Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Jul 21;40:100828. doi: 10.1016/j.bbih.2024.100828
Abstract
Recently, the diagnosis of autism spectrum disorder (ASD) has increased from 1 in 150 to every 1 in 36 children in the United States, warranting a need for novel prevention and therapeutic strategies. Broad-spectrum cannabidiol oil, free from delta-9-tetrahydrocannabinol, the psychoactive component of cannabis, may be one such therapeutic. It has a high safety profile and is frequently used as a complementary and integrative intervention by persons experiencing symptoms of anxiety, stress, and inflammation. Using a neurodevelopmental rat model of ASD (based on neuroinflammation induced by stress and terbutaline exposure during pre- and postnatal development), we sought to prevent the development of ASD-like behaviors in male offspring by administering broad-spectrum cannabidiol oil to dams throughout pregnancy (10 mg/kg, i.p., daily, embryonic days 3–16). To assess an ASD-like phenotype in the offspring, we used three behavioral measures relevant to three core ASD symptoms: 1) social communication (time spent vocalizing when alone); 2) repetitive behavior (marbles buried during a marble burying test); and 3) social interaction (time spent interacting with a novel conspecific during the three-chamber social interaction test). Broad-spectrum cannabidiol oil given during pregnancy decreased scores for all three ASD-related behavioral responses, resulting in an overall significant prevention of the ASD-like phenotype. These findings highlight the potential of broad-spectrum cannabidiol oil as a complementary and integrative approach for prevention of stressor-induced sequelae relevant to development of an ASD-like phenotype.
Highlights
- •
CBD was tested in preventing autistic-like behaviors produced by neuroinflammation.
- •
CBD prevented an overall autistic-like phenotype from developing in male rats.
- •
CBD may be an appropriate complementary prenatal neuroinflammatory preventative.
1. Introduction
Autism spectrum disorder (ASD) has risen in prevalence from 2000 to 2020 in the U.S. (Zablotsky et al., 2019; Maenner et al., 2023). This increase in prevalence highlights a need for prevention strategies (Insel and Scolnick, 2006). A suspected causal component of ASD is the pattern of cytokines expressed by maternal immune cells during pregnancy (Meyer et al., 2006, 2008, 2011). Recently, a paradigm shift in thought concerning stress and inflammation has spurred the development of environmental rodent models of ASD (Bronson et al., 2014; Bercum et al., 2015; Fonken et al., 2018; Frank et al., 2020; Smith et al., 2020). Indeed, within our rat developmental model of ASD, which utilizes maternal stress and terbutaline administration in early postnatal development of the offspring (terbutaline is a tocolytic β2 adrenergic receptor agonist known to be an environmental risk factor for ASD in humans and ASD-like behaviors in rats), an increase in neuroinflammatory markers have been observed (Bercum et al., 2015; Smith et al., 2020). Additionally, rat offspring in this model display behavioral deficits in social interaction, repetitive behaviors, and social communication, all of which are hallmark characteristics of ASD (American Psychiatric Association, 2013; Bercum et al., 2015; Smith et al., 2020).
Broad-spectrum cannabidiol (CBD) hemp extract oil may be an effective intervention for preventing neuroinflammation and/or cytokine imbalances in maternal, placental, and fetal tissue. Cannibidiol acts as an agonist at cannabinoid receptor 2 on microglia, which results in microglial cells expressing anti-inflammatory molecules (Komorowska-Muller and Schmole, 2020). Additionally, CBD is lipophilic and capable of crossing the placenta (Chayasirisobhon, 2020), which is important because maternal cytokines are capable of crossing the placental barrier as well. Here, we conducted a brief investigation of CBD’s therapeutic potential as a preventative intervention in our rat developmental model of ASD by assessing behavioral outcomes in a small cohort of male rats. We hypothesized that daily administration of CBD given to dams during pregnancy would prevent the development of ASD-like behaviors in male offspring.
2. Materials and methods
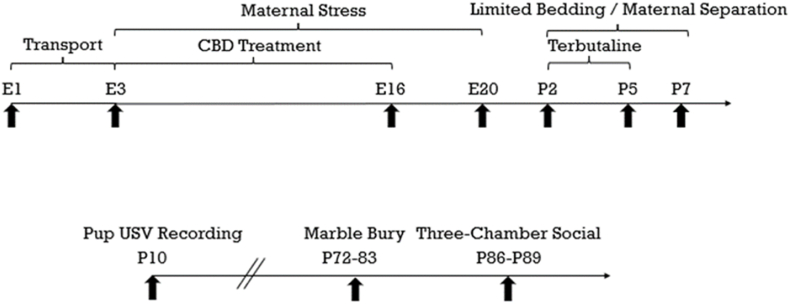
On E1 (embryonic day 1), timed mated Sprague Dawley dams were shipped and arrived on E3 (Fig. 1, N = 12; ENVIGO (now Inotiv), Indianapolis, IN, USA). Dams were randomly assigned to one of four groups: No-Stress/No-Terbutaline + Vehicle (No ST/Veh), No-Stress/No-Terbutaline + CBD (No ST/CBD), Stress/Terbutaline + Vehicle (ST/Veh), Stress/Terbutaline + CBD (ST/CBD) (n = 3 per group). Dams were given diamond twists (Teklad 7979C.CS Certified/Irradiated, Cat. No. NC1738670, ENVIGO) for environmental enrichment and were single housed. Food and water were provided ad libitum (20 ± 1 °C; 12 h light/dark cycle, 0700–1900). All procedures were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee and were conducted in an AAALAC-accredited facility.
Fig. 1.
2.1. Cannabidiol administration
Dams received CBD daily from E3 – E16. CBD was administered (1000 h) 1 h prior to the shock stress regimen. CBD solutions were made fresh immediately before injection (dissolved in 2% Tween 80 in sterile saline, 10 mg/kg, i.p.; 100 mg/mL CBD, organic MCT oil, CO2 extracted, batch #CB20219, Colorado Botanicals, Denver, CO, USA). Supplemental Fig. 1 contains the certificate of analysis performed by Botanacor Laboratories™ (Denver, CO, USA).
2.2. Maternal stress shock regimen
Dams began the maternal stress shock regimen on E3 (see Smith et al., 2020 for details). Briefly, the regimen consisted of an initial habituation day (E3) followed by a shock day (E4; two 1 mA foot shocks, 1 s duration, 60 s interval; Modular Shock Floor Model H10-11R/M-TC-SF and H13-15 Precision-Regulated Shocker, Coulbourn Instruments, Allentown, PA, USA) and two context days (E5 and E6). This schedule (shock-context-context) was repeated from E7 – E20 (E10 and E17 were rest days). This procedure was conducted between the hours 1100–1300.
2.3. Cross-fostering
Dams and their litters were left undisturbed from E21 through P1 (postnatal day 1). On P2, pups were counted, females were culled (n = 59), and males were cross-fostered to dams within their treatment groups (N = 36). Only male pups were examined in this study due to the prevalence of ASD among boys being 3.8 times higher than for girls (Maenner et al., 2023). No dam fostered more than six pups.
2.4. Limited bedding
On P2 – P7, dams and litters assigned to the stress condition were housed in custom cages (25.4 cm diameter x 30.4 cm high cylindrical plastic cage) containing ∼21 g of bedding, one paper towel (22.8 cm × 10.1 cm), six pieces of food (∼30 g, daily), and ad libitum water. Cage bottoms were changed daily with fresh bedding, food, and paper towel (Ivy et al., 2008; Molet et al., 2014; Smith et al., 2020).
2.5. Maternal separation
On P2 – P7, dams were separated from their litters and each pup was placed into an individual cage with one paper towel to lay on for 3 h (room temperature 21 °C).
2.6. Terbutaline administration
As rats are an altricial species, P2–P5 corresponds to the second or third trimester in humans. Terbutaline has been used to delay preterm labor occurring during these two periods, but can have detrimental effects on development as it can cross the placenta (Rhodes et al., 2003; Perna et al., 2014). Terbutaline was administered on P2 – P5 (10 mg/kg, 5 mg/mL, max volume injected ∼ 0.028 mL at P5, USP, i.p., dissolved in sterile saline; CAS 23031-32-5, Spectrum Chemical Mfg. Corp., New Brunswick, NJ, USA).
2.7. Behavioral tests
Behavioral tests were conducted on days P10 (ultrasonic vocalizations (USV)), P72–P83 (marble burying test), and P86–P89 (three-chamber social interaction test) (Fig. 1).
2.7.1. Ultrasonic vocalizations
Pups were placed in a plastic mouse cage with two paper towels on the bottom for 3 min alone inside a sound-attenuating chamber. A Ciel Electronique (Cat. No. CDP102, St Andre De La Roche, France) bat detector was set to transduce frequencies between 40 and 50 kHz and Audacity® open source sound software (version 3.3.3, London, UK) was used to record. Recordings were analyzed using custom-written Matlab software (R2021b, version 9.11.0.1769968, Natick, MA, USA).
2.7.2. Marble burying test
Rats were randomized into four equally sized cohorts and assigned a three-day testing schedule (see Smith et al., 2020 for details). Briefly, rats underwent two days of 30-min habituation sessions to the test environment followed by a test day. On test day, animals were given 10 min alone in a plastic rat cage containing 18 marbles in a 3 x 6 grid on ∼5 cm of bedding. The number of marbles buried were counted at the end of the test. A marble was considered buried if: 1) at least 2/3 of the marble was buried and 2) it appeared to have not been walked on.
2.7.3. Three-chamber social interaction test
Animals were randomized into four equally sized cohorts and assigned a testing day. The amount of time spent socially exploring a conspecific juvenile was measured (see Smith et al., 2020 for details). Briefly, the test consisted of three stages run in succession. These stages were: 1) a 5-min habituation stage, 2) an animal vs empty cage stage, and 3) a familiar animal vs unfamiliar animal stage. Social exploration was considered to be any behavior displaying direct active engagement, interest, or investigation of the conspecific animal(s).
2.8. Data and statistical analysis
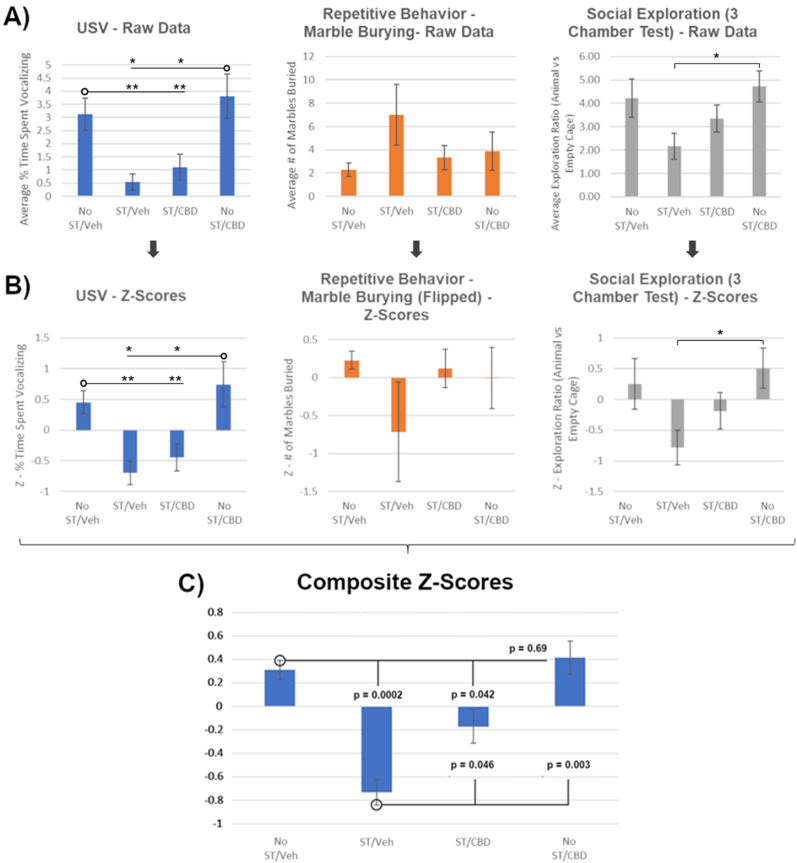
An ASD-like score was created by converting the three behavioral test scores into Z-scores (=−), which were then averaged to form a composite score (El-Kordi et al., 2013; Bercum et al., 2015; Smith et al., 2020). The marble burying test Z-scores were inverted so that when combined with the USV and three-chamber social interaction test scores, ASD-like behaviors would all be heading in the same direction and accurately depict a negative score.
3. Results
One dam in the No ST/CBD group was excluded from the study due to dehydration on E8. All other dams gave birth (No ST/Veh, mean litter size = 11.5 ± 0.14, male n = 7, female n = 16; ST/Veh, mean litter size = 7.6 ± 1.22, male n = 9, female n = 14; ST/CBD, mean litter size = 7.3 ± 0.96, male n = 10, female n = 12; No ST/CBD, mean litter size = 9 ± 0.9, male n = 10, female n = 17). One dam from each group (not including the No ST/CBD group) was excluded from fostering due to cross-fostering constraints. A dam in the ST/Veh group developed a mammary infection, which led to significant litter weight loss and euthanasia of her litter on P12 (n = 4). One pup in the No ST/CBD group and one in the ST/CBD group were found dead on P3 and P4, respectively.
Fig. 2A and B depict the three behavioral tests (raw data and Z-scores, respectively). There was a trend toward increased ASD-like behavior in the ST/Veh rats, reflected in all 3 behavioral tests, with a main effect of ST on USVs and Social Exploration reaching significance via a two-way ANOVA (F(1,26) = 17.102, p = 0.0003 and F(1,26) = 5.34, p = 0.029, respectively). Post hoc analysis (Fisher’s LSD) for USVs found ST/Veh rats were significantly different compared to No ST/Veh and No ST/CBD rats (p = 0.0017 and p = 0.0169, respectively), but for Social Exploration ST/Veh rats were only significantly different from No ST/CBD rats (p = 0.0228). Behavioral severity seen in ST/Veh rats decreased (non-significantly) with CBD treatment (ST/CBD) and approached that of No ST/Veh and No ST/CBD rats.
Fig. 2.
As ASD is composed of deficits in three core behavioral domains, we combined these tests into one ASD-like score. To justify this combination, a multivariate ANOVA was first conducted to compare the effects of treatment on the separate tests. There was a main effect of CBD treatment (F(2,81) = 7.65; p = 0.0009) but no main effect of the separate behavioral tests (F(2,81) = 0.24; p = 0.7887) or interaction (F(4,81) = 0.88; p = 0.4793). As there was no effect of separate tests and to decrease their variability, we proceeded with combining them into a composite Z-score reflecting an ASD-like behavioral measure (Fig. 2C). Two-way ANOVA of the ASD-like composite Z-score revealed a significant effect of stress/terbutaline (F(1,26) = 16.80, p = 0.0003) but no effect of CBD (F(1,26) = 2.80, p = 0.1060) or interaction (F(1,26) = 1.49, p = 0.2329). Post-hoc analysis (Fisher’s LSD) indicated a significant behavioral abnormality for the ST/Veh rats (p = 0.0002) compared to No ST/Veh rats. CBD reduced ASD-like behavioral composite Z-scores significantly (p = 0.046). There was no effect of CBD alone relative to No ST/Veh rats.
4. Discussion
Exposure to stress and terbutaline resulted in an overall ASD-like behavioral phenotype similar to that previously observed in male rats (Bercum et al., 2015; Smith et al., 2020). These offspring had impairments to social communication by P10 and social interaction by P86. When combined with our repetitive behavioral measurement, a clear deviation from normal behavior was observed.
Administering CBD during pregnancy provided protection to the stress/terbutaline offspring. However, from these data it is unclear if CBD was protecting against prenatal stress (E1 – E20) or all adverse manipulations. CBD is lipophilic and found in mouse pups for up to four days after last prenatal exposure; it’s therefore possible that CBD was still present in the rat pups postnatally (Swenson et al., 2023). Additionally, CBD may have been present in the dams’ breast milk. Cannabinoids can be detected in breast milk of nursing human mothers up to six days from last use (smoked cannabis) and, in studies utilizing rats and mice, were discovered to accumulate in adipose, liver, and muscle tissue (Bertrand et al., 2018; Moss et al., 2021; Child et al., 2022; Johnson et al., 2022). As such, CBD may have been present in small quantities that were slowly being released by adipose tissues used for metabolism and breast milk production.
If CBD was not present in the rat pups or dams during postnatal manipulations, the protection observed here may have stemmed from lasting prenatal neuronal and microglial changes. Previous research using this model has explored an alternative prevention strategy utilizing heat-killed Mycobacterium vaccae NCTC 11659 (M. vaccae) (Reber et al., 2016; Smith et al., 2020; Amoroso et al., 2021). M. vaccae has been found to immunize against psychosocial stressors that elicit sterile inflammation and has demonstrated highly significant behavioral protection against the development of ASD-like behaviors (Reber et al., 2016; Smith et al., 2020; Amoroso et al., 2021). The two behaviors assessed by Smith et al. (2020) were social interaction and repetitive behaviors. We observed similar results, indicating that CBD may be acting through comparable immunoregulatory mechanisms. M. vaccae induces regulatory T cell expansion in peripheral tissues and is capable of inducing expression of the anti-inflammatory cytokine interleukin (IL) 4 in the central nervous system (Fonken et al., 2018; Frank et al., 2020; Smith et al., 2020). Interestingly, CBD also induces expansion of regulatory T cells (Dhital et al., 2017; Nichols and Kaplan, 2020; Angelina et al., 2022). As CBD can bind to endocannabinoid receptors and these receptors are present in immune cells, one speculative explanation of how CBD is protecting against the observed behavioral deficits may be that CBD is acting through anti-inflammatory and immunoregulatory mechanisms.
Understanding the potential negative effects of CBD administration during pregnancy is important. Negative effects of prenatal CBD administration (50 mg/kg, oral gavage) were noticed only in demanding behavioral test paradigms in mice (Swenson et al., 2023). Here, it is a promising finding that a lower prenatal dosage of CBD (10 mg/kg, i.p.) did not produce readily observable negative behavioral outcomes.
5. Conclusions
This study examined the potential for prenatal administration of CBD to prevent negative neurodevelopmental behavioral effects of stress/terbutaline exposures. The behavioral improvements in the male offspring of CBD-treated animals are promising. Future research will be needed to uncover the mechanisms underlying the protective effects of CBD.
CRediT authorship contribution statement
Jeremy A. Taylor: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation. Zachariah Z. Smith: Methodology. Michael E. Anderson: Methodology. Evan M. Holbrook: Formal analysis, Data curation. Isabella S. Elkinbard: Methodology. Jon D. Reuter: Writing – review & editing, Project administration, Funding acquisition, Conceptualization. Christopher A. Lowry: Writing – review & editing, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Daniel S. Barth: Writing – review & editing, Validation, Supervision, Resources, Project administration, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank A. Trimble for experimental assistance. This work was supported by the Institute of Cannabis Research, Colorado State University Pueblo (ICR-FY23-Lowry); a Research & Innovation Seed Grant Program (RISGP) award, University of Colorado Boulder (grant RISGP FY23 – LOWRY); and the Department of Defense (grant EP2170067).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100828.
Contributor Information
Jeremy A. Taylor, Email: Jeremy.Taylor@colorado.edu.
Zachariah Z. Smith, Email: Zachariah.Smith@colorado.edu.
Michael E. Anderson, Email: michael.anderson-2@colorado.edu.
Evan M. Holbrook, Email: Evan.Holbrook@colorado.edu.
Isabella S. Elkinbard, Email: Isabella.Elkinbard@colorado.edu.
Jon D. Reuter, Email: Jon.Reuter@colorado.edu.
Christopher A. Lowry, Email: Christopher.Lowry@colorado.edu.
Daniel S. Barth, Email: Daniel.Barth@colorado.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association. 2013 [Google Scholar]
- Amoroso M., Langgartner D., Lowry C.A., Reber S.O. Rapidly growing Mycobacterium species: the long and winding road from tuberculosis vaccines to potent stress-resilience agents. Int. J. Mol. Sci. 2021;22(23) doi: 10.3390/ijms222312938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelina A., Pérez-Diego M., López-Abente J., Rückert B., Nombela I., Akdis M., et al. Cannabinoids induce functional Tregs by promoting tolerogenic DCs via autophagy and metabolic reprogramming. Mucosal Immunol. 2022;15(1):96–108. doi: 10.1038/s41385-021-00455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercum F.M., Rodgers K.M., Benison A.M., Smith Z.Z., Taylor J., Kornreich E., et al. Maternal stress combined with terbutaline leads to comorbid autistic-like behavior and epilepsy in a rat model. J. Neurosci. 2015;35(48):15894–15902. doi: 10.1523/JNEUROSCI.2803-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K.A., Hanan N.J., Honerkamp-Smith G., Best B.M., Chambers C.D. Marijuana use by breastfeeding mothers and cannabinoid concentrations in breast milk. Pediatrics. 2018;142(3) doi: 10.1542/peds.2018-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson S.L., Bale T.L. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal anti-inflammatory treatment. Endocrinology. 2014;155(7):2635–2646. doi: 10.1210/en.2014-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayasirisobhon S. Mechanisms of action and pharmacokinetics of Cannabis. Perm. J. 2020;25:1–3. doi: 10.7812/TPP/19.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child R.B., Tallon M.J. Cannabidiol (CBD) dosing: plasma pharmacokinetics and effects on accumulation in skeletal muscle, liver and adipose tissue. Nutrients. 2022;14(10):2101. doi: 10.3390/nu14102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhital S., Stokes J.V., Park N., Seo K.S., Kaplan B.L. Cannabidiol (CBD) induces functional Tregs in response to low-level T cell activation. Cell. Immunol. 2017;312:25–34. doi: 10.1016/j.cellimm.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kordi A., Winkler D., Hammerschmidt K., Kästner A., Krueger D., Ronnenberg A., et al. Development of an autism severity score for mice using Nlgn4 null mutants as a construct-valid model of heritable monogenic autism. Behav. Brain Res. 2013;251:41–49. doi: 10.1016/j.bbr.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Fonken L.K., Frank M.G., Gaudet A.D., Maier S.F. Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain Behav. Immun. 2018;73:133–148. doi: 10.1016/j.bbi.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.G., Fonken L.K., Watkins L.R., Maier S.F. Acute stress induces chronic neuroinflammatory, microglial and behavioral priming: a role for potentiated NLRP3 inflammasome activation. Brain Behav. Immun. 2020;89:32–42. doi: 10.1016/j.bbi.2020.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R., Scolnick E.M. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol. Psychiatr. 2006;11(1):11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy A.S., Brunson K.L., Sandman C., Baram T.Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.T., de Abreu G.H.D., Mackie K., Lu H.C., Bradshaw H.B. Cannabinoids accumulate in mouse breast milk and differentially regulate lipid composition and lipid signaling molecules involved in infant development. BBA Advances. 2022;2 doi: 10.1016/j.bbadva.2022.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowska-Müller J.A., Schmöle A.C. CB2 receptor in microglia: the guardian of self-control. Int. J. Mol. Sci. 2020;22(1):19. doi: 10.3390/ijms22010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Schedlowski M., Yee B.K. Immunological stress at the maternal–foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav. Immun. 2006;20(4):378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U., Murray P.J., Urwyler A., Yee B.K., Schedlowski M., Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatr. 2008;13(2):208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr. Res. 2011;69(8):26–33. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Avishai‐Eliner S., Baram T.Z. Naturalistic rodent models of chronic early‐life stress. Dev. Psychobiol. 2014;56(8):1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M.J., Bushlin I., Kazmierczak S., Koop D., Hendrickson R.G., Zuckerman K.E., Grigsby T.M. Cannabis use and measurement of cannabinoids in plasma and breast milk of breastfeeding mothers. Pediatr. Res. 2021;90(4):861–868. doi: 10.1038/s41390-020-01332-2. [DOI] [PubMed] [Google Scholar]
- Maenner M.J., Warren Z., Williams A.R., Amoakohene E., Bakian A.V., Bilder D.A., et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2020. MMWR Surveillance Summaries. 2023;72(2):1. doi: 10.15585/mmwr.ss7202a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J.M., Kaplan B.L. Immune responses regulated by cannabidiol. Cannabis and Cannabinoid Research. 2020;5(1):12–31. doi: 10.1089/can.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna R., Loughan A., Perkey H., Tyson K. Terbutaline and associated risks for neurodevelopmental disorders. Child Development Research. 2014;2014(1) [Google Scholar]
- Rhodes M.C., Nyska A., Seidler F.J., Slotkin T.A. Does terbutaline damage the developing heart? Birth Defects Res. Part B Dev. Reproductive Toxicol. 2003;68(6):449–455. doi: 10.1002/bdrb.10043. [DOI] [PubMed] [Google Scholar]
- Reber S.O., Siebler P.H., Donner N.C., Morton J.T., Smith D.G., Kopelman J.M., et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc. Natl. Acad. Sci. USA. 2016;113(22):E3130–E3139. doi: 10.1073/pnas.1600324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Z.Z., Kubiak R.A., Arnold M.R., Loupy K.M., Taylor J.A., Crist T.G., et al. Effects of immunization with heat-killed Mycobacterium vaccae on autism spectrum disorder-like behavior and epileptogenesis in a rat model of comorbid autism and epilepsy. Brain Behav. Immun. 2020;88:763–780. doi: 10.1016/j.bbi.2020.05.034. [DOI] [PubMed] [Google Scholar]
- Swenson K.S., Gomez Wulschner L.E., Hoelscher V.M., Folts L., Korth K.M., Oh W.C., Bates E.A. Fetal cannabidiol (CBD) exposure alters thermal pain sensitivity, problem-solving, and prefrontal cortex excitability. Mol. Psychiatr. 2023;28(8):3397–3413. doi: 10.1038/s41380-023-02130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotsky B., Black L.I., Maenner M.J., Schieve L.A., Danielson M.L., Bitsko R.H., et al. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics. 2019;144(4) doi: 10.1542/peds.2019-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.