 nm. Calibration curves with their respective standards were made.
Flavonoid analysis by HPLC
A reversed-phase C18 column (250 mm×4.6 mm, Inertsil ODS-3) was used. The solvent system and the operational conditions were as described by Justesen et al. (1998) with slight modifications. The mobile phase consisted of MeOH : H2O (30 : 70, v/v) with 0.1% TFA (A) and MeOH with 0.1% TFA (B). The gradient was 25–86% B in 40 min followed by 86% B for 5 min and a gradient step from 86 to 25% B for 5 min at a flow-rate of 1 ml min–1 and at 25°C. A 20 μl aliquot of resuspended hydrolyzed samples was injected. Retention times for aglycones were as follows: apigenin 23.02 min, kaempferol 21.95 min, luteolin 18.37 min, quercetin 16.37 min, isovitexin 5.32 min, vitexin 4.71 min and orientin 3.64 min; and for apigenin-7-O-Glc 10.7 min and luteolin-7-O-Glc 7.42 min. Flavones and flavonols were detected at their maximal UV absorbance (quercetin, 255 nm; kaempferol, 265.8 nm; apigenin, isovitexin, vitexin and apigenin-7-O-Glc, 270 nm; and orientin, luteolin and luteolin-7-O-Glu, 350 nm). The flow rate was 1 ml min–1 at 25°C. Calibration curves with their respective standards were made. The standards apigenin and vitexin were dissolved in MeOH : dimethylsulfoxide (DMSO) (7 : 3), orientin in MeOH : DMSO (8 : 2, v/v), apigenin-7-O-Glc and luteolin-7-O-Glc in MeOH : DMSO (9 : 1, v/v) and the remainder only in MeOH.
The optimum APCI conditions for LC-MS analyses were as described above.
Acid hydrolysis for flavonoids
A 500 μl aliquot of the MeOH : H2O fraction from the extraction was hydrolyzed at 90°C for 60 min with 500 μl of 4 N HCl to which 2 mg of the antioxidant tert-butylhydroquinone was added. Hydrolysates were extracted with ethyl acetate three times. The organic phase was dried over anhydrous NaSO4 and evaporated with N2 gas.
Statistics
All data were analyzed by MultiExperiment Viewer MEV 4.0 software (Saeed et al. 2003). For analyses involving two, and three or more groups, paired t-tests and analysis of variance (ANOVA) were used, respectively, with α = 0.05 for significance.
Funding
Consejo Nacional de Ciencia y Tecnología (Mexico) partial grant (to I.J.F-S).
ACKNOWLEDGEMENTS
We thank J. Fei for growing C. sativa ‘Kompolti’ plants, A. Hazekamp for technical assistance with the cannabinoid and flavonoid analyses by LC-MS and HPLC, and Dr. A. Garza-Ortiz for technical assistance with me-olivetolate hydrolysis.
REFERENCES
| Abe I, Takahashi Y, Morita H, Noguchi H. Benzalacetone synthase: a novel polyketide synthase that plays a crucial role in the biosynthesis of phenylbutanones in Rheum palmatum, Eur. J. Biochem. , 2001, vol. 268 (pg. 3354-3359) Google ScholarCrossRefPubMed |
| Abe T, Morita H, Noma H, Kohno T, Noguchi H, Abe I. Structure function analyses of benzalacetone synthase from Rheum palmatum, Bioorg. Med. Chem. Lett. , 2007, vol. 17 (pg. 3161-3166) Google ScholarCrossRefPubMed |
| Austin MB, Noel JP. The chalcone synthase superfamily of type III polyketide synthases, Nat. Prod. Rep. , 2003, vol. 20 (pg. 79-110) Google ScholarCrossRefPubMed |
| Clark MN, Bohm BA. Flavonoid variation in Cannabis L, Bot. J. Linn. Soc. , 1979, vol. 79 (pg. 249-257) Google ScholarCrossRef |
| Chiron H, Drouet A, Lieutier F, Payer HD, Ernst D, Sanderman HJ. Gene induction of stilbene biosynthesis in Scot pine in response to ozone treatment, wounding and fungal infection, Plant Physiol. , 2000, vol. 124 (pg. 865-872) Google ScholarCrossRefPubMed |
| Choi YH, Kim HK, Hazekamp A, Erkelens C, Lefeber A.WM, Verpoorte R. Metabolomic differentiation of Cannabis sativa cultivars using 1H NMR spectroscopy and principal component analyses, J. Nat. Prod. , 2004, vol. 67 (pg. 953-957) Google ScholarCrossRefPubMed |
| Dhar A, Lee KS, Dhar K, Rosazza J.PN. Nocardia sp. vanillic acid decarboxylase, Enzyme Microb. Technol. , 2007, vol. 41 (pg. 271-277) Google ScholarCrossRef |
| Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology, Chem. Biol. , 2007, vol. 14 (pg. 741-756) Google ScholarCrossRefPubMed |
| Di Marzo V, De Petrocellis L. Plant, synthetic and endogenous cannabinoids in medicine, Annu. Rev. Med. , 2006, vol. 57 (pg. 553-574) Google ScholarCrossRefPubMed |
| Eckermann C, Schröder G, Eckermann S, Strack D, Schmidt J, Schneider B, et al. Stilbenecarboxylate biosynthesis: a new function in the family of chalcone synthase-related proteins, Phytochemistry , 2003, vol. 62 (pg. 271-286) Google ScholarCrossRefPubMed |
| ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids, Life Sci. , 2005, vol. 78 (pg. 539-548) Google ScholarCrossRefPubMed |
| Erhard D, Pohnert G, Gross EM. Chemical defense in Elodea nuttallii reduces feeding and growth of aquatic herbivorous Lepidoptera, J. Chem. Ecol. , 2007, vol. 33 (pg. 1646-1661) Google ScholarCrossRefPubMed |
| Fellermeier M, Eisenreich W, Bacher A, Zenk MH. Biosynthesis of cannabinoids: incorporation experiments with 13C-labeled glucoses, Eur. J. Biochem. , 2001, vol. 268 (pg. 1596-1604) Google ScholarCrossRefPubMed |
| Fellermeier M, Zenk MH. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol, FEBS Lett. , 1998, vol. 427 (pg. 283-285) Google ScholarCrossRefPubMed |
| Flores-Sanchez IJ, Verpoorte R. Secondary metabolism in cannabis, Phytochem. Rev. , 2008, vol. 7 (pg. 615-639) Google ScholarCrossRef |
| Funa N, Awakawa T, Horinouchi S. Pentaketide resorcylic acid synthesis by type III polyketide synthase from Neurospora crassa, J. Biol. Chem. , 2007, vol. 282 (pg. 14476-14481) Google ScholarCrossRefPubMed |
| Fung SY, Brussee J, Van der Hoeven R.AM, Niessen W.MA, Scheffer J.JC, Verpoorte R. Analysis of proposed aromatic precursors of hop bitter acids, J. Nat. Prod. , 1994, vol. 57 (pg. 452-459) Google ScholarCrossRef |
| Gaisser S, Trefzer A, Stockert S, Kirschning A, Bechthold A. Cloning of an avilamycin biosynthetic gene cluster from Streptomyces viridochromogenes Tü57, J. Bacteriol. , 1997, vol. 179 (pg. 6271-6278) Google ScholarCrossRefPubMed |
| Gaucher GM, Shepherd MG. Isolation of orsellinic acid synthase, Biochem. Biophys. Res. Commun. , 1968, vol. 32 (pg. 664-671) Google ScholarCrossRefPubMed |
| Gehlert R, Kindl H. Induced formation of dihydrophenanthrenes and bibenzyl synthase upon destruction of orchid mycorrhiza, Phytochemistry , 1991, vol. 30 (pg. 457-460) Google ScholarCrossRef |
| Gorham J. Reinhold L, Harbome JB, Swain T. The stilbenoids, Progress in Phytochemistry , 1980, vol. Vol. 6 OxfordPergamon Press(pg. 203-252) |
| Gould KS, Lister C. Andersen ØM, Markham KR. Flavonoid functions in plants, Flavonoids: Chemistry, Biochemistry and Applications , 2006Boca Raton, FLCRC Press-Taylor & Francis Group(pg. 397-441) |
| Hazekamp A, Peltenburg-Looman A, Verpoorte R, Giroud C. Chromatographic and spectroscopic data of cannabinoids from Cannabis sativa L, J. Liq. Chromatogr. Relat. Technol. , 2005, vol. 28 (pg. 2361-2382) Google ScholarCrossRef |
| Hazekamp A, Simons R, Peltenburg-Looman A, Sengers M, van Zweden R, Verpoorte R. Preparative isolation of cannabinoids from Cannabis sativa by centrifugal partition chromatography, J. Liq. Chromatogr. Relat. Technol. , 2004, vol. 27 (pg. 2421-2439) Google ScholarCrossRef |
| Hoelzl J, Petersen M. Chemical constituents of Hypericum spp, Med. Aromat. Plant-Ind. Profiles , 2003, vol. 31 (pg. 77-93) |
| Holley JH, Hadley KW, Turner CE. Constituents of Cannabis sativa L. XI: Cannabidiol and cannabichromene in samples of known geographical origin, J. Pharm. Sci. , 1975, vol. 64 (pg. 892-895) Google ScholarCrossRefPubMed |
| Horper W, Marner FJ. Biosynthesis of primin and miconidin and its derivatives, Phytochemistry , 1996, vol. 41 (pg. 451-456) Google ScholarCrossRef |
| Huang Z, Dostal L, Rosazza J.PN. Purification and characterization of a ferulic acid decarboxylase from Pseudomonas fluorescens, J. Bacteriol. , 1994, vol. 176 (pg. 5912-5918) Google ScholarCrossRefPubMed |
| Jacobs M, Rubery PH. Naturally occurring auxin transport regulators, Science , 1988, vol. 241 (pg. 346-349) Google ScholarCrossRefPubMed |
| Jeandet P, Douillet-Breuil AC, Bessis R, Debord S, Sbaghi M, Adrian M. Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity and metabolism, J. Agric. Food Chem. , 2002, vol. 50 (pg. 2731-2741) Google ScholarCrossRefPubMed |
| Justesen U, Knuthsen P, Leth T. Quantitative analyses of flavonols, flavones and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection, J. Chromatogr. A , 1998, vol. 799 (pg. 101-110) Google ScholarCrossRefPubMed |
| Klingauf P, Beuerle T, Mellenthin A, El-Moghazy S.AM, Boubakir Z, Beerhues L. Biosynthesis of the hyperforin skeleton in Hypericum calycimun cell cultures, Phytochemistry , 2005, vol. 66 (pg. 139-145) Google ScholarCrossRefPubMed |
| Kozubek A, Tyman JH. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity, Chem. Rev. , 1999, vol. 99 (pg. 1-26) Google ScholarCrossRefPubMed |
| Kreuzaler F, Hahlbrock K. Enzymatic synthesis of aromatic compounds in higher plants: formation of naringenin (5,7,4′-trihydroxyflavanone) from p-coumaroyl coenzyme A and malonyl coenzyme A, FEBS Lett. , 1972, vol. 28 (pg. 69-72) Google ScholarCrossRefPubMed |
| Kushima H, Shoyama Y, Nishioka I. Cannabis. XII: variations of cannabinoid contents in several strains of Cannabis sativa L. with leaf-age, season and sex, Chem. Pharm. Bull. , 1980, vol. 28 (pg. 594-598) Google ScholarCrossRef |
| Liu B, Raeth T, Beuerle T, Beerhues L. Biphenyl synthase, a novel type III polyketide synthase, Planta , 2007, vol. 225 (pg. 1495-1505) Google ScholarCrossRefPubMed |
| Lois R, Buchanan BB. Severe sensitivity to ultraviolet radiation in an Arabidopsis mutant deficient in flavonoid accumulation. II. Mechanisms of UV-resistance in Arabidopsis, Planta , 1994, vol. 194 (pg. 504-509) Google ScholarCrossRef |
| Mahlberg PG, Hammond CT, Turner JC, Hemphill JK. Rodriguez E, Healey PL, Mehta I. Structure, development and composition of glandular trichomes of Cannabis sativa L, Biology and Chemistry of Plant Trichomes , 1984New YorkPlenum Press(pg. 23-51) |
| Money T, Comer FW, Webster G.RB, Wright IG, Scott AI. Pyrone studies. I: biogenetic-type synthesis of phenolic compounds, Tetrahedron , 1967, vol. 23 (pg. 3435-2448) Google ScholarCrossRef |
| Morimoto S, Komatsu K, Taura F, Shoyama Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa, Phytochemistry , 1998, vol. 49 (pg. 1525-1529) Google ScholarCrossRefPubMed |
| Morimoto S, Tanaka Y, Sasaki K, Tanaka H, Fukamizu T, Shoyama Y, et al. Identification and characterization of cannabinoids that induce cell death through mitochondrial permeability transition in Cannabis leaf cells, J. Biol. Chem. , 2007, vol. 282 (pg. 20739-20751) Google ScholarCrossRefPubMed |
| Morimoto S, Taura F, Shoyama Y. Biosynthesis of cannabinoids in Cannabis sativa L, Curr. Top. Phytochem. , 1999, vol. 2 (pg. 103-113) |
| Mosbach K, Ehrensvard U. Studies on lichen enzymes. Part I. Preparation and properties of a depside hydrolyzing esterase and of orsellinic acid decarboxylase, Biochem. Biophys. Res. Commun. , 1966, vol. 22 (pg. 145-150) Google ScholarCrossRefPubMed |
| Napoli CA, Fahy D, Wang HY, Taylor LP. White anther: a petunia mutant that abolishes pollen flavonol accumulation, induces male sterility, and is complemented by a chalcone synthase transgene, Plant Physiol. , 1999, vol. 120 (pg. 615-622) Google ScholarCrossRefPubMed |
| Novak P, Krofta K, Matousek J. Chalcone synthase homologues from Humulus lupulus: some enzymatic properties and expression, Biol. Plant. , 2006, vol. 50 (pg. 48-54) Google ScholarCrossRef |
| Okada Y, Yamazaki Y, Suh DY, Sankawa U. Bifunctional activities of valerophenone synthase in Hop (Humulus lupulus L.), J. Amer. Soc. Brew. Chem. , 2001, vol. 59 (pg. 163-166) |
| Paniego NB, Zuurbier K.WM, Fung SY, van der Heijden R, Scheffer J.JC, Verpoorte R. Phlorisovalerophenone synthase, a novel polyketide synthase from hop (Humulus lupulus L.) cones, Eur. J. Biochem. , 1999, vol. 262 (pg. 612-616) Google ScholarCrossRefPubMed |
| Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable, Anal. Biochem. , 1977, vol. 83 (pg. 346-356) Google ScholarCrossRefPubMed |
| Pettersson G. An orsellinic acid decarboxylase isolated from Gliocladium roseum, Acta Chem. Scand. , 1965, vol. 19 (pg. 2013-2021) Google ScholarCrossRef |
| Potter D. Guy GW, Whittle BA, Robson PJ. Growth and morphology of medicinal cannabis, The Medicinal Uses of Cannabis and Cannabinoids , 2004LondonPharmaceutical Press(pg. 17-54) |
| Pryce RJ. Metabolism of lunularic acid to a new plant stilbene by Lunularia cruciata, Phytochemistry , 1972, vol. 11 (pg. 1355-1364) Google ScholarCrossRef |
| Pryce RJ, Linton L. Lunularic acid decarboxylase from the liverwort Conocephalum conicum, Phytochemistry , 1974, vol. 13 (pg. 2497-2501) Google ScholarCrossRef |
| Raharjo TJ. Studies of cannabinoid biosynthesis in Cannabis sativa L.: the polyketide synthase, PhD Thesis , 2004Leiden University, The Netherlands |
| Raharjo TJ, Chang WT, Choi YH, Peltenburg-Looman A.MG, Verpoorte R. Olivetol as product of a polyketide synthase in Cannabis sativa L, Plant Sci. , 2004, vol. 166 (pg. 381-385) Google ScholarCrossRef |
| Raharjo TJ, Chang WT, Verberne MC, Peltenburg-Looman A.MG, Linthorst H.JM, Verpoorte R. Cloning and over-expression of a cDNA encoding a polyketide synthase from Cannabis sativa, Plant Physiol. Biochem. , 2004, vol. 42 (pg. 291-297) Google ScholarCrossRefPubMed |
| Robert N, Ferran J, Breda C, Coutos-Thevenot P, Boulay M, Buffard D, et al. Molecular characterization of the incompatible interaction of Vitis vinifera leaves with Pseudomonas syringae pv. pisi: expression of genes coding for stilbene synthase and class 10 PR protein, Eur. J. Plant Pathol. , 2001, vol. 107 (pg. 249-261) Google ScholarCrossRef |
| Rothschild M, Rowan MR, Fairbairn JW. Storage of cannabinoids by Arctia caja and Zonocerus elegans fed on chemically distinct strains of Cannabis sativa, Nature , 1977, vol. 266 (pg. 650-651) Google ScholarCrossRefPubMed |
| Roy B, Dutta BK. In vitro lethal efficacy of leaf extract of Cannabis sativa on the larvae of Chironomous samoensis Edward: an insect of public health concern, Indian J. Exp. Biol. , 2003, vol. 41 (pg. 1338-1341) Google ScholarPubMed |
| Rozema J, Bjorn LO, Bornman JF, Gaberscik A, Hader DP, Trost T, et al. The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analyses of the evolution of UV-absorbing compounds, J. Photochem. Photobiol. B: Biol. , 2002, vol. 66 (pg. 2-12) Google ScholarCrossRef |
| Rupprich N, Kindl H. Stilbene synthases and stilbenecarboxylate synthases, I: enzymatic synthesis of 3,5,4′-trihydroxystilbene from p-coumaroyl coenzyme A and malonyl coenzyme A, Hoppe Seyler Z. Physiol. Chem. , 1978, vol. 359 (pg. 165-172) |
| Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, et al. TM4: a free, open-source system for microarray data management and analysis, Biotechniques , 2003, vol. 34 (pg. 374-378) Google ScholarPubMed |
| Samappito S, Page J, Schmidt J, De-Eknamkul W, Kutchan TM. Aromatic and pyrone polyketides synthesized by a stilbene synthase from Rheum tataricum, Phytochemistry , 2003, vol. 62 (pg. 313-323) Google ScholarCrossRefPubMed |
| Schöppner A, Kindl H. Purification and properties of a stilbene synthase from induced cell suspension cultures of peanut, J. Biol. Chem. , 1984, vol. 259 (pg. 6806-6811) Google ScholarPubMed |
| Schröder J. A family of plant-specific polyketide synthases: facts and predictions, Trends Plant Sci. , 1997, vol. 2 (pg. 373-378) Google ScholarCrossRef |
| Schröder J, Heller W, Hahlbrock K. Flavanone synthase: simple and rapid assay for the key enzyme of flavonoid biosynthesis, Plant Sci. Lett. , 1979, vol. 14 (pg. 281-286) Google ScholarCrossRef |
| Shoyama Y, Yagi M, Nishioka I. Biosynthesis of cannabinoid acids, Phytochemistry , 1975, vol. 14 (pg. 2189-2192) Google ScholarCrossRef |
| Sirikantaramas S, Morimoto S, Shoyama Y, Ishikawa Y, Wada Y, Shoyama Y, et al. The gene controlling marijuana psychoactivity; molecular cloning and heterologous expression of Δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L, J. Biol. Chem. , 2004, vol. 279 (pg. 39767-39774) Google ScholarCrossRefPubMed |
| Sirikantaramas S, Taura F, Tanaka Y, Ishikawa Y, Morimoto S, Shoyama Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes, Plant Cell Physiol. , 2005, vol. 46 (pg. 1578-1582) Google ScholarCrossRefPubMed |
| Springob K, Lukacin R, Ernwein C, Groning I, Matern U. Specificities of functionally expressed chalcone and acridone synthases from Ruta graveolens, Eur. J. Biochem. , 2000, vol. 267 (pg. 6552-6559) Google ScholarCrossRefPubMed |
| Stöckigt J, Zenk MH. Chemical syntheses and properties of hydroxycinnamoyl-coenzyme A derivatives, Z. Naturforsch. C , 1975, vol. 30 (pg. 352-358) Google ScholarPubMed |
| Stratford M, Plumridge A, Archer DB. Decarboxylation of sorbic acid by spoilage yeasts is associated with the PAD1 gene, Appl. Environ. Microbiol. , 2007, vol. 73 (pg. 6534-6542) Google ScholarCrossRefPubMed |
| Taura F, Sirikantaramas S, Shoyama Y, Yoshikai K, Shoyama Y, Morimoto S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa, FEBS Lett. , 2007, vol. 581 (pg. 2929-2934) Google ScholarCrossRefPubMed |
| Turner J, Hemphill J, Mahlberg PG. Gland distribution and cannabinoid content in clones of Cannabis sativa L, Amer. J. Bot. , 1977, vol. 64 (pg. 687-693) Google ScholarCrossRef |
| Turner J, Hemphill J, Mahlberg PG. Quantitative determination of cannabinoids in individual glandular trichomes of Cannabis sativa L. (Cannabaceae), Amer. J. Bot. , 1978, vol. 65 (pg. 1103-1106) Google ScholarCrossRef |
| Turner J, Hemphill J, Mahlberg PG. Interrelationships of glandular trichomes and cannabinoid content. I: developing pistillate bracts of Cannabis sativa L. (Cannabaceae), Bull. Narc. , 1981, vol. 33 (pg. 59-69) Google ScholarPubMed |
| Valant-Vetschera KM, Wollenweber E. Andersen ØM, Markham KR. Flavones and flavonols, Flavonoids: Chemistry, Biochemistry and Applications , 2006Boca Raton, FLCRC Press-Taylor & Francis Group(pg. 617-748) |
| Vanhoenacker G, Van Rompaey P, De Keukeleire D, Sandra P. Chemotaxonomic features associated with flavonoids of cannabinoid-free cannabis (Cannabis sativa subsp. sativa L.) in relation to hops (Humulus lupulus L.), Nat. Prod. Lett. , 2002, vol. 16 (pg. 57-63) Google ScholarCrossRefPubMed |
| Vogt T, Wollenweber E, Taylor LP. The structural requirements of flavonols that induce pollen germination of conditionally male fertile Petunia, Phytochemistry , 1995, vol. 38 (pg. 589-592) Google ScholarCrossRef |
| Whitehead IM, Dixon RA. Chalcone synthase from cell suspension cultures of Phaseolus vulgaris L, Biochem. Biophys. Acta , 1983, vol. 747 (pg. 298-303) |
| Williamson EM, Evans FJ. Cannabinoids in clinical practice, Drugs , 2000, vol. 60 (pg. 1303-1314) Google ScholarCrossRefPubMed |
| Ylstra B, Busscher J, Franken J, Hollman P.CH, Mol J.NM, van Tunen AJ. Flavonols and fertilization in Petunia hybrida: localization and mode of action during pollen tube growth, Plant J. , 1994, vol. 6 (pg. 201-212) Google ScholarCrossRef |
| Zuurbier K.WM, Leser J, Berger T, Hofte A.JP, Schröder G, Verpoorte R, et al. 4-Hydroxy-2-pyrone formation by chalcone and stilbene synthase with nonphysiological substrates, Phytochemistry , 1998, vol. 49 (pg. 1945-1951) Google ScholarCrossRefPubMed |
Abbreviations:
- APCIatmospheric pressure chemical ionization
- BASbenzalacetone synthase
- BBSbibenzyl synthase
- BUSisobutyrophenone synthase
- CBCAcannabichromenic acid
- CBDcannabidiol
- CBDAcannabidiolic acid
- CBGcannabigerol
- CBGAcannabigerolic acid
- CBNcannabinodiol
- CHSchalcone synthase
- Δ8-THCΔ8-tetrahydrocannabinol
- Δ9-THCΔ9-tetrahydrocannabinol
- Δ9-THCAΔ9-tetrahydrocannabinolic acid
- Δ9-THVAΔ9-tetrahydrocannabivarinic acid
- DMSOdimethylsulfoxide
- DOXP/MEPdeoxyxylulose phosphate/methyl-erythritol phosphate
- DTTdithiothreitol
- GPPgeranyl diphosphate
- HEDS/HvCHShomoeriodictyol/eriodictyol synthase
- LC-MSliquid chromatography–mass spectrometry
- PiBPphlorisobutyrophenone
- PiVPphlorisovalerophenone
- PKSpolyketide synthase
- PVPPpolyvinylpolypyrrolidone
- Rtretention time
- STCSstilbene carboxylate synthase
- STSstilbene synthase
- TFAtrifluoroacetic acid
- VPSphlorisovalerophenone synthase
© The Author 2008. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For permissions, please email: journals.permissions@oxfordjournals.org
Supplementary data
Supplementary Data
– zip file
Related articles in
-
- Identification of Reference Genes for RT-qPCR Expression Analysis in Arabidopsis and Tomato Seeds
- Nitrogen Partitioning in the Photosynthetic Apparatus of Plantago asiatica Leaves Grown Under Different Temperature and Light Conditions: Similarities and Differences Between Temperature and Light Acclimation
- Two WUSCHEL-related homeobox Genes, narrow leaf2 and narrow leaf3, Control Leaf Width in Rice
- Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green
- Stress Enhances the Synthesis of Secondary Plant Products: The Impact of Stress-Related Over-Reduction on the Accumulation of Natural Products