Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Oct 27;14:25642. doi: 10.1038/s41598-024-76340-x
Abstract
The medicinal plant Cannabis sativa L. (C. sativa) is currently being extensively studied to determine the full extent of its therapeutic pharmacological potential. Δ9-tetrahydocannabinol (THC) and cannabidiol (CBD) are the most thoroughly investigated compounds. We aimed to explore the anticancer activity of cannabinoids mixture isolated from the Lebanese C. sativa plant in ratios comparable to the local medicinal plant, to elucidate its mechanism of action in breast cancer cells in vitro. Cells were subjected to cytotoxicity assay, cell cycle analysis, Annexin V/PI dual staining, cell death ELISA, immunofluorescence, in addition to western blot analysis of apoptotic and autophagy markers. We further evaluated the anti-metastatic effect of cannabinoids on MDA-MB-231 using the scratch wound-healing, trans-well migration and invasion assays. Our results revealed the promising therapeutic benefits of CBD/THC on inhibiting the growth of breast cancer cells by promoting cellular fragmentation, phosphatidylserine translocation to the outer membrane leaflet and DNA fragmentation in both cell lines while inhibiting the motility of the triple negative breast cancer cells. In our study, CBD/THC mixture was found to exhibit a pro-apoptotic activity via the activation of the mitochondrial apoptotic pathway, independent from ROS production while also suggesting the activation of a caspase-dependent apoptotic pathway. Even though autophagy was altered upon exposure to the cannabinoid mixture, our data suggested that it is not the mechanism responsible of inducing cell death. In conclusion, our study demonstrates the promising therapeutic benefits of CBD and THC isolated from the Lebanese C. sativa plant on breast cancer cells in vitro.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76340-x.
Introduction
Since the beginning of human civilization, Cannabis sativa L. (C. sativa) commonly known as marijuana or cannabis has been used for both therapeutic and recreational purposes1. This herbaceous plant is a dioecious flowering plant native to Central Asia and member of the Cannabaceae family. As a result of the genus’ diverse phenotypes, cannabis is taxonomically divided into three subspecies: Cannabis sativa, Cannabis ruderalis, and Cannabis indica2. In contrast to ruderalis, the sativa and indica species have been substantially developed for their therapeutic and commercial advantages3. C. sativa is the most widely used illicit substance globally, despite the fact that numerous nations have legalized its production and consumption due to its significant clinical interest4.
Before the use of cannabis in Western medical systems, C. sativa has been used medicinally for at least 1800 years in India and China, according to ancient writings5. This plant has been documented for the treatment of various ailments including chronic pain, constipation, fever, inflammation, meningitis, dermatological and urinary disorders1,3,6. Currently, research studies are being conducted to determine the full extent of the therapeutic potential of cannabis plants. Indeed, it is well established that its biochemical constituents are predominantly responsible for the various functions associated with its consumption. More than 500 compounds including cannabinoids, terpenoids and flavonoids, were found to be present in the flowering C. sativa plant7,8. Chief among these major phytochemical compounds are the psychoactive Δ9-tetrahydocannabinol (THC) and the non-psychoactive but medicinally relevant cannabidiol (CBD)9. THCs are a particular class of cannabinoids with terpenophenolic structure and make up approximately 20% of the total plant weight. Currently, eighteen THC isoforms are known to exist in the cannabis plant with Δ9-THC being the most thoroughly investigated compound10. Similarly, CBD has received a great interest for its pharmacotherapeutic value11. Numerous studies have supported the use of CBD and THC in the treatment of various conditions including nausea, vomiting, sleep and neurodegenerative disorders as summarized by Khalsa et al. in their review12.
In-depth-investigation has been carried out in the past twenty years to explore the clinical and biological prospects of cannabinoids, alone or in combination, in the treatment of various diseases, including cancer. Recent studies showed that cannabinoids exert anticancer activity in almost all types of cancer through the suppression of cell proliferation, migration and angiogenesis along with the activation of apoptosis and autophagy upon exposure to CBD and THC, alone or in combination13,14. It was demonstrated that THC compounds can halt the progression of breast, brain, leukemia, lung, melanoma, prostate, pancreatic, hepatocellular, and colon cancer in vitro and in vivo15. Other studies investigated the anti-cancer properties of CBD in breast, lung, glioma, myeloma, colon and prostate cancer among many others16. However, very few studies focused on the combination of cannabinoids on cancer cells. A study conducted by Marcu et al. on glioblastoma, demonstrated that combining CBD with THC may increase the efficacy of treatment via the synergistic inhibition of cell proliferation and the induction of apoptosis17. This was similar to the results of a study conducted on melanoma cells exposed to equal amounts of CBD and THC18. Another study showed a synergistic effect of CBD and THC on the suppression of leukemic cell growth particularly when combined with chemotherapeutic drugs which might improve patients survival19.
More recently, a study conducted by Schoeman et al., focused on assessing the anticarcinogenic effect of different CBD and THC mixtures on breast cancer20. This study provided further evidence on the promising antiproliferative effect of cannabinoids mixtures on breast cancer cells with no cytotoxic effect on normal cells10. In fact, breast cancer is one of the most frequent malignancies among women and a leading cause to death worldwide. More than 1.8 million new instances of breast cancer are discovered every year, and the incidence rates for the disease are still rising21. Modern molecular analysis of breast tumors neglects morphological subtypes and classify them by the positive or negative expression of three hormone receptors: progesterone receptor (PR), estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2)22. Triple negative breast cancer (TNBC) cells are regarded as the most aggressive type of breast cancer cell lines due to the negative expression of the three receptors. Chemotherapy is therefore the primary systemic treatment used in this case since these cells are not susceptible to endocrine and molecularly targeted therapy23. Common breast cancer cells include the hormone-independent (PR–, ER– and HER2–) MDA-MB-231 and the hormone-dependent (PR+, ER+ and HER2+) MCF-7 cells, both developed from individuals with ductal invasive carcinoma24. Despite the many contemporary medicinal techniques used to treat breast cancer tumors, patients may experience severe symptoms including fatigue, nausea, hair loss and depression hence why complementary and alternative medicine is of interest and employed nowadays25.
In this study we aim to investigate the anticancer activity of a mixture of cannabinoids isolated from the Lebanese C. sativa plant. In fact, a previous study conducted by Shebaby et al. reported the chemical characterization of the Lebanese C. sativa plant revealing its high CBD to THC content26. In this study, a mixture of CBD and THC (3:1) in ratios comparable to their abundance in the Lebanese plant was used to elucidate the mechanism of action in breast cancer cells, given the fact that such medicinal formulation is able to selectively inhibit the proliferation of MDA-MB-231 and MCF-7 cells with no adverse effect on normal cells10.
Material and methods
Breast cancer cell culture
Two human breast cancer cell lines (MDA-MB-231 and MCF-7) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). According to the ATCC guidelines for growth medium, cell cultures were maintained under standard conditions (37 °C and 5% CO2 humidified environment). MDA-MB-231 and MCF-7 cells were cultured and allowed to grow into a monolayer in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% Fetal Bovine Serum (FBS, Gibco, Dublin, Ireland) and 1% antibiotics (100U/mL penicillin and 100 μg/mL streptomycin)27. Cells were checked every day using the ZOE Fluorescent Cell Imager and split when reaching a 70–80% confluency using trypsin–EDTA (Sigma-Aldrich, St. Louis, MO, USA). The trypan exclusion method and a hemocytometer were used to assess and determine the viability of the cells before plating.
Plant extract preparation
The Drug Enforcement Office in Zahle, Beqaa Governorate, provided the dried samples of a Lebanese cannabis strain in October 2019 from the Beqaa valley in Yammoune (Yammoune: 34◦07′46.4′′North, 36◦01′40.8′′East; altitude, 1375 ± 10 m). The plant material was securely stored at the Lebanese American University and a plant specimen was deposited at the University Department of Natural Sciences’ herbarium (ID, 2019–0011). Lebanese Cannabis flower sample (10 g) was air-dried and extraction using ethanol was performed as previously described by Shebaby et al. yielding 1.17 g of crude cannabis oil extract (COE)26. The phytochemical compounds of the Lebanese C. sativa plant were separated via liquid column chromatography (LCC). The column ran for four days at different rates using a normal-phase silica gel column and a 4:3:3 ratio of chloroform, hexane, and diethyl ether, respectively, as the eluent mobile phase. The samples were collected in test tubes and the solvent was evaporated using the rotary evaporator. Following that, the collected samples were analyzed via thin layer chromatography (TLC) that was carried out for every five tubes. The Retention Factor (Rf) was calculated by measuring the traveled distances using the ImageJ software and tubes with similar TLC behavior were combined. The tubes were later evaluated by Gas chromatography coupled to Mass spectrometry using a Shimadzu GCMS-QP2020NX as previously described26.
The extract preparation of CBD and THC compounds to be used on cancer cells was achieved by dissolving 12.5 mg of THC and 37.5 mg of CBD in ethanol. Following ethanol evaporation using rotary evaporator, the CBD and THC (3:1) were then dissolved in 1 mL DMSO reaching a final concentration of 50 mg/mL stock solution, stored at − 20 °C for later use. The composition and percentages of CBD and THC in this purified preparation were further confirmed (Supplementary Table 1 and Figure S1) by GC–MS analysis as previously described26.
Cell viability assay
The viability of MDA-MB-231 and MCF-7 was assessed using the MTS (Promega, Wisconsin, USA) cell viability reagent. Cells were harvested, counted and prepared for seeding in triplicates at a density of 1 × 105 cells/mL overnight. Cells were then treated with various concentrations of CBD/THC mixture (10, 12.5, 15, 17.5, 20 and 25 μg/mL) for 24 and 48 h. After the desired incubation period, MTS reagent was used to quantify cell viability and determine cell proliferation. Following removal of cell culture medium, 100uL of the MTS working solution prepared according to the manufacturer’s instructions was added to each well28. This assay is based on using the MTS tetrazolium salt, which when combined with metabolically active cells results in the formation of a formazan dye which could be detected at 492 nm using the Varioskan™ LUX multimode microplate reader. The findings of three separate trials are included and the relative cell proliferation was calculated as follows:
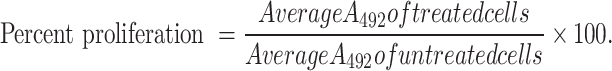 |
Cell cycle progression analysis using flow cytometer
Both, MDA-MB-231 and MCF-7 cells were seeded at a density of 1 × 105 cells/mL in 6 well-plates overnight followed by treatment for 24 and 48 h with 12.5, 15 and 17.5 μg/mL of the cannabinoid mixture and 30uM cisplatin (Abcam, Cambridge, United Kindgom), used as positive control. After the desired incubation period, culture medium was collected, and cells were washed with PBS and detached using trypsin–EDTA reagent. Cells were centrifuged, washed, fixed with ice-cold PBS and 70% ethanol and stored at − 80 °C, overnight29. Fixed cells were centrifuged the following day, counted using hemocytometer, and stained with 50 μg/mL Propidium Iodide (Sigma-Aldrich, St. Louis, MO, USA) and 0.5 μg/mL RNase (Roche, Basel, Switzerland) for 45 min in the dark. Using the Incyte mode on the Guava® easyCyteTM flow cytometer (Luminex Corporation, Austin, TX, USA), the DNA content was assessed and distributed among the various cell cycle phases according to the degree of PI binding relative to the control. Cells with DNA contents less than 2n were classified into the pre-G0/G1 phase, those with DNA content between 2 and 4n into the synthesis phase (S phase) while cells with 2n and 4n DNA contents were grouped into the G0/G1 and G2/M phases, respectively.
Apoptosis investigation using flow cytometer
Both cell lines were seeded (1 × 105 cells/mL) in 6 well-plates and treated with 12.5, 15 and 17.5 μg/mL CBD/THC and 30uM cisplatin for 24 and 48 h. After desired incubation period, cells were washed using PBS and detached using trypsin. After centrifugation (1400 g, 5 min and 4 °C), cells were counted, and stained for 5 min with the staining solution (1X Binding buffer, 1X PI and 1X Annexin-V) in the dark. Flow cytometry (Luminex Corporation, Austin, TX, USA) was used to obtain the data (5,000 events/sample) which was analyzed using the Incyte mode on the Guava® easyCyteTM software. The cell population in the four quadrants was examined and defined as follows: the lower left quadrant (Ann–/PI–) denotes viable cells, the lower right (Ann+/PI) and upper right (Ann+/PI+) quadrants represent cells at early and late stages of apoptosis, respectively, while the upper left quadrant (Ann–/PI+) represents necrotic cells.
Cell death detection enzyme-linked immunosorbent assay (ELISA)
Breast cancer cells were plated overnight in a 6 well-plate at a density of 1 × 105 cells/mL. The following day, cells were treated with 12.5, 15 and 17.5 μg/mL CBD/THC mixture and 30 μM cisplatin, in fresh medium, for a duration of 24 h. After the desired incubation period, and using the Cell Death detection ELISA kit (Roche, Basel, Switzerland), a sandwich enzyme-immunoassay procedure was followed as previously described by El Khoury et al.30.This assay is used to measure cytoplasmic histone-associated DNA fragments (mono- and oligo-nucleosomes) released into the cytoplasm of cells undergoing apoptosis. Following the manufacturer’s instructions, cells were collected, lysed and transferred into a pre-coated microplate with anti-histone antibody with high specificity to H1, H2A, H2B, H3 and H4 histone subunits. After sufficient time, the wells were washed followed by the addition of anti-DNA antibody conjugated to a peroxidase enzyme (anti-DNA-POD) that binds to single- and double-stranded DNA. Upon the addition of the ABTS (2,2’-azino-di-[3-ehtylbenzthiazoline sulfonate (6)]) peroxidase substrate, the absorbance A405 was measured using the Varioskan™ LUX multimode microplate reader, allowing for the determination of the retained peroxidase enzyme in the immunocomplex. The results were used to calculate the specific enrichment of mono- and oligo-nucleosomes released into the cytoplasm of apoptotic cells using the following formula:
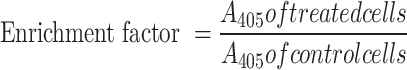 |
Inhibition of caspases using Z-VAD-FMK
Breast cancer cells were seeded in 96 well-plates at a density of 1 × 105 cells/mL and then treated with caspases inhibitor, Z-VAD-FMK (50 μM) for 30 min prior to the addition of the CBD/THC (12.5, 15 and 17.5 μg/mL) mixture for 24 and 48 h. To assess the caspase-dependent cytotoxic effect of the treatment, MTS viability reagent was used as described previously to quantify metabolically active cells by spectrophotometry using the Varioskan™ LUX multimode microplate reader at 492 nm. The effect of Z-VAD-FMK inhibition on the proliferation of both cell lines was assessed and compared to cells treated only with CBD/THC mixture.
Inhibition of autophagy using chloroquine and wortmannin
Breast cancer cells were seeded in 96 well-plates at a density of 1 × 105 cells/mL. To evaluate the autophagy-dependent cell death, breast cancer cells were pre-exposed to 50 nM wortmannin (Wort) or 10 μM chloroquine (CQ) for 30 min, inhibitors of autophagosome and autophagolysosome formation, respectively. Cells were then treated with increasing concentrations of CBD/THC mixture for 24 h followed by the addition of MTS viability reagent, as detailed above. Cell viability was determined by quantifying formazan formation relative to control, untreated cells, which was further compared to cells treated with CBD/THC only.
Immunofluorescence
Breast cancer cells were seeded at 1 × 105 cells/mL in 6 well plates overnight, and then treated with CQ (10uM), Wort (50 nM), CQ + Wort, and CBD/THC (10 and 12. 5 μg/mL) with and without Wort (50 nM) for 24 h. The following day, cells were prepared for immunofluorescence. As such, cells were washed with PBS (1X), fixed using 4% paraformaldehyde (PFA) and permeabilized using 0.1% Triton-X. Blocking using 1% bovine serum albumin (BSA) was performed for 1 h at room temperature followed by primary antibody incubation (anti-LC3B) overnight. The following day, washing and secondary goat anti-rabbit IgG antibody carrying Alexa fluor 488, was performed along with DAPI staining prior to visualizing the samples using the ZOE Fluorescent Cell Imager31.
Wound healing assay
To evaluate the migration properties of aggressive breast cancer cell lines post-exposure to cannabinoids (CBD and THC), a wound healing assay was performed. Cells were cultured overnight, in 24 well-plates, at a density of 2 × 105 cells/mL and were allowed to reach a confluent monolayer. The following day, a scratch using a sterile micropipette tip was performed across the middle of the well, creating a wound region. Each well was gently washed with PBS to remove non-adherent cells before adding fresh medium with the various concentrations of treatment (IC50/4 = 3.125 μg/mL and IC50/2 = 6.25 μg/mL). These sub-IC50 concentrations were used to confirm that any alterations in wound healing reflect alterations in migration rather than cytotoxic effects. Using the ZOE fluorescent cell imager microscope, images were taken at 0 and 24 h after wounding and the wound widths were analyzed using the ImageJ software. The data reported represents the average of 3 separate trials, each including measurements at 16 different points for each experimental condition.
Trans membrane migration and invasion assays
To further evaluate the effectiveness of the CBD/THC mixture on the motility of aggressive breast cancer cells, migration and invasion assays were performed using trans-well chambers (24-well chamber; 8 μm pore size). On the first day, TNBC cells were cultured and starved for 24 h in incomplete media supplemented with 0.5% v/v FBS. The following day, cells were collected and seeded in the upper side of the trans-well chamber at a density of 5 × 105 cells/mL in serum-free medium for the migration assay or in pre-coated inserts with Matrigel for the invasion assay. Matrigel (Corning, NY, United States) was thawed on ice at 4 °C, diluted in serum-free medium (1:4), and finally 60uL were added to each insert, 1 h prior to the addition of cells with treatment (IC50/4 and IC50/2). In the migration assay, the cells were incubated for 24 h while the assessment of cell invasion was performed after 48 h. Following the desired incubation period, inserts were washed twice with PBS and a cotton swab was used to remove the remaining cells from the upper side of the insert. Cells on the lower side of the insert were fixed with PFA (4%) for 2 min, then permeabilized using methanol (100%) for 20 min and stained for 15 min with DAPI (1 μg/mL). Membranes were then washed and observed on the inverted fluorescent microscope and several images from different fields were used to quantify the number of migratory and invasive cells using the ImageJ software.
Protein extraction, quantification and preparation
To evaluate the effect of the CBD/THC mixture on the expression of regulatory proteins, MDA-MB-231 and MCF-7 cells were cultured in petri dishes and allowed to grow overnight. The following day, cells were treated with 12.5, 15 and 17.5 μg/mL cannabinoid mixture. After 12 or 24 h, cells were scraped and lysed using a lysis buffer prepared with protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA) and EDTA solution to prevent the proteolytic degradation during protein extraction. Lysate was centrifuged at 18,000 g for 10 min at 4 °C and the lysate was aliquoted and stored at − 80 °C for later use32. Protein quantification was performed using the DC Protein Assay reagents (BioRad, Hercules, CA, USA) following the manufacturer’s instructions. Laemmli buffer solution containing β-mercaptoethanol was used for the preparation of protein samples (25–50 μg), subjected to 100 °C for 10 min before loading into the polyacrylamide gels.
Cytoplasmic cellular fractionation
Subcellular fractionation was performed to separate nuclear, membrane and cytoplasmic cellular fractions. A protocol adapted from Abcam was followed to perform this protein extraction. Both breast cancer cell lines were seeded at a density of 1 × 105 cells/mL overnight followed by the treatment with 12.5, 15 and 17.5 μg/mL of CBD/THC for 24 h. After the desired incubation period, cell lysis was performed on ice using a fractionation buffer prepared with protease inhibitors. Cells were then scraped and centrifuged at 2800 g for 5 min. The supernatant containing the cytoplasmic, mitochondrial and membrane factions was centrifuged again at 13,600 g (5 min) allowing the separation of the mitochondrial fraction in the pellet from the cytoplasmic fraction in the supernatant33,34. Each fraction was collected and quantified using the DC Protein Assay reagents. Proteins were prepared in Laemmli buffer solution containing β-mercaptoethanol, then denatured at 100 °C for 10 min before loading into the polyacrylamide gels.
Western blot analysis
The effect of CBD and THC mixture on the protein expression was evaluated in both cell lines, via Western Blotting. Protein samples were loaded onto the gel to be separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred to polyvinylidene difluoride (PVDF) membranes which were subsequently blocked in 5% BSA solution for 1 h at room temperature and then incubated with primary antibody, overnight at 4°C35. All primary antibodies were diluted according to the manufacturer’s recommendation in blocking buffer solution as follows: 1:3000 anti-β-Actin (Santa Cruz Biotechnology, Dallas, TX, USA) used as loading control, and 1:1000 anti-Bax, anti-Bcl2, anti-cleaved PAPRP, anti-cytochrome c, anti-cleaved caspase 3, anti-beclin1, anti-LC3B, anti-MMP2 and anti-MMP9. Membranes were then washed and incubated with HRP-conjugated secondary antibody (BioRad, Hercules, CA, USA) for 1 h at room temperature36. The chemiluminescence Clarity Western ECL Substrates (BioRad, Hercules, CA, USA) were used to detect proteins expression through the revelation of blot images using the ChemiDoc imaging instrument. ImageJ software was used for quantitative analysis of the target protein bands as normalized to the endogenous β-actin control.
Detection of reactive oxygen species
Breast cancer cell lines were seeded in 96 well-plates at a density of 2 × 105 cells/mL and allowed to adhere overnight. The following day, MDA-MB-231 and MCF-7 cells were treated for 45 min with 25 μM of the cell-permeant 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) according to the manufacturer’s recommendation (Abcam, Cambridge, United Kingdom). After the desired incubation time, cells were washed and treated with 12.5, 15 and 17.5 μg/mL of CBD/THC mixture diluted in culture media without phenol red for 2 h and 24 h. Hydrogen peroxide (H2O2) was used as a potent ROS inducer and NAC, a potent ROS inhibitor was used. The oxidative conversion of H2DCFDA to its highly fluorescent 2′,7′-dichlorofluorescein (DCF) upon ROS presence, was quantified using the fluorometric Varioskan™ LUX multimode microplate reader37.
Statistical analysis
All the reported data represents the mean value ± standard deviation of three independent trials for each experiment. Statistical analyses were performed using GraphPad Prism8 by calculating p-value using t-tests, one- or two-way ANOVA depending on the experiment. GraphPad Prism8 was used for the preparation of the figures. All significant differences were reported as follows: * indicating a p-value: 0.01 < p < 0.05, ** indicating a p-value: 0.001 < p < 0.01, and *** indicating a p-value: 0.0001 < p < 0.001.
Results
Cannabinoids mixture inhibits the proliferation of breast cancer cell lines by promoting cellular fragmentation
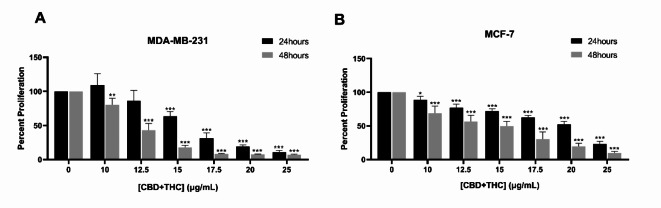
We investigated the efficacy of two major compounds isolated from the Lebanese C. sativa plant, CBD and THC, on the viability and proliferation of breast cancer cell lines. Cytotoxicity assay was performed using the MTS reagent. As reported in Fig. 1, CBD/THC mixture (3:1) showed a significant inhibition of MDA-MB-231 and MCF-7 proliferation in a dose- and time- dependent manner. After 24 and 48 h, both cell lines showed a gradual decrease in absorption value (A492) upon treatment as compared to the untreated cells. A significant decrease in MDA-MB-231 proliferation was determined reaching 86.4%, 63.57% and 31.37% at 12.5, 15 and 17.5 μg/mL 24 h post-treatment, respectively, while reporting a significantly higher effect after 48 h of treatment reaching 43.2%, 17.79% and 8.4% at 12.5, 15 and 17.5 μg/mL, respectively (Fig. 1A). The half-maximal inhibitory concentrations (IC50) on MDA-MB-231 cells are 16.18 μg/mL at 24 h and 12.12 μg/mL at 48 h treatment. However, a milder yet significant effect was observed on MCF-7 cell line, showing a decrease in cell proliferation reaching 77.11%, 71.99% and 62.6% after 24 h and 56.51%, 49.80% and 30.57% after 48 h of treatment with 12.5, 15 and 17.5 μg/mL, respectively (Fig. 1B). The mixture of CBD and THC was found to exhibit a noticeably higher inhibitory effect on the proliferation of cells as compared to the individual compounds in concentrations that are comparable to the mixture (Supplementary Fig. 2A & B). The IC50 concentrations are 19.43 μg/mL after 24 h and 13.62 μg/mL after 48 h of treatment. Thus, our results indicate a dose- and time-dependent anti-proliferative effect on both breast cancer cell lines, MDA-MB-231 and MCF-7 cells. Based on these results, subsequent experiments were performed using CBD/THC mixture concentrations (12.5, 15 and 17.5 μg/mL) the closest to IC50 at 24 and 48 h on both cell lines.
Fig. 1.
Fig. 2.
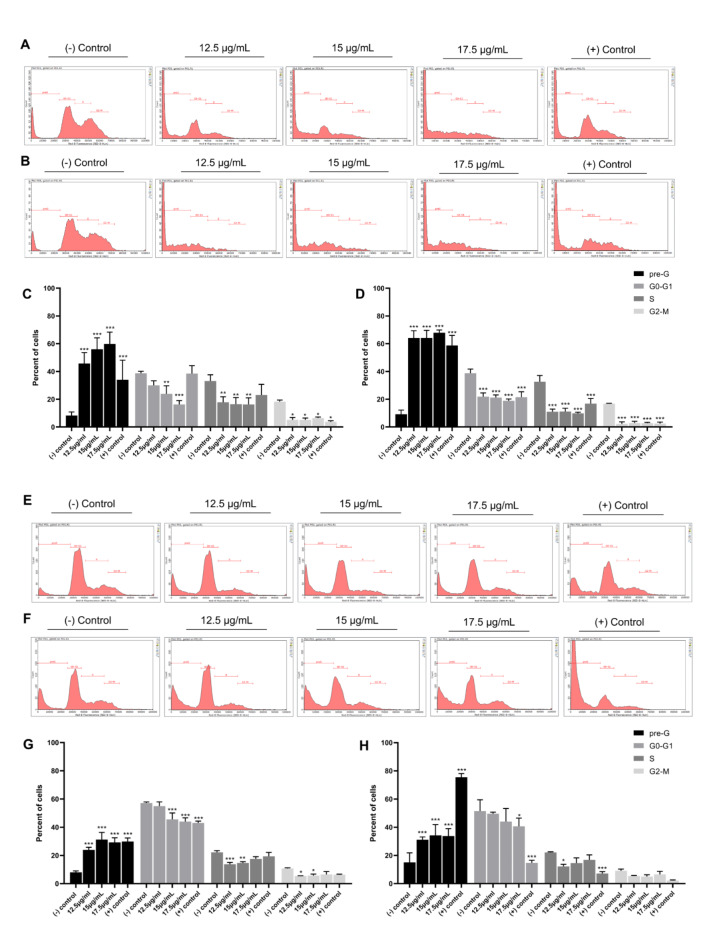
Cell cycle analysis of MDA-MB-231 and MCF-7 cells in response to CBD/THC treatment was then performed by evaluating the DNA content using the flow cytometer in accordance with PI binding affinity. Cannabinoid mixture significantly decreased the G0-G1 (DNA = 2n), S (2n < DNA < 4n) and G2/M (DNA = 4n) content while increasing the pre-G (DNA < 2n) content in both cell lines in a dose- and time-dependent manner (Fig. 2). This mixture significantly decreased the G0-G1 content in MDA-MB-231 cells from 38.7% in control cells to 16.26% at 24 h and from 38.76% to 18.86% at 48 h of 17.5 μg/mL treatment. Along with this, a significant increase in the pre-G subpopulation was noticed from 8.32% and 9.17% in control cells to 59.86% and 67.96% at 17.5 μg/mL treatment at 24 and 48 h, respectively (Fig. 2C and D). The effect of treatment on MCF-7 cell cycle progression was milder when compared to MDA-MB-231 cell line. The data showed a significant decrease from 57.10% (24 h) and 51.46% (48 h) in control cells to 44.09% (24 h) and 40.63% (48 h) at 17.5 μg/mL in G0-G1 content while showing a significant increase in the pre-G content from 8.05% (24 h) and 14.98% (48 h) in control cells reaching 29.22% (24 h) and 33.78% (48 h) at the highest concentration of treatment (17.5 μg/mL) (Fig. 2G and H). Our data suggests that CBD/THC mixture inhibits the proliferation of breast cancer cells by promoting cellular fragmentation rather than inducing a cell cycle arrest.
Cannabinoid mixture promotes the activation of the apoptotic cell death mechanism in breast cancer cells
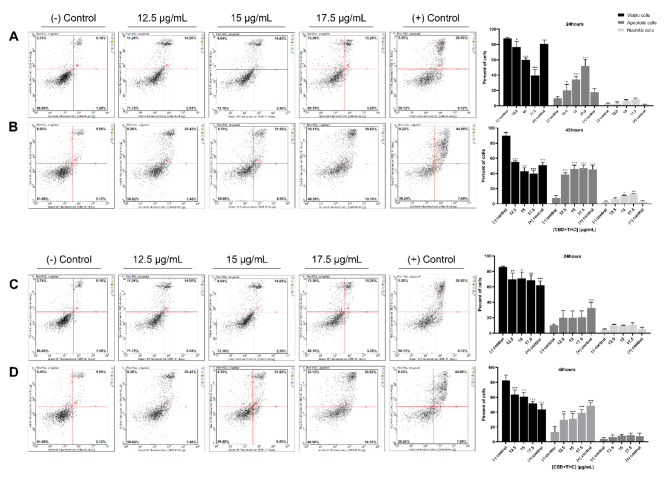
To determine whether the mixture of CBD and THC promotes the activation of the programmed apoptotic cell death mechanism, MDA-MB-231 and MCF-7 cells were treated with 12.5, 15 and 17.5 μg/mL of CBD and THC for 24 and 48 h and analyzed using flow cytometry following Annexin V and PI dual staining. As shown in Fig. 3, a dose- and time-dependent increase in apoptotic cells was observed in both cell lines. The TNBC cells showed a significant decrease in the percentage of viable cells (Ann–/PI–) from 87.97% in control cells to 39.37% upon exposure to 17.5 μg/mL along with a significant increase in the total percentage of apoptotic cells (Ann+/PI– and Ann+/PI+) from 9.59% to 52.02% at the highest concentration of treatment (Fig. 3A). The effect was noticeably higher after 48 h of treatment, where the results showed a significant decrease from 89.63% to 39.81% in viable cells (Ann–/PI–) and a significant increase from 7.70% to 47.16% in apoptotic cells (Ann+/PI– and Ann+/PI+) at 17.5 μg/mL of CBD/THC treatment (Fig. 3B). On the other hand, the results on MCF-7 cells showed a decrease in the percentage of viable cells (Ann–/PI–) from 85.75% to 68.63% coupled to an increase in the total apoptotic cells (Ann+/PI– and Ann+/PI+) from 10.12% to 20.63% at 17.5 μg/mL as compared to control cells (Fig. 3C). A higher effect was observed after 48 h of treatment as shown by the significant decrease in viable cells (Ann–/PI–) from 82.82% to 51.69% along with a significant increase in the total apoptotic cell population (Ann+/PI– and Ann+/PI+) from 13.16% to 39.17% upon exposure to 17.5 μg/mL of CBD/THC (Fig. 3D). Our results demonstrate the promising effect of this cannabinoid mixture on promoting apoptosis in MDA-MB-231 and MCF-7 cell lines in a dose- and time-dependent manner.
Fig. 3.
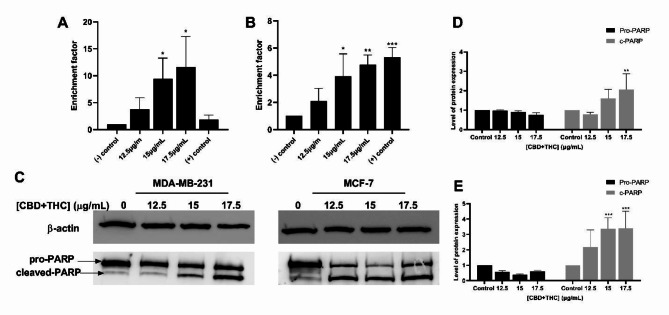
To further explore the potent pro-apoptotic activity of CBD/THC mixture on breast cancer cell lines, DNA fragmentation, a major hallmark of apoptosis was quantified using the ELISA method. Both cell lines showed a significant increase in the enrichment of DNA fragments while increasing the concentration of cannabinoid treatment. MDA-MB-231 cells reported a significant increase reaching 3.76, 9.48 and 11.64 upon exposure to 12.5, 15 and 17.5 μg/mL of treatment, respectively. The effect was noticed to be higher than the positive control used, Cisplatin (30uM) in this case (Fig. 4A). CBD and THC mixture showed similar effects on MCF-7 cells, with an increase in enrichment factor reaching 2.1, 3.93 and 4.77 at 12.5, 15 and 17.5 μg/mL treatment, respectively. Cisplatin, used as a positive control, showed a similar effect to CBD and THC treatment on DNA fragmentation in this cell line (Fig. 4B). At a molecular level, DNA fragmentation was further evaluated through PARP cleavage analysis via western blotting (Fig. 4C). Cannabinoid treatment induced a significant increase in the cleaved-PARP expression in both breast cancer cell lines reaching 2- and 3.5-fold increase at the highest concentration of treatment (17.5 μg/mL) in MDA-MB-231 and MCF-7 cells, respectively (Fig. 4D–E). These results confirmed the promising effect of the cannabinoid mixture on promoting DNA fragmentation in MDA-MB-231 and MCF-7 cells, a major hallmark of apoptosis.
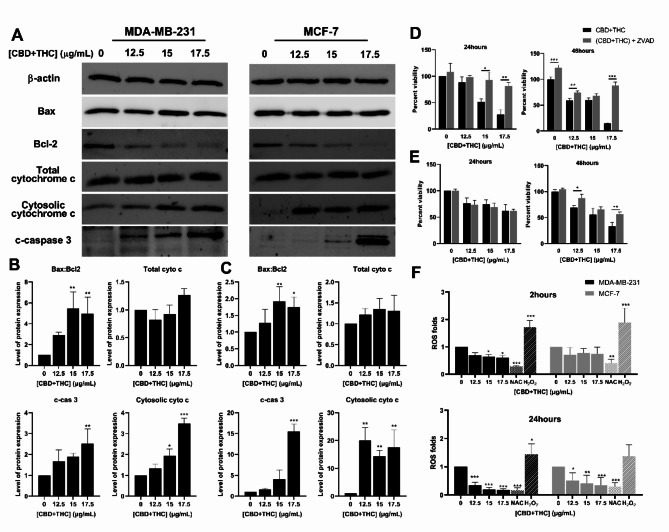
Fig. 4.
To decipher the molecular pathways through which the cannabinoid mixture inhibits the proliferation of breast cancer cell lines, western blot analysis of regulatory apoptotic markers was performed. The expression of major proteins involved in the activation or repression of the apoptotic pathway was assessed. The expression of the pro-apoptotic protein Bax was not altered and remained constant while the expression of the anti-apoptotic Bcl-2 protein was significantly reduced as compared to the control in both cell lines treated with CBD/THC (Fig. 5). The ratio of Bax to Bcl2 was calculated and found to be significantly increasing dose-dependently in both cell lines. The data showed a 1.7- fold increase in MCF-7 cells and 5- fold increase in MDA-MB-231 cells upon treatment with the highest concentration of CBD and THC (17.5 μg/mL) (Fig. 5B–C). These results reveal the promising pro-apoptotic activity of cannabis compounds on breast cancer cells via the activation of the mitochondrial-dependent pathway. To further confirm the activation of the intrinsic apoptotic pathway, cytochrome c release into the cytosol was assessed. Thus, we aimed at evaluating the expression of cytochrome-c isolated from total and cytosolic protein fractions via western immunoblotting. No significant change in the total cytochrome c expression was observed in both cell lines. However, subcellular fractionation revealed the increase in the cytosolic cytochrome c expression in MCF-7 (15-folds increase) and MDA-MB-231 (3.5-folds increase) (Fig. 5B–C). The release of cytochrome c into the cytoplasm further supports the activation of the mitochondrial-dependent apoptotic pathway.
Fig. 5.
The activation of the execution apoptotic pathway through caspase-3 cleavage was evaluated to further confirm the prominent effect of cannabinoids on promoting apoptosis in breast cancer cells. Western blot analysis revealed a consistent upregulated expression of cleaved caspase-3 after 24 h of treatment. A significant increase in the cleaved-caspase 3 expression in both breast cancer cell lines was noted, reaching 2.5- and 15.52-fold increase at the highest concentration of treatment (17.5 μg/mL) in MDA-MB-231 and MCF-7 cells, respectively (Fig. 5B–C). These results demonstrate the pro-apoptotic activity of CBD/THC mixture as revealed by the activation of apoptosis via the execution pathway. Moreover, the caspase inhibitor, Z-VAD-FMK, was used to further determine the role of caspases in promoting cell death. Therefore, MTS assay was performed on both cell lines pre-treated with Z-VAD for 30 min followed by the addition of CBD/THC mixture for 24 and 48 h. The viability of MDA-MB-231 cells significantly decreased, reaching 28% post treatment with 17.5 μg/mL of CBD/THC. However, the cell viability was found to be significantly higher, reaching approximately 80%, when the TNBC cells were treated for 24 h with Z-VAD in combination with 17.5 μg/mL of CBD/THC (Fig. 5D). On the other hand, Z-VAD treatment did not affect the response of MCF-7 cells to the cannabinoid treatment for 24 h. Cell viability assay revealed a decrease in percent viability of MCF-7 cells reaching 60% when treated with cannabinoids alone or in combination with Z-VAD (Fig. 5E). However, a significantly higher effect of Z-VAD on reversing the anti-proliferative effect of the cannabinoid mixture in MDA-MB-231 and MCF-7 cells was observed reaching 88% and 57% viability, respectively, as compared to 14% and 33% viability when treated with 17.5 μg/mL cannabinoids alone. These results further support our previous findings demonstrating the activation of apoptosis in a caspase-dependent manner, via the onset of the execution apoptotic pathway.
To determine if the activation of the intrinsic apoptotic pathway in this case was due to oxidative stress, DCFDA assay was performed to quantify the production of ROS in MDA-MB-231 and MCF-7 cells exposed to the cannabinoid treatment. The TNBC cells exhibited a weak yet significant decrease in ROS levels upon exposure for 2 h to the cannabinoid mixture reaching 0.6-fold upon treatment with the highest concentration of CBD/THC mixture (17.5 μg/mL). However, the instant ROS production levels were found to be moderately decreased in MCF-7 cells after 2 h of treatment reaching approximately 0.75-fold. Therefore, we aimed at assessing the time-dependent effect of CBD/THC mixture on inhibiting ROS production after 24 h of treatment. Our results showed an enhanced inhibition of ROS production in MDA-MB-231 and MCF-7 cell lines in a dose-dependent manner. A significant decrease in ROS production was observed reaching 0.17 in MDA-MB-231 and 0.2 in MCF-7 cells upon exposure to 17.5 μg/mL for 24 h (Fig. 5F). Our results were compared to the potent ROS inhibitor, NAC, and the ROS inducer H2O2 used on both cell lines. The dose- and time-dependent inhibition of ROS formation demonstrates the antioxidant properties of this treatment suggesting that the activation of the intrinsic apoptotic pathway is not dependent on oxidative stress, but rather on internal stimuli responsible for its activation.
Cannabinoids mixture promotes alteration to the regulatory role of autophagy in breast cancer cells
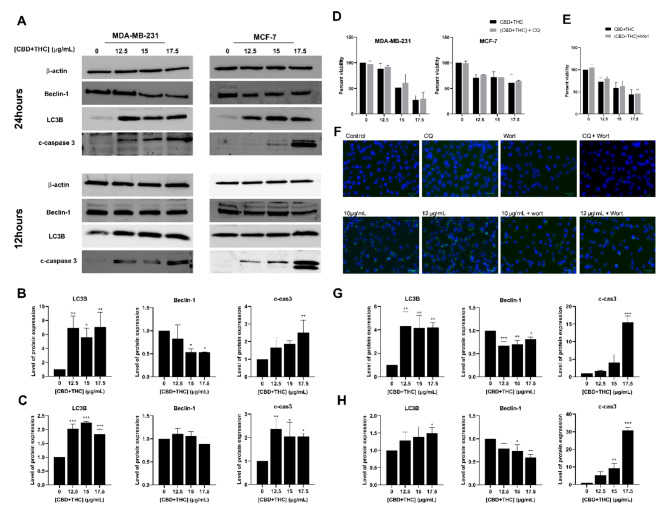
To further elucidate whether the mixture of CBD and THC can promote the activation of another cell death mechanism, western immunoblotting of major autophagic markers, namely LC3B and Beclin-1, was performed. Our results showed a significant increase in the expression level of the autophagosome membrane-bound protein, LC3B, in breast cancer cells upon treatment with CBD/THC for 24 h (Fig. 6). The quantification of LC3B revealed a sevenfold and fourfold increased expression in MDA-MB-231 and MCF-7 cells, respectively. However, our results showed a significant dose-dependent decrease in the expression level of Beclin-1 in both breast cancer cell lines. The quantification of the blots showed a decreased expression reaching 0.5 in MDA-MB-231 and 0.7 in MCF-7 cells upon treatment with cannabinoid mixture. Additionally, the expression of LC3B, Beclin-1 and cleaved caspase 3 proteins was also evaluated after 12 h of treatment to assess the time-dependent alterations in autophagy. Our western blot analysis revealed a significant 1.5-fold increase in the expression of LC3B in MDA-MB-231 and MCF-7 cells while also promoting a downregulated expression of Beclin-1 reaching 0.8 and 0.5 in MDA-MB-231 and MCF-7, respectively. The expression of cleaved caspase 3 was also evaluated 12 h post-treatment and was found to be significantly upregulated in both cell lines further confirming an earlier activation of apoptosis. Given the consistent increase in LC3B observed, we aimed to investigate whether autophagy is responsible for breast cancer cell death; hence, cytotoxicity assay was performed following the treatment of breast cancer cells with the cannabinoid mixture alone or in combination with autophagy inhibitors, CQ or Wort. MTS assay was conducted, and no significant effect was observed between the cells treated with CBD/THC alone or in combination with CQ for 24 h. The MDA-MB-231 cell viability reached 27% following treatment with 17.5 μg/mL CBD/THC mixture and 30% upon treatment with CBD/THC and CQ for 24 h. Similarly, MCF-7 cell viability decreased reaching 61% and 65% upon treatment with CBD/THC mixture alone and in combination with CQ, respectively (Fig. 6D). Since CQ inhibits the last stages of autophagy, namely autophagic flux, we then aimed at assessing the effect of Wort, an inhibitor of earlier stages of autophagy. Our results revealed no reversal effect upon exposure of breast cancer cells to cannabinoids alone or in combination with Wort reaching 44% and 46% viability, respectively, supporting therefore our previous findings using CQ (Fig. 6E). To further confirm the ability of Wort to reduce the formation of complexes needed for phagophore formation, visual assessment was performed via immunofluorescence using LC3B antibody. Microscopic images revealed the ability of Wort to reverse the effect of CQ as observed by the reduced accumulation of autophagosomes in the cytosol. Immunostaining also showed an even more pronounced accumulation of autophagosomes in the cytosol of cells treated with CBD/THC as compared to cells treated with CQ alone. Taking these results together, we can suggest that the mixture of CBD/THC is promoting cellular death via apoptosis activation rather than autophagy while also acting as an autophagic flux inhibitor in breast cancer cells.
Fig. 6.
Cannabinoids mixture exhibits anti-metastatic properties by inhibiting the migratory and invasive capacities of TNBC cells
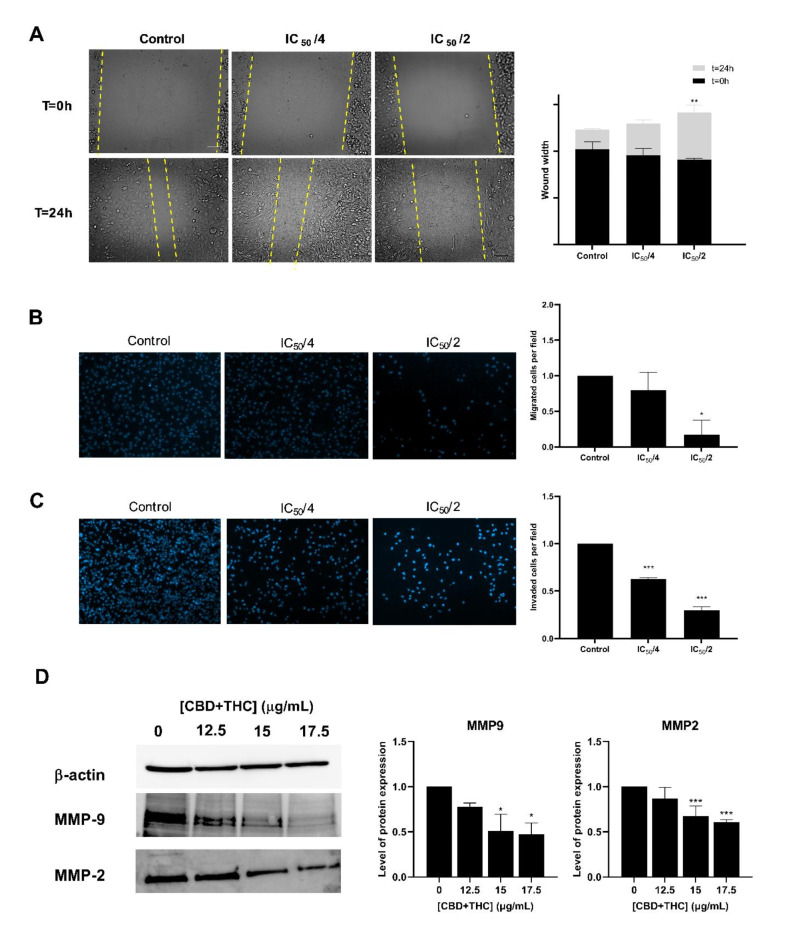
From a different perspective, we aimed at assessing the anti-metastatic properties of CBD and THC on the most aggressive type of breast cancer, namely TNBC cells. Wound healing and trans-well migration and invasion assays were performed. In the wound healing assay, the TNBC cells were co-cultured with low doses of CBD/THC mixture (IC50/4 and IC50/2) knowing to exhibit a mild effect on cell viability, following the scratch formation. In Fig. 7, images from time points 0 and 24 h are shown along with the quantification results of the wound closure (Fig. 7A). Our results showed that the cannabinoid mixture significantly inhibited the migration of MDA-MB-231 cells across the wound space after 24 h, as compared to the control cells. A significant increase in the wound width was observed upon exposure of the MDA-MB-231 cells to 6.25 μg/mL (IC50/2) of CBD/THC for 24 h. To further confirm the results of wound healing assay, trans-well migration assay was performed. Our results suggested that after 24 h of treatment, the motility of the TNBC cells was significantly inhibited as revealed by the prominent decrease in the number of migrated cells when compared to untreated cells (Fig. 7B) A significant decrease in migrated cells was noticed reaching 0.8 and 0.2 upon exposure to 3.12 and 6.25 μg/mL, respectively. On the other hand, we tested the inhibitory effect of CBD/THC mixture on inhibiting cell invasion through a Matrigel coat that resembles the extracellular environment. Our data revealed a significant inhibitory effect of this cannabinoid’s mixture on the invasive capacities of MDA-MB-231 cells following CBD/THC treatment (6.25 μg/mL) for 48 h reaching 0.3 folds (Fig. 7C). The anti-metastatic effect was further elucidated at a molecular level as revealed by the ability of CBD/THC to downregulate the expression of Matrix Metalloproteinases (MMP) (Fig. 7D). Our data showed a significant downregulated expression of MMP2 and MMP9, reaching 0.6 and 0.5, respectively upon exposure to 17.5 μg/mL of CBD/THC. Taking these results together, we can conclude that the CBD/THC mixture exhibits an inhibitory effect on the metastatic ability of TNBC cells.
Fig. 7.
Discussion
Humans have traditionally used plants for various purposes as an unbounded reservoir of bioactive molecules that play a therapeutic role in the treatment of several ailments including tumor progression. Since the beginning of human civilization, Cannabis sativa L. has been used for both recreational and therapeutic purposes1,38. Multiple studies conducted recently discovered a wide range of natural compounds, namely CBD and THC, that selectively target cancer cells while sparing healthy cells from damage. In-depth-investigation has been carried out in the past twenty years to explore the clinical and biological prospects of cannabinoids, alone or in combination, in the treatment of various diseases including cancer. Recently, Schoeman et al., aimed at studying the antiproliferative properties of medicinal (high CBD) and recreational (high THC) cannabis plants on two different breast cancer cell lines, MDA-MB-231 and MCF-7 cells20. Given their promising results, we aimed at identifying in the current study the anticancer mechanism of these cannabinoids isolated from the Lebanese C. sativa strain. Thus, a mixture of CBD and THC (3:1) in ratios comparable to the Lebanese medicinal plant was used to elucidate the mechanism of action in breast cancer cells, knowing that such formulation was found to selectively inhibit the proliferation of MDA-MB-231 and MCF-7 cells with no adverse effect on normal cells20,26.
The prominent effect of C. sativa major compounds, CBD and THC, was previously established on various types of cancer. The non-psychoactive cannabidiol (CBD) was previously reported to inhibit the growth of breast, glioma, leukemia, lung and colon cancer cells39,40. Similarly, various studies on THC revealed its cytotoxic effect on breast, leukemia, lung, hepatocellular and prostate cancers15,41,42. Our results demonstrate that the combination of the two most abundant cannabinoids, namely CBD and THC, exhibit a noticeably higher inhibitory effect on breast cancer cells as compared to the effect of the individual compounds. This is in accordance to previous studies reporting the synergistic effect of combining CBD and THC on lung and glioblastoma cells17,43. The mixture of CBD and THC in equal amounts (1:1) was found to exhibit an anti-proliferative effect on leukemic cells by enhancing their sensitivity to cytarabine and vincristine19, while more recently, a study on high THC and high CBD strains, revealed a notable inhibition of breast cancer cell viability20. These results were further supported in our study, where a high CBD to THC (3:1) mixture revealed a significant antiproliferative effect in a dose- and time-dependent manner on MDA-MB-231 and MCF-7 cells. Interestingly, cannabinoids were previously found to exhibit a selective inhibitory effect on cancer cells without affecting the growth of healthy cells44. A study conducted by Shrivastava et al. showed that CBD exhibit a selective effect on cancer cells with no cytotoxic activity on normal mammary cells, namely MCF-10A45, similar to other studies evaluating the THC compound and revealing its protective effect on normal cells10,46. It was previously suggested that the differential expression of the cannabinoid receptors on the surface of cancer and normal cells, might be a possible explanation for their selective effect on cancer cells while protecting normal cells from their cytotoxic and apoptotic activity47. Based on the prominent dose- and time-dependent effect reported in this study on both cell lines, subsequent experiments were performed using CBD/THC mixture concentrations which are closest to the IC50 at 24 and 48 h on MDA-MB-231 and MCF-7 cells.
A series of experiments were performed to decipher the molecular mechanisms activated in breast cancer cells exposed to the cannabinoid mixture. We were interested in determining whether this effect is due to cell cycle arrest, using flow cytometry. Our analysis of the cell cycle progression showed a significant upregulation in cellular fragmentation of MDA-MB-231 and MCF-7 cells as revealed by the consistent increase in the pre-G subpopulation. This is in accordance to previous studies evaluating the role of CBD monotreatment or cotreatment with THC in inducing cell death rather than cell cycle arrest in cervical and multiple myeloma cell lines, respectively48,49. These studies among several others reveal that the anticancer activity of CBD/THC mixture is directly associated with the activation of a programmed cell death mechanism. Thus, we hypothesized that the cellular death observed in our study might be due to the activation of apoptosis. To unravel whether this mechanism is indeed activated and responsible for this effect, flow cytometry analysis of cells dually stained with Annexin V and Propidium Iodide was performed. Our data suggested a significant increase in the percentage of apoptotic cells (Ann+ and PI+/-) confirming that CBD/THC mixture promotes the activation of apoptosis in breast cancer cell lines. Additionally, assessing DNA fragmentation might provide further confirmation of apoptosis induction, since the activity of endonucleases promotes the cleavage of chromosomal DNA into oligonucleosomal fragments that could be detected via the ELISA method50. In our study, a significant increase in the enrichment of DNA oligomers was observed in both cell lines, confirming the pro-apoptotic activity of CBD and THC on MDA-MB-231 and MCF-7 cells, in vitro. Previous studies reported a similar pro-apoptotic effect of cannabinoids on different types of cancer, revealed by the phosphatidylserine translocation to the outer membrane in breast cancer cells and the induction of DNA fragmentation in gastric cancer cells45,51. These hallmarks are the results of regulatory proteins that allowed the activation of apoptosis, found to be in this study, the major mechanism through which breast cancer cells undergo death upon exposure to the mixture of CBD and THC.
As a first step in the initiation of the intrinsic apoptotic pathway, it is crucial to determine the balance between different apoptotic markers such as the pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins, that trigger mitochondrial dysfunction and dictate cellular fate52. In this study, western blot analysis revealed a downregulation in the expression of the Bcl-2 protein with no alteration in the expression of Bax in both cell lines. It is suggested that the Bax/Bcl-2 ratio is of a great importance to determine the loss of the mitochondrial membrane integrity since it acts as a “rheostat” in regulating mitochondrial function53. Our results confirmed the significant upregulation in the ratio of Bax/Bcl2 upon exposure of MDA-MB-231 and MCF-7 cells to CBD/THC mixture, suggesting therefore the perturbation of the outer mitochondrial membrane. This in turn allows the formation of pores and the subsequent release of apoptogenic factors such as cytochrome c to the cytoplasm which was also evaluated and confirmed in our study. Given these results, we can conclude that the intrinsic apoptotic pathway is activated in the two breast cancer cell lines similarly to previous studies in which cannabinoids, CBD or THC, were able to promote the activation of the mitochondrial-dependent apoptotic pathway via the increase in Bax/Bcl-2, the loss of mitochondrial membrane integrity, and the release of cytochrome into the cytosol54–56. Next, we suggested that the activation of this pathway might be due to an internal oxidative stress caused by an increase in ROS formation. Therefore, an instant and time-dependent ROS production assay was performed on both cell lines. Our data show that this mixture exhibits a protective effect on both breast cancer cells via its antioxidant activity, which could be partly due to the high CBD content, previously established for its antioxidant properties. It has been recently demonstrated that cannabinoids can promote the activation of an oxidative stress-independent apoptotic pathway in pancreatic cancer cells through the mitochondrial axis57,58.
The execution pathway is the final terminal stage of apoptosis leading to the onset of apoptotic trademarks including membrane blebbing, cell shrinkage and DNA fragmentation59. To further confirm the induction of the execution apoptotic pathway, the expression of cleaved caspase 3, a key regulatory protein of this pathway, was evaluated and found to be upregulated in MDA-MB-231 and MCF-7 after 24 h of treatment. Caspase 3 is known to be cleaved to its active forms during apoptosis. Therefore, in order to confirm the activation of the execution pathway, we assessed the role of the active caspase 3 in promoting DNA fragmentation via PARP cleavage from 116 to 89 kDa and 24 kDa fragments. PARP is a major protein that plays a crucial role in DNA repair through its DNA-binding domain that recognizes DNA lesions and recruits the DNA repair machinery60. The data obtained revealed a significant increase in cleaved PARP expression in both breast cancer cell lines. These results are consistent with the observed increase in apoptosis demonstrated by the flow cytometry and cell death ELISA performed. To further confirm that the cannabinoids mixture promotes a caspase-dependent cell death, a pan caspase inhibitor, Z-VAD-FMK, was used. The combination of CBD and THC with Z-VAD revealed a significantly higher viability in MDA-MB-231 and MCF-7 cells after 24 and 48 h of treatment, respectively. Thus, the presence of Z-VAD reversed, in a time-dependent manner, the cytotoxic effect of CBD/THC mixture, which confirmed the induction of a caspase-dependent apoptotic pathway. In fact, several studies demonstrated the apoptotic activity of cannabinoids compounds on different types of cancer by promoting apoptosis via the activation of the execution mechanism. CBD and THC monotreatment were demonstrated to induce apoptosis via caspase3 along with PARP cleavage in lung, colon and breast cancer cells45,51,61–63.
It was previously established that cannabinoids, might also promote the activation of the autophagy programmed cell death mechanism in various types of cancer. In this study, we were interested in evaluating the role of CBD and THC cotreatment on autophagy in breast cancer cell lines by investigating the levels of several protein markers correlated to autophagy. Our results showed that the cannabinoid mixture caused an increase in the levels of the autophagosome membrane-bound protein, LC3B, in both breast cancer cells. This is in line with previous studies reporting an enhanced expression of the LC3B protein in melanoma and breast cancer cell lines18,45. It was also found that CBD monotreatment promotes a modest increase in LC3B expression as compared to its combination with THC, which greatly enhanced its expression in multiple myeloma cells64. These studies together, correlated the upregulation in LC3B to an increase in inducible autophagy above baseline level. It is noteworthy to mention that an increase in LC3B could be due to an increase in autophagy induction or to an inhibition of the last stages of autophagy, namely autophagic flux. Moreover, a crosstalk between autophagy and apoptosis is controversial, therefore, Beclin-1, a protein tightly regulated by apoptotic caspases and involved in early stages of autophagy was also evaluated in this study. Interestingly, our results revealed a downregulated expression of Beclin-1 in both cell lines treated with the cannabinoid mixture. Previous reports provided evidence that Beclin-1, a caspase substrate and member of the class III PI3K complex, is cleaved upon the activation of caspase-3 during apoptosis65,66. In this study, we found that the overexpression of Beclin-1 is no longer detected, which could be due to its cleavage by caspases, particularly the cleaved caspase 3 form upon the induction of apoptosis. Since we observed an increase in LC3B and contradicting results from Beclin-1 in addition to the data obtained upon CQ treatment that was not able to revert the cytotoxic effect of CBD/THC, visual assessment of the autophagosome membrane-bound LC3B protein was performed. Microscopic evaluation confirmed the accumulation of autophagic vesicles in the cytosol upon exposure of the cells to CBD/THC. However, this observation could be attributed to an induction of autophagy above basal level, or it could be correlated to the ability of the treatment to inhibit autophagic flux. As such, immunostaining results revealed autophagosome accumulation in cells treated with CBD/THC similar and even more pronounced than those treated with CQ alone. Thus, this might suggest that cannabinoids treatment is even more efficient than CQ in inhibiting autophagic flux and might explain therefore why no reversal of cellular death was observed upon combination of CBD/THC with the inhibitor. Given that, we aimed at evaluating Wort, another inhibitor of earlier stages of autophagy to assess its ability to completely reverse the death promoted by the drug. Similarly, we found that Wort was not able to abolish CBD/THC induced cell death while also observing a visual decrease in cell count upon exposure of the cells to the cannabinoid’s mixture along with Wort. This further supports our previous findings that breast cancer cell death is mostly correlated to apoptosis rather than autophagy and that the apoptotic pathways are more efficient in promoting cellular death. In fact, apoptosis was found to be triggered earlier as observed by the activation of caspase 3 through its cleavage at 12 h even when Beclin-1 was still not significantly downregulated. The activity of Beclin-1 is lost upon the translocation of its cleaved fragment into the mitochondria, stimulating the susceptibility of the cells to apoptotic death66. Therefore, autophagy might not be the major mechanism through which breast cancer cells are dying since apoptosis is activated at earlier stages, repressing basal levels of autophagy. Although recent studies have provided valuable information into the mechanisms through which the major cannabinoids namely CBD and THC promote cell death, the specific pathways and receptors mediating their effect remain unclear. The two major cannabinoid receptors CB1 and CB2 through which the phytocannabinoids might exert their physiological effect were previously established for their role in the endocannabinoid system67. However, several studies reported the low affinity of CBD to both of these receptors suggesting its possible interaction with other channels such as the receptor potential vanilloid (TRPV) and Orphan-G-protein-coupled receptor 55 (GPR55) among others16,68. Taking these together, the therapeutic effects of cannabinoids are more complex since they might involve several receptors beyond the well-established receptors, CB1 and CB2.
Metastasis continues to be the leading cause of death in cancer patients. Previous reports revealed the prominent role of cannabinoids in inhibiting the migration and invasion of various types of cancer including breast, cervical and lung cancer cells69,70. In this study, we evaluated the inhibitory effect of the CBD/THC mixture on the motility of TNBC cell line which is considered the most aggressive type of breast cancer. The wound healing assay showed an impairment of MDA-MB-231 motility, similar to the results of the trans-well migration assay in which we assessed the ability of treated cells to move across a porous membrane. Our data revealed a noticeable and significant suppression of cell migration as compared to the control. The process of metastasis is not solely dependent on the migratory capacity of cancer cells but also on their invasive properties and their ability to degrade extracellular matrix71. Cancer cells acquire their invasive properties by the expression of several invasive markers, particularly MMPs. These proteins were previously established for their proteolytic activity and ability to degrade extracellular matrix (ECM), hence promoting cell invasion and progression72. Therefore, we investigated the effect of CBD/THC mixture on suppressing the invasion of breast cancer cells by assessing the ability of MDA-MB-231 to degrade Matrigel and move across the Boyden chamber membrane. Our results are in accordance with previous studies that showed a decrease in the invasive ability of urothelial cell carcinoma, prostate, glioma and glioblastoma stem cells among others1,16,73,74. Previous studies reported the anti-metastatic activity of CBD or THC to be mediated via the downregulation of MMPs among several other pro-metastatic factors16. In fact, Elbaz and his colleagues reported that in breast cancer, CBD treatment can inhibit cell invasion by suppressing the secretion of MMP-2 and MMP-969. Previous studies reported that cannabinoids including CBD, might exhibit their anti-metastatic effect in a CB1/CB2-dependent or independent manner75. In the current study, the mixture of CBD/THC revealed an anti-metastatic activity on TNBC cells via a downregulation in the MMPs expression, namely MMP-2 and MMP-9. Taking these results together, we can conclude that the mixture of major cannabinoids isolated from the Lebanese cannabis plant, not only promote the activation of programmed cell death mechanism, but also suppress the migratory and invasive capacities of aggressive breast cancer cells, in vitro. To date, several studies evaluated the anti-cancer properties of cannabinoids on various types of cancer revealing their promising anti-proliferative, pro-apoptotic, anti-invasive and anti-metastatic properties on various types of cancer, in vitro and in vivo76.
Conclusion
Before the use of cannabis in Western medical systems, C. sativa has been used for at least 1800 years. It is well established that its biochemical constituents are predominantly responsible for the various functions associated with its consumption. Our study showed that CBD and THC isolated from the Lebanese cannabis strains, in ratios comparable to the medicinal plants, exhibit promising effect on breast cancer cell lines. The anticancer activity of this mixture was revealed by its ability to promote cellular fragmentation, phosphatidylserine translocation and DNA fragmentation while inhibiting the motility of aggressive breast cancer cells. Our results showed a pro-apoptotic activity on MDA-MB-231 and MCF-7 cells via the activation of the intrinsic apoptotic pathway. Moreover, we found that even if autophagy was altered in breast cancer cell lines, it is not the major mechanism leading to cellular death. Also, we demonstrated that this mixture was effective in halting the progression of breast cancer cells via the suppression of cancer cell migration and invasion. Further investigations are required to further understand the mechanisms of actions responsible for the observed effect. Specifically, studying the interaction between the two programmed cell death mechanisms, apoptosis and autophagy, will provide further insight into the intricate mechanisms involved. Additionally, identifying the receptors involved in mediating the observed therapeutic effect is crucial for developing targeted therapy. Evaluating the anticancer properties of this cannabinoid mixture on other breast cancer cell lines is also essential to understand the broader activity of the mixture across different breast cancer subtypes. This work paves the way for future investigations that need to be done in vivo and at a later stage, in clinical trials to enhance cancer patients’ health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Performing experiments, Data collection and Formal analysis: M.Y. Figures and First draft preparation: M.Y. Plant extract collection and analysis reported: W.S, J.I, S.A.T and M.E.H. Conceptualization and final revision of the manuscript: M.M, H.Y.N and S.R. Funding acquisition, Methodology, Project administration and Supervision: S.R. All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded by intramural funds from the Department of Natural Science, Lebanese American University, to secure space, equipment and consumables.
Data availability
All data generated and analyzed in this study are mentioned in this manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peeri, H. & Koltai, H. Cannabis biomolecule effects on cancer cells and cancer stem cells: Cytotoxic, anti-proliferative, and anti-migratory activities. Biomolecules12, 491 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blebea, N. M., Hancu, G., Vlad, R. A. & Pricopie, A. Applications of capillary electrophoresis for the determination of cannabinoids in different matrices. Molecules28, 638 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niloy, N. et al. Effect of cannabis on memory consolidation, learning and retrieval and its current legal status in India: A review. Biomolecules13, 162 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall, W. et al. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. The Lancet394, 1580–1590 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Schanknecht, E., Bachari, A., Nassar, N., Piva, T. & Mantri, N. Phytochemical constituents and derivatives of Cannabis sativa: Bridging the gap in Melanoma treatment. Int J Mol Sci24, 859 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Brito Siqueira, A. L. G. et al. Phytocannabinoids: Pharmacological effects, biomedical applications, and worldwide prospection. J. Tradit. Complement. Med.13, 575–587 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koltai, H. & Shalev, N. Anti-cancer activity of Cannabis sativa phytocannabinoids: Molecular mechanisms and potential in the fight against ovarian cancer and stem cells. Cancers14, 4299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarasiuk, A., Mirocha, G. & Fichna, J. Current status of complementary and alternative medicine interventions in the management of pancreatic cancer—An overview. Curr. Treat. Options Oncol.24, 1852–1869 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bautista, J. L., Yu, S. & Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, bioactivities, and biotechnology. ACS Omega6, 5119–5123 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prateeksha, P. et al. Tetrahydrocannabinols: Potential cannabimimetic agents for cancer therapy. Cancer Metastasis Rev.10.1007/s10555-023-10078-2 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Atalay Ekiner, S., Gęgotek, A. & Skrzydlewska, E. The molecular activity of cannabidiol in the regulation of Nrf2 system interacting with NF-κB pathway under oxidative stress. Redox Biol.57, 102489 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalsa, J. H. et al. Review: Cannabinoids as medicinals. Curr. Addict. Rep.9, 630–646 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida, C. F., Teixeira, N., Correia-da-Silva, G. & Amaral, C. Cannabinoids in breast cancer: Differential susceptibility according to subtype. Molecules27, 156 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velasco, G., Sánchez, C. & Guzmán, M. Anticancer mechanisms of cannabinoids. Curr. Oncol.23, S23–S32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomko, A. M., Whynot, E. G., Ellis, L. D. & Dupré, D. J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in Cannabis. Cancers12, 1985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovalchuk, O. & Kovalchuk, I. Cannabinoids as anticancer therapeutic agents. Cell Cycle19, 961–989 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcu, J. P. et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther.9, 180–189 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong, J. L. et al. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Invest. Dermatol.135, 1629–1637 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Scott, K. A., Dalgleish, A. G. & Liu, W. M. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int. J. Oncol.51, 369–377 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Schoeman, R., de la Harpe, A., Beukes, N. & Frost, C. L. Cannabis with breast cancer treatment: Propitious or pernicious?. 3 Biotech12, 54 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prodhan, A. S. U. et al. Breast cancer management in the Era of Covid-19; key issues, contemporary strategies, and future implications. BCTT15, 51–89 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardiff, R. D. & Borowsky, A. D. At last: Classification of human mammary cells elucidates breast cancer origins. J. Clin. Invest.124, 478–480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin, L., Duan, J.-J., Bian, X.-W. & Yu, S. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Research22, 61 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turashvili, G. & Brogi, E. Tumor heterogeneity in breast cancer. Front. Med.4, 227 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huntley, A. L., de Valois, B., Dog, T. L. & Borrelli, F. Complementary and alternative medicine and cancer survivorship. Evid. Based Complement Alternat. Med.2012, 850429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shebaby, W. et al. In vivo and in vitro anti-inflammatory activity evaluation of Lebanese Cannabis sativa L. ssp. indica (Lam.). J. Ethnopharmacol.270, 113743 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Nafeh, G. et al. Urtica dioica leaf infusion enhances the sensitivity of triple-negative breast cancer cells to cisplatin treatment. Pharmaceuticals16, 780 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayyed, K. et al. Acute cytotoxicity, genotoxicity, and apoptosis induced by petroleum VOC emissions in A549 cell line. Toxicol. Vitro83, 105409 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Idriss, M., Hodroj, M. H., Fakhoury, R. & Rizk, S. Beta-tocotrienol exhibits more cytotoxic effects than gamma-tocotrienol on breast cancer cells by promoting apoptosis via a P53-independent PI3-kinase dependent pathway. Biomolecules10, 577 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Khoury, M. et al. Malva pseudolavatera leaf extract promotes ROS induction leading to apoptosis in acute Myeloid Leukemia cells in vitro. Cancers12, 435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husein, D. M. et al. Severe pathogenic variants of intestinal sucrase-isomaltase interact avidly with the wild type enzyme and negatively impact its function and trafficking. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis.1868, 166523 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Baz, J. et al. Enhanced potency of a chloro-substituted polyaromatic platinum(II) complex and its platinum(IV) prodrug against lung cancer. Chem. Biol. Interact.388, 110834 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Subcellular Fractionation Protocol. https://www.abcam.com/en-fr/technical-resources/protocols/subcellular-fractionation.
- 34.Tannous, S., Haykal, T., Dhaini, J., Hodroj, M. H. & Rizk, S. The anti-cancer effect of flaxseed lignan derivatives on different acute Myeloid Leukemia cancer cells. Biomed. Pharmacother.132, 110884 (2020). [DOI] [PubMed] [Google Scholar]
- 35.El Hayek, L. et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci.39, 2369–2382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younes, M. et al. The selective anti-proliferative and pro-apoptotic effect of A. cherimola on MDA-MB-231 breast cancer cell line. BMC Complement. Med. Ther.20, 343 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haykal, T. et al. Annona Cherimola Seed extract activates extrinsic and intrinsic apoptotic pathways in leukemic cells. Toxins11, 1–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leinen, Z. J. et al. Therapeutic potential of Cannabis: A comprehensive review of current and future applications. Biomedicines11, 2630 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massi, P. et al. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J. Pharmacol. Exp. Ther.308, 838–845 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Massi, P., Solinas, M., Cinquina, V. & Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol.75, 303–312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allister, S. D. M. et al. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J. Neurooncol.74, 31–40 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Takeda, S. et al. Δ9-Tetrahydrocannabinol disrupts estrogen-signaling through up-regulation of estrogen receptor β (ERβ). Chem. Res. Toxicol.10.1021/tx4000446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mashabela, M. D. & Kappo, A. P. Anti-cancer and anti-proliferative potential of cannabidiol: A cellular and molecular perspective. Int. J. Mol. Sci.25, 5659 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salazar, M. et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest.119, 1359–1372 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrivastava, A., Kuzontkoski, P. M., Groopman, J. E. & Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther.10, 1161–1172 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Fulda, S. Autophagy in cancer therapy. Front. Oncol.7, 128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermanson, D. J. & Marnett, L. J. Cannabinoids, endocannabinoids and cancer. Cancer Metastasis Rev.30, 599–612 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Arencibia, M., Molina-Holgado, E. & Molina-Holgado, F. Effect of endocannabinoid signalling on cell fate: Life, death, differentiation and proliferation of brain cells. Br. J. Pharmacol.176, 1361–1369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukhele, S. T. & Motadi, L. R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Alternat. Med.16, 335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matassov, D., Kagan, T., Leblanc, J., Sikorska, M. & Zakeri, Z. Measurement of Apoptosis by DNA Fragmentation. In Apoptosis Methods and Protocols (ed. Brady, H. J. M.) 1–17 (Humana Press, Totowa, 2004). [DOI] [PubMed]
- 51.Jeong, S. et al. Cannabidiol promotes apoptosis via regulation of XIAP/Smac in gastric cancer. Cell Death Dis.10, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khodapasand, E., Jafarzadeh, N., Farrokhi, F., Kamalidehghan, B. & Houshmand, M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer?. Iran. Biomed. J.19, 69–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volkmann, N., Marassi, F. M., Newmeyer, D. D. & Hanein, D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ21, 206–215 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gross, C., Ramirez, D. A., McGrath, S. & Gustafson, D. L. Cannabidiol induces apoptosis and perturbs mitochondrial function in human and canine glioma cells. Front. Pharmacol.12, 725136 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malheiro, R. F., Carmo, H., Carvalho, F. & Silva, J. P. Cannabinoid-mediated targeting of mitochondria on the modulation of mitochondrial function and dynamics. Pharmacol. Res.187, 106603 (2023). [DOI] [PubMed] [Google Scholar]
- 56.Sarafian, T. A., Kouyoumjian, S., Khoshaghideh, F., Tashkin, D. P. & Roth, M. D. Δ9-tetrahydrocannabinol disrupts mitochondrial function and cell energetics. Am. J. Physiol. Lung Cell. Mol. Physiol.284, L298–L306 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Atalay, S., Jarocka-Karpowicz, I. & Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants9, 21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emhemmed, F. et al. Cannabis sativa extract induces apoptosis in human pancreatic 3D cancer models: Importance of major antioxidant molecules present therein. Molecules27, 1214 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vermeulen, K., Van Bockstaele, D. R. & Berneman, Z. N. Apoptosis: Mechanisms and relevance in cancer. Ann. Hematol.84, 627–639 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Mashimo, M. et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem.296, 100046 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leelawat, S. et al. Anticancer activity of Δ9-tetrahydrocannabinol and cannabinol in vitro and in human lung cancer xenograft. Asian Pacific J. Trop. Biomed.12, 323 (2022). [Google Scholar]
- 62.Lombard, C., Nagarkatti, M. & Nagarkatti, P. S. Targeting cannabinoid receptors to treat leukemia: Role of cross-talk between extrinsic and intrinsic pathways in Δ9-tetrahydrocannabinol (THC)-induced apoptosis of Jurkat cells. Leukemia Res.29, 915–922 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Rieder, S. A., Chauhan, A., Singh, U., Nagarkatti, M. & Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology215, 598–605 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nabissi, M. et al. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget7, 77543–77557 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho, D.-H. et al. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett.274, 95–100 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Fan, Y.-J. & Zong, W.-X. The cellular decision between apoptosis and autophagy. Chin. J. Cancer32, 121–129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Velasco, G., Hernández-Tiedra, S., Dávila, D. & Lorente, M. The use of cannabinoids as anticancer agents. Progress Neuro-Psychopharmacol. Biol. Psychiatry64, 259–266 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Hosami, F., Ghadimkhah, M. H., Salimi, V., Ghorbanhosseini, S. S. & Tavakoli-Yaraki, M. The strengths and limits of cannabinoids and their receptors in cancer: Insights into the role of tumorigenesis-underlying mechanisms and therapeutic aspects. Biomed. Pharmacother.144, 112279 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Elbaz, M. et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of cannabidiol in breast cancer. Mol. Oncol.9, 906–919 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramer, R., Merkord, J., Rohde, H. & Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol.79, 955–966 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Martin, T. A., Ye, L., Sanders, A. J., Lane, J. & Jiang, W. G. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. In Madame Curie Bioscience Database [Internet] (Landes Bioscience, 2013).
- 72.Cabral-Pacheco, G. A. et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci.21, 9739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anis, O. et al. Cannabis-derived compounds Cannabichromene and Δ9-tetrahydrocannabinol interact and exhibit cytotoxic activity against urothelial cell carcinoma correlated with inhibition of cell migration and cytoskeleton organization. Molecules26, 465 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Reilly, E. et al. Cannabidiol inhibits the proliferation and invasiveness of prostate cancer cells. J. Nat. Prod.86, 2151–2161 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dariš, B., Verboten, M. T., Knez, Ž & Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn J. Basic Med. Sci19, 14–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cherkasova, V. et al. Use of Cannabis and cannabinoids for treatment of cancer. Cancers14, 5142 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed in this study are mentioned in this manuscript.