Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Oct 18;12(6):e1219. doi: 10.1002/prp2.1219
Abstract
The endocannabinoid system has garnered attention as a potential therapeutic target in a range of pathological disorders. Cannabinoid receptors type 2 (CB2) are a class of G protein‐coupled receptors responsible for transmitting intracellular signals triggered by both endogenous and exogenous cannabinoids, including those derived from plants (phytocannabinoids) or manufactured synthetically (synthetic cannabinoids). Recent recognition of the role of CB2 receptors in fibrosis has fueled interest in therapeutic targeting of CB2 receptors in fibrosis. Fibrosis is characterized by the alteration of the typical cellular composition within the tissue parenchyma, resulting from exposure to diverse etiological factors. The pivotal function of CB2 agonists has been widely recognized in the regulation of inflammation, fibrogenesis, and various other biological pathologies. The modulation of CB2 receptors, whether by enhancing their expression or activating their function, has the potential to provide benefits in numerous conditions, particularly by avoiding any associated adverse effects on the central nervous system. The sufficient activation of CB2 receptors resulted in the complete suppression of gene expression related to transforming growth factor β1 and its subsequent fibrogenic response. Multiple reports have also indicated the diverse functions that CB2 agonists possess in mitigating chronic inflammation and subsequent fibrosis development in various types of tissues. While currently in the preclinical stage, the advancement of CB2 compounds has garnered significant attention within the realm of drug discovery. This review presents a comprehensive synthesis of various independent experimental studies elucidating the pivotal role of identified natural and synthetic CB2 agonists in the pathophysiology of organ fibrosis, specifically in the cardiac, hepatic, and renal systems.
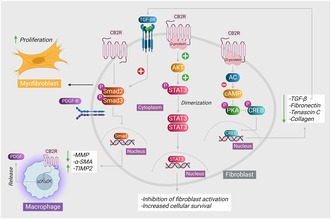
Graphical illustration of the mechanistic action of CB2 agonists on fibroblast.
Abbreviations
- 2‐AG
- 2‐arachidonoyl glycerol
- cAMP
- cyclic adenosine monophosphate
- CB1
- cannabinoid receptors type 1
- CB2
- cannabinoid receptors type 2
- Cnr2
- cannabinoid receptor 2
- CNS
- central nervous system
- ECM
- extracellular matrix
- ECS
- endocannabinoid system
- FAAH
- fatty acid amide hydrolase
- GSH
- glutathione
- HOCl
- hypochlorous acid
- IL‐1β
- interleukin‐1 beta
- IL‐2
- interleukin‐2
- IL‐6
- interleukin‐6
- LPS
- Lipopolysaccharide
- MAGL
- monoglyceride lipase
- MAPK
- mitogen‐activated protein kinases
- MMP
- matrix metalloproteinase
- mRNA
- messenger RNA
- NAFLD
- non‐alcoholic fatty liver disease
- Nrf2
- nuclear factor erythroid 2‐related factor 2
- PDGF
- platelet‐derived growth factor
- siRNA
- small interfering RNA
- SOD
- superoxide dismutase
- TAC
- transverse aortic constriction
- TGF‐β
- tumor growth factor‐beta
- Th1
- type 1 T helper cells
- THC
- Tetrahydrocannabinol
- TNF‐α
- tumor necrosis factor alpha
- α‐SMA
- alpha smooth muscle actin
1. INTRODUCTION
Organ fibrosis is correlated with perturbed cellular homeostasis and aberrant architectural organization of the organ. This phenomenon occurs as a reaction to an etiological agent, typically a chronic inflammatory response that continuously causes damage to the endothelium or epithelium tissue of the organ. Consequently, the original functional tissue is gradually replaced by connective tissue. During the process of replacement, as observed in numerous instances, the regeneration of the endothelium tissue tends to be inadequate owing to the varying regenerative and proliferative capacities exhibited by different organs. Therefore, this process results in a vigorous accumulation of extracellular matrix (ECM) proteins by the activated mesenchymal cells. As a consequence, this leads to the alteration of the impacted organ, resulting in the development of scar tissue, increased thickness, and rigidity, ultimately leading to compromised functionality.1This pathological anomaly has the potential to impact various organs, encompassing the integumentary system, cardiovascular system, respiratory system, hepatic system, and renal system. While wound healing serves as a vital adaptive process necessary for addressing cellular damage and restoring injured tissue, an inadequate response fails to effectively restore tissue homeostasis, leading to the development of fibrosis.2Inflammation serves as the initial stage in the process of cellular repair and clearance. Nevertheless, chronic inflammation plays a pivotal role in the initiation of fibrosis by facilitating the secretion of fibrogenic cytokines and instigating an abnormal buildup of collagen fibers.3Hence, anti‐inflammatory drugs are extensively employed as a cornerstone in the therapeutic approach toward fibrosis, owing to the compelling body of evidence elucidating the intricate connection between inflammation and this pathological fibrosis.4
The endocannabinoid system (ECS) is comprised of endocannabinoids, which are signaling molecules, and their corresponding cannabinoid receptors. Additionally, enzymes play a crucial role in regulating the overall turnover of these compounds within the ECS. 2‐arachidonoyl glycerol (2‐AG) and arachidonoyl ethanolamide (anandamide) are the two main endogenous endocannabinoids that are the most well studies. The production of those ligands is stimulated upon request in response to an external injury. They are fatty in nature and synthesized from phospholipids. There are two enzymes responsible for their degradation. Fatty acid amide hydrolase hydrolyzes AEA and monoglyceride lipase degrades 2‐AG.5The neuromodulatory effect of the ECS has sparked significant interest as a potential therapeutic target in various pathological diseases. The effect exerted by endogenous endocannabinoids is mediated through their interaction with cannabinoid receptors type 1 (CB1) and cannabinoid receptors type 2 (CB2) receptors, which are G‐protein‐coupled receptors. The CB1 receptors exhibit a higher degree of prevalence within the anatomical components of the central nervous system (CNS), including the basal ganglia and hippocampus. These receptors are found in the axon terminal of cholecystokinin and glutamatergic neurons. In comparison, the expression of CB2 receptors within the CNS is significantly lower when compared to the expression of CB1 receptors.6, 7
The CB2 receptors are predominantly located on microglia cells and cerebral vasculature. It has been observed that their expression is said to be stimulated in response to nerve injury.8Studies have additionally elucidated a prevailing expression of CB2 receptors in various anatomical locations, including cardiomyocytes,9the gastrointestinal tract,10the liver,11bone,12and the reproductive system13, 14with high expression in immune cells including leukocytes, lymphocytes and other cell populations.15The indication of therapeutic potential in various pathological conditions has been observed through the effects mediated by the CB2 receptor in several models. In response to the inflammatory trigger, there has been a notable upregulation of CB2 receptors, while the introduction of CB2 agonists has resulted in a reduction in the secretion of pro‐inflammatory mediators from fibroblast cells within the inflamed dermal tissue.16
Due to their heightened expression on various immune cells such as macrophages, natural killer cells, T and B cells, eosinophils, and leukocytes, CB2 receptors exhibit a distinct affinity toward these cellular components17; a significant anti‐inflammatory effect has been attributed to them.18The direct effect of CB2 receptors on fibroblast cells is illustrated in Figure 1. CB2 receptor activation has been observed to confer anti‐inflammatory properties by inhibiting the progression of the inflammatory cascade and the secretion of inflammatory cytokines, including interleukin‐2 (IL‐2), interleukin‐1 beta (IL‐1β), tumor necrosis factor alpha (TNF‐α), interleukin‐8, and interleukin‐6 (IL‐6).19This effect can be mediated by either synthetic or natural cannabinoids. CB2 receptors are predominantly expressed in response to an inflammatory stimulus owing to their extensive presence on circulating immune cells and cells derived from macrophages. Simultaneously, it has been reported that the activation of CB2 receptors exhibits a deficiency in the undesirable psychoactive effects that impose restrictions on their medical application.
FIGURE 1.
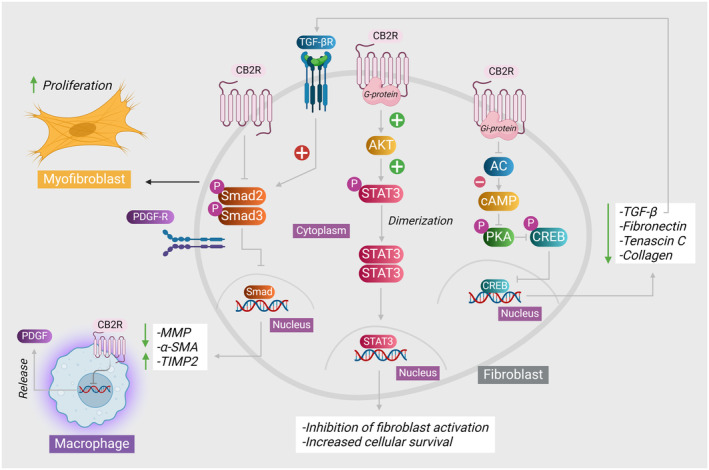
These effects are primarily mediated by CB1 receptors. The restricted expression of CB2 receptors in the brain that is limited to microglia and brain endothelial cells supports the fact that CB2 receptors mediate no psychotropic effects which are largely mediated through CB1 receptors.20, 21, 22Therefore, the advancement of therapies based on cannabinoids that eliminate the undesired effects would be appropriate by specifically targeting the CB2 receptors.23Due to the intricate interplay between inflammation and fibrosis, the advantageous characteristic of cannabinoids in this context has been extrapolated and harnessed as an antifibrotic approach in fibrosis of various organs.24The cannabis plant and its derivatives have demonstrated notable involvement in the management of various afflictions, including pain, epilepsy, and other neurodegenerative disorders25as well as fibrosis.26This review comprehensively aims to elucidate the role of CB2 receptor agonists, encompassing both phytocannabinoids and synthetic ligands, in mitigating the advancement of fibrosis across diverse models of fibrosis in various organs.
2. CB2 AGONISTS IN CARDIAC FIBROSIS
Myocardial fibrosis refers to a form of cardiac remodeling characterized by the pathological alteration of the heart, involving cellular and molecular abnormalities, functional decline, and phenotypic modifications. The pathogenesis of cardiac fibrosis encompasses the occurrence of necrotic and apoptotic cellular demise, heightened inflammatory response, and oxidative insult caused by prolonged stimulation of receptors on cardiomyocytes or pressure‐induced strain on the heart tissue due to uncontrolled blood pressure.27The ECS has been observed to exert regulatory influence on various physiological processes within the cardiovascular system. In this context, novel methodologies grounded in the ECS are currently under scrutiny for their potential therapeutic applications in the realm of cardiovascular ailments. Studies have shown the potential role of CB2 receptor activation in protecting heart from various injuries including inflammatory and oxidative ones taking advantage of expressed CB2 receptors in cardiomyocytes, endothelial cells, and smooth muscle cells, alongside immune cells within the cardiac region.28
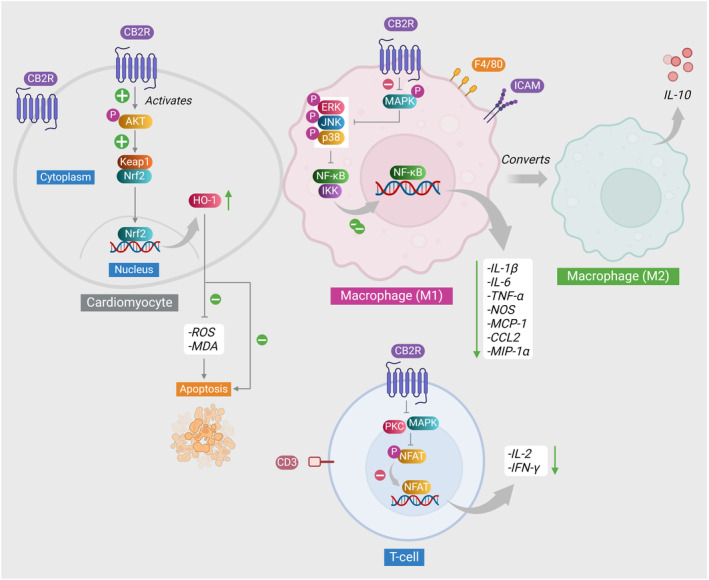
Numerous investigations have elucidated that the stimulation of CB2 receptors induces a range of outcomes, including the suppression of inflammatory responses and the inhibition of fibrotic processes. In a model of myocardial fibrosis, the activation of CB2 receptors by AM1241 demonstrated a mitigating effect on the fibrosis that was induced after myocardial infarction. The i.p. injection of AM1241 in mice was observed to stimulate the activation of the CB2 receptor in the cardiac region. This activation led to the subsequent initiation of the Akt/nuclear factor erythroid 2‐related factor 2 (Nrf2)/HO‐1 signaling pathway, specifically in the cardiomyocytes of the heart. It was demonstrated to expedite the translocation of Nrf2 to the cellular nucleus, where it functioned to enhance the transcription of molecules involved in antioxidant defense. The potential mechanism underlying the prevention of fibrosis by inhibiting the activation of TGF‐β1/Smad3 pathway and subsequent accumulation of collagen is believed to rely on the dependence of Akt/Nrf2/HO‐1‐dependent signaling cascade.29The protective mechanism of CB2 receptors is demonstrated in Figure 2. The efficacy of AM1241 in mitigating the induced fibrosis following myocardial infarction induced by left anterior descending ligation was further validated. Masson’s trichrome staining revealed a notable decrease in the fibrotic region within the group that received the CB2 agonist AM1241, in comparison to the group that received phosphate buffer saline. This phenomenon was observed in parallel with a reduction in the synthesis and secretion of fibrotic cytokines TGF‐β1 and platelet‐derived growth factor (PDGF) by mesenchymal stem cells derived from adipose tissues.30
FIGURE 2.
Additionally, the administration of AM1241 has exhibited a mitigating effect on atrial fibrosis through the restoration of TGF‐β, matrix metalloproteinase (MMP), collagen I, and collagen III expression levels to a state that is relatively comparable to normal levels subsequent to the infusion of angiotensin II in mice.31The obtained consistent results provided additional confirmation regarding the involvement of AM1241 in the observed phenomenon. The administration of AM1241 to mice with myocardial infarction has shown a notable improvement in reducing fibrosis development in an experimental model of myocardial infarction. The administration of AM1241 has additionally facilitated cellular proliferation subsequent to myocardial infarction by augmenting the population of progenitor cells within the cardiac tissue. The findings unveiled an increased expression of the fluorescence‐stained c‐kit+ and Runx1+ proteins, both of which serve as markers for progenitor cells, in MI mice treated with AM1241.32Table 1 summarizes the cardioprotective mechanisms of various CB2 agonists in different fibrosis models.
TABLE 1.
The cardioprotective role of different cannabinoids.
| Compound | Dose, duration, and route of administration | Mechanisms of protection | Reference |
|---|---|---|---|
| AM1241 | 20 mg/kg, i.p. for 7 days to C57BL/6 male mice | ↑Akt/Nrf2/HO‐1 | [29] |
| AM1241 | 20 mg/kg, i.p. for 28 days to mice | ↓ROS, MDA, ↑p‐Akt
↑p‐Erk, ↑p‐Stat3, ↓TGF‐β1, PDGF |
[30] |
| AM1241 | 20 mg/kg, i.p. injected for 7 days to C57BL/6 mice | ↓MDA, TNF‐α, IL‐6, ↑c‐kit+, Runx1+
↑Nrf2, HO‐1 |
[32] |
| AM1241 | 20 mg/kg, i.p. for 21 days to C57BL/6 mice | ↓TGF‐β, collagen I, collagen III, MMP | [31] |
| EHP101 | 5, 10, and 20 mg/kg, p.o. for 2 weeks to C57‐BL/6 mice | ↓NFAT, ↓ERK1 + 2, ↓IL1β, ↓IL6, ↓Col1A2, ↓CCL2, ↓CD3+, F4/80+ cells, Cdk1, Top2a, Ki67 | [33] |
| JWH133 | 3 mg/kg, i.p., single injection to CB2−/− mice
0.5 μM to fibroblasts |
↓TNF‐α, ↓α‐SMA
↑MMP‐2 |
[34] |
| AM1241, JWH133 | AM 1241 (5 μM) and JWH‐133 (1 μM) 30 min prior to quetiapine‐to‐myocardial HL‐1 cells | ↓p‐MLKL, ↓Inflammation
↓fibrosis |
[35] |
| 2‐Arachidonoylglycerol | 10 μg/kg for 4 weeks to C57BL/6 mice | ↓TGF‐β1
↓p‐Smad2/3 |
[36] |
| HU910 | 10 mg/kg, i.p. for 14 days to C57BL/6J mice | ↓CD45+ cells, ↓ MIP1α, IL1α, and TLR4, ↓E‐selectin, VCAM‐1, ICAM‐1, ↓COX1,2, ↓3‐NT, and MDA | [37] |
Similarly, it has been demonstrated that oral administration of the lipid‐based formulation (VCE‐004) of EHP‐101, a synthetic cannabidiol aminoquinone, has antifibrotic effects on the heart. The observed phenomenon demonstrated the inhibition of interstitial and perivascular collagen deposition in the heart interstitium, induced by angiotensin‐II. Additionally, it prevented the transformation of fibroblasts into profibrotic‐activated myofibroblasts. Furthermore, the population of T cells (CD3+ cells) and macrophages (F4/80+ cells) exhibited a notable reduction in the left ventricle of the heart upon administration of EHP‐101, mirroring the effects observed in the positive control, losartan. In order to investigate the potential underlying mechanism behind this antifibrotic effect, a transcriptomic analysis was conducted to examine the genes that were either upregulated or downregulated in response to EHP‐101. The analysis has unveiled a reduction in the transcriptional activity of genes associated with epithelial–mesenchymal transition, as well as E2F targets that govern the activity of transcription factors and influence the modulation of fibrosis. A subset of the E2F target genes encompasses cyclin‐dependent kinase 1 (Cdk1), DNA topoisomerase II alpha (Top2a), and marker of proliferation Ki67 (Mki67). The aforementioned study has examined the antifibrotic function of AJULEMIC ACID, a synthetic CB2 receptor ligand,38and showed efficacy in decreasing the extent of induced fibrosis and accumulated collagen in the heart, with a lower potency compared to EHP‐101.33
In further substantiation of the antifibrotic impact of CB2 agonism, it has been documented that both JWH‐133 and AM1241 exhibited protective properties against quetiapine‐induced cardiotoxicity and mitigated the initiation of cardiac fibrosis. Following administration of JWH‐133 and AM1241, the progression of inflammatory cells and cytokines infiltrating the myocardium cells was observed to be impeded. Additionally, the buildup of fibrotic fibers on the myocardium cells was found to be suppressed. The observed effects were counteracted upon pretreatment with AM630, a specific antagonist of the CB2 receptor. Furthermore, it was observed that this pretreatment even intensified the toxic effect induced by quetiapine.35
Furthermore, it has been demonstrated that the cannabinoid naturally synthesized within the body exhibits advantageous impacts on cardiac fibrosis, which arises as a complication of long‐term diabetes induction. The fibrotic region, which exhibited positive staining, displayed a notable reduction in streptozotocin‐induced diabetic mice that were administered 2‐arachidonoylglycerol. The mice treated with 2‐arachidonoylglycerol exhibited consistently lower collagen content in comparison to the mice with untreated diabetes. The administration of 2‐arachidonoylglycerol resulted in a suppression of protein expression of TGF‐β1, p‐Smad2/3, and Smad2/3, which play crucial roles as regulators in the process of fibrosis.36
Moreover, the vulnerability of mice lacking CB2 receptors to develop cardiac fibrosis was assessed in an ischemia/reperfusion model. The echocardiography examination revealed a notable degree of cardiac remodeling in the heart, characterized by an augmented expansion in the dimensions of the left ventricle and a heightened average of the cardiac myocyte‐stained sections. Mice lacking the CB2 gene (CB2−/−) exhibited an increased susceptibility to the formation of cardiac fibrosis, characterized by a higher occurrence of fiber disarray and accumulation of collagenous fibrils in the myocardium, in comparison to mice with the normal CB2 gene (wild‐type mice). The molecular analysis revealed an increase in the stimulated messenger RNA (mRNA) expression of fibrogenic markers such as TGF‐β1, collagen 1, and collagen 3. In contrast, the mRNA levels of these fibrogenic markers in the wild‐type mice that underwent ischemia/reperfusion surgery were comparable to those of mice that underwent sham surgery. Furthermore, the investigation revealed that fibroblasts obtained from CB2−/− mice hearts exhibited heightened vulnerability to oxidative stress and the initiation of apoptosis upon exposure to hydrogen peroxide. This was accompanied by a significant increase in the release of TNF‐α and a positive detection of alpha smooth muscle actin (α‐SMA). The introduction of JWH133 into cellular systems has exhibited a safeguarding effect against the initiation of apoptosis, mitigated irregular cardiac remodeling, and enhanced the secretion and activity of MMP‐2 in cells of the wild type, while no discernible impact was observed in the knocked‐out cells. This observation underscores the selectivity of JWH133 toward CB2 receptors.34
Moreover, the model utilizing CB2−/− was subsequently employed to elucidate the impact of CB2 receptor deficiency on the enhancement of fibrosis. The ventricles of the CB2−/− group exhibited a greater accumulation of fibrotic tissue in comparison to the wild‐type group. This was accompanied by a pronounced activation of B‐cells subsequent to the induction of infarction.39Similarly, the hearts of mice lacking CB2 receptors exhibited maladaptation to pressure overload. Following transverse aortic constriction (TAC) surgery, there was an observable presence of confluent and well‐defined patchy regions exhibiting heightened cell density, suggesting the substitution of healthy cardiomyocytes with fibrotic tissue. Mice lacking the CB2 receptor (Cnr2−/−) exhibited collagenous regions that were more densely stained, displaying a non‐compacted pattern. These mice also showed reduced production of the arachidonic acid, endocannabinoid 2‐AG, and anandamide, in comparison to their Cnr2+/+ counterparts. The absence of the CB2 receptor resulted in a compromised antioxidant defense mechanism in the cardiac tissue, accompanied by reduced levels of Hmox1 protein expression. This reduction serves as an indicator of impaired differentiation of M2 macrophages, indicating a disrupted polarization of macrophages.
Simultaneously, an intriguing observation was made regarding the upregulation of pro‐inflammatory cytokines, which enhanced the inflammatory response in mice lacking the Cnr2 gene (Cnr2−/−) following TAC surgery, in comparison to mice with intact Cnr2 gene (Cnr2+/+ mice).40In accordance with the established pattern, the absence of CB2 receptors in myocardial ischemia and reperfusion (I/R) model resulted in a significant increase in replacement fibrosis, which served as a compensatory mechanism for the loss of cardiomyocytes. Notably, the microinfarcted regions in Cnr2−/− mice exhibited augmented collagen deposition. Furthermore, it is noteworthy to mention that Cnr2−/− mice exhibited only a partial regression of fibrosis 60 days post‐I/R injury, in contrast to the complete regression observed in mice of the wild type. The justification for this observation stems from the prevalence of irreversible collagen isoforms and the absence of the lysyl oxidase enzyme, which is responsible for facilitating the cross‐linking of collagen fibers in Cnr2−/− mice.41This exemplifies the intricate and interconnected relationship between endocannabinoids and CB2 receptors, which play a crucial role in mediating cardioprotection and preserving the integrity of cardiomyocytes. The implicated mechanisms involve the expression of CB2 receptors and the subsequent anti‐inflammatory effect when CB2 receptors are activated in the heart. The data provided highlights the crucial significance of the endocannabinoid–CB2 receptor axis in the cardiac response to diverse stimuli.
3. CB2 AGONISTS IN LIVER FIBROSIS
Chronic liver diseases, which can be triggered by excessive alcohol consumption, viral hepatitis, or non‐alcoholic fatty liver disease (NAFLD), ultimately lead to the development of liver fibrosis and cirrhosis. These conditions pose a significant risk to patients, as they can result in severe complications such as heightened portal pressure, encephalopathy, and in the most severe instances, hepatocellular carcinoma. The ECS has been discovered to play a significant role in regulating the development and progression of liver diseases, as well as providing protective effects. The upregulation of CB2 receptors has been observed in Kupffer cells and hepatic myofibroblasts as a protective response to liver injury. This upregulation is believed to have an anti‐inflammatory and antifibrogenic effect, which helps to mitigate the progression of fibrosis.42
Previous studies have reported on the involvement of endocannabinoids in the pathogenesis of liver fibrosis. The study conducted by Siegmund and Schwabe in 2008 demonstrated that a lack of CB2 receptors was associated with a higher likelihood of experiencing enhanced fibrogenesis and increased proliferation of myofibroblasts. The activation of CB2 receptors has been documented to exhibit inhibitory properties on the proliferation of hepatic myofibroblasts. Furthermore, it has been observed to promote apoptosis, inducing a cytotoxic effect on both hepatic myofibroblasts and hepatic stellate cells, thereby reducing their viability.43The activation of CB2 receptors has been documented to exhibit inhibitory properties on the proliferation of hepatic myofibroblasts. Furthermore, it has been observed to promote apoptosis, inducing a cytotoxic effect on both hepatic myofibroblasts and hepatic stellate cells, thereby reducing their viability.44The signaling pathway associated with CB2 has been recognized as an antifibrogenic mechanism, as evidenced by multiple scientific investigations. δ9‐Tetrahydrocannabinolic acid (Δ9‐THCA), which serves as the precursor to Δ9‐tetrahydrocannabinol (Δ9‐THC), the primary and most prevalent compound found in Cannabis sativa, has been the subject of scientific inquiry due to its potential antifibrogenic and anti‐inflammatory properties. The administration of Δ9‐THCA has effectively impeded the initiation of hepatic fibrosis caused by CCl4, as evidenced by a notable reduction in the fibrotic region, decreased levels of α‐SMA, and diminished expression of tenascin C. The treatment with Δ9‐THCA resulted in a significant reduction in the gene expression of fibrotic markers Col1a2, Col3a1, and TGFβ. Furthermore, a reduction in the infiltration of T lymphocytes and macrophages in the liver resulted in a decrease in the induced hepatic inflammation. Comparable phenomena were documented in a 23‐week experimental setup involving the induction of liver fibrosis through a high‐fat diet.45
In a comparable pattern, the administration of cannabigerol has exhibited a safeguarding effect against the initiation of hepatic fibrosis and inflammation. This has been substantiated by observing a reduction in the presence of α‐SMA (alpha‐smooth muscle actin) in liver tissue sections, as well as by impeding the infiltration of immune cells, specifically leukocytes and macrophages, into the liver.46However, the utilization of the CB2 agonist HU910 has demonstrated a significant reduction in liver fibrosis. This reduction is achieved by mitigating the infiltration of leukocytes and the secretion of proinflammatory cytokines, as well as vascular inflammation markers. Additionally, HU910 restores the hepatic microcirculation, transforming it into a state of intact and healthy vasculature in mice with bile duct ligation.37Mechanisms implicated in antifibrotic effect of CB2 agonists are collectively illustrated in Table 2.
TABLE 2.
The hepatoprotective role of different cannabinoids.
| Compound | Dose, duration, and route of administration | Mechanisms of protection | Reference |
|---|---|---|---|
| Δ9‐Tetrahydrocannabinolic Acid | 20 or 40 mg/kg, i.p. for 2 weeks to C57BL/6 mice | ↓α‐SMA, tenascin C, ↓ALT, ↓Col1a2, Col3a1, Acta2, TGFβ, ↓Il1b, and Il6
↓CD3, F4/80+ cells |
[45] |
| JWH133 | 3 mg/kg, i.p. for 3 weeks to wild‐type and CB2−/− C57BL/6 mice
5 μM to hepatic stellate cell |
↓ IL‐6, IL‐1β, TNF‐α, and iNOS, ↓ p‐P38, NF‐κB/p65
↓ Collagen I |
[47] |
| JWH133 | 10 mg/kg, i.p. for 3 days to C57BL/6 mice | ↓F4/80+ cells, ↓PKA, ↓Ubiquitin‐specific peptidase 4 | [48] |
| JWH133 | 1 mg/kg, s.c. for 9 days to Wistar rats | ↓α‐SMA, Collagen I
↑MMP‐2, ↑caspase‐3 ↓CD68+ cells |
[24] |
| HU910 | 10 mg/kg, i.p. for 14 days to C57BL/6J mice | ↓CD45+ cells, ↓IL1β, IL6, MIP1α, TNFα
↓E‐selectin, VCAM‐1, ICAM‐1, ↓ALT and ALP |
[37] |
| Cannabigerol | 2.46, 24.6 mg/kg, i.p. 3 times a week for 2 weeks to C57BL/6 mice | ↓α‐SMA, collagen
↓CD45+ cells |
[46] |
| β‐Caryophyllene | 5 mg/kg, p.o. for 2 weeks to Wistar rats | ↓collagen
↑CB2 expression |
[49] |
| AM1241 | 3 and 6 mg/kg, i.p. for 3 weeks to Wistar rats | ↓NF‐κβ p65, ↑GSH, SOD ↓MDA, ↓TNF‐α, IL‐1β and IL‐6
↓Vimentin, ↑E‐cadherin |
[50] |
| AM1241 | 3 mg/kg, i.p. to C57BL/6J mice | ↓PDGF, ↓Col‐III | [51] |
| AM1241 | 1 mg/kg, i.p. for 5 weeks to rats | ↓PDGF, ↓α‐SMA, collagen, ↑MMP
↓TIMP |
[52] |
| JWH‐015 | 1 μmol/L to hepatic myofibroblasts and stellate cells | ↓forskolin‐stimulated cAMP, ↓PDGF
↑COX‐2 |
[44] |
In order to gain more understanding of the functionality of CB2 receptors, cellular entities were subjected to transfection with CB2 small interfering RNA (siRNA), resulting in the suppression of CB2 receptor activity and subsequent reduction in receptor expression levels when compared to the group transfected with negative control siRNA. The observed downregulation of CB2 receptors was found to be correlated with an augmented secretion of pro‐inflammatory cytokines from Kupffer cells upon stimulation by lipopolysaccharide (LPS). Furthermore, the process of phosphorylation of p38 MAPK and the subsequent nuclear translocation of NF‐κB/p65 exhibited heightened levels in CB2 knocked‐out cells as compared to the control cells. Using an alternative methodology, the identical investigation assessed the impact of CB2 receptor activation on the activation of Kupffer cells induced by LPS. The administration of JWH133 effectively suppressed the release of IL‐6, IL‐1β, TNF‐α, and inducible nitric oxide synthase from activated Kupffer cells in response to LPS stimulation. The expression levels of p‐P38 MAPK and activated NF‐κB/p65 were observed to be significantly reduced in cells transfected with negative control siRNA, but not in cells with CB2 knockout, following treatment with JWH133. The antifibrotic effects were further examined in mice with CCl4‐induced liver fibrosis, encompassing both CB2−/− and wild‐type specimens. JWH133 has exhibited a mitigating effect on the artificially induced pathological fibrotic lesion, as evidenced by a reduction in the presence of stained α‐SMA and collagen I in the wild‐type mice. However, this effect was not observed in the CB2−/− CCl4‐challenged mice.47
Moreover, the administration of JWH133 resulted in a notable decrease in the accumulation of collagen fibers around the veins and portal areas, accompanied by a reduction in the distortion of the liver’s structure. This was further supported by the preservation of the liver’s functional tissue, as confirmed through Sirius red staining.24The observed deletion of CB2 receptors was additionally demonstrated to intensify hepatic injury and fibrosis upon exposure to CCl4. In the fibrotic model induced in mice lacking the CB2 receptor gene (CB2−/−), a notable augmentation in cellular necrosis and infiltration of inflammatory cells was observed. Additionally, collagen fibers, which are found in various regions of the liver tissue, were observed to be more widely distributed in the CB2−/− model group compared to the control group. This increased distribution of collagen fibers was accompanied by an upregulation of α‐SMA and TGF‐β1 expression. Simultaneously, the observed protein expression levels of A20 and phosphorylated NF‐κB p65, along with the mRNA levels of IL‐6 and TNF‐α, exhibited significant upregulation in the CB2−/− model mice in comparison to the wild‐type mice that were exposed to CCl4.53
Furthermore, additional investigations have demonstrated that the progression of fibrosis was augmented in mice lacking the CB2 receptor. In an experimental setup involving bile duct ligation, the fibrogenic response of both CB2‐deficient and wild‐type mice was assessed. In individuals lacking CB2, there was an increased presence of type 1 T helper cells (Th1) or Th2 markers, as well as Th17 markers such as Rora, Rorct, and Il23r, along with heightened levels of IL‐17 in the liver, as compared to their counterparts with a normal CB2 gene. The observed increase in the immune response was correlated with an augmented fibrotic response, as evidenced by elevated levels of collagen I and α‐SMA mRNA expression. Interestingly, wild‐type mice demonstrated a remarkable expression of the CB2 receptors, as evidenced by the augmented mRNA expression of Cnr2, the enzymes Dagla and Daglb responsible for synthesizing 2‐AG, and a diminished expression of the enzyme Mgll responsible for degrading 2‐AG. Conversely, the mice lacking the CB2 receptor gene showed no discernible impact on the expression of these components. Furthermore, the administration of IL‐17 has resulted in a notable augmentation of collagen I, asma, and Tgfb mRNA expression in a population of CB2‐deficient mice. In order to gain a deeper understanding of the impact of CB2 receptor activation, Th17 lymphocytes undergoing differentiation in a controlled culture were subjected to treatment with JWH‐133. The findings indicated a notable decrease in the quantity of IL‐17+ cells and the production of IL‐17, accompanied by a reduction in the intensity of stained phospho‐STAT5. The treatment of macrophages with JWH‐133 resulted in the inhibition of the activation of the pro‐inflammatory M1 phenotype and the downregulation of the expression of Il1b, Il6, and chemokine ligand 2 genes.54Mice lacking the CB2 gene (CB2−/−) have also exhibited an increased vulnerability to hepatic steatosis and fibrogenesis following prolonged exposure to ethanol. A notable augmentation in inflammation, activation of hepatic stellate cells, and accumulation of collagen were observed in comparison to both CB1−/− and wild‐type mice.55
The presence of endogenous cannabinoids has also been observed, with a notable increase in the concentration of anandamide, up to 10 to 20 times higher, in fibrotic mice when compared to control mice. The increased abundance of anandamide functions as a defensive mechanism, providing protection against artificially induced fibrosis.56In rats subjected to bile duct ligation, there was a notable downregulation in the gene expression of CB2 receptors. This downregulation was found to be linked with an increased occurrence of hepatic fibrosis. However, the administration of β‐caryophyllene, a naturally occurring cannabinoid agonist, has been observed to reduce the accumulation of collagen in the central vein and portal tract of liver of the rats.49Furthermore, the ameliorative effects of curcumin on hepatic fibrosis were observed to be facilitated through the modulation of CB2 receptor expression and the subsequent restoration of gene expression to normal levels after exposure to CCL4.57The antifibrotic protective effects of a curcumin derivative known as C66 were observed in a study. These effects were found to be associated with the upregulation of CB2 receptors, as evidenced by both mRNA and protein analysis.58
Subsequent investigations delved into the examination of the impact of CB2 receptor activation on the reversal of fibrosis initiation or progression. The administration of AM1241 exhibited a protective effect against the release of inflammatory cytokines induced by thioacetamide. The levels of TNF‐α, IL‐1β, and IL‐6 were constrained by the presence of AM1241, which additionally exhibited an antioxidant impact by augmenting the activity of superoxide dismutase (SOD) and the concentration of reduced glutathione (GSH). The findings additionally showcased the antifibrotic impact of AM1241, which effectively reinstated the expression of E‐cadherin while concurrently reducing the expression of vimentin in hepatocytes. AM1241 exhibited a downregulatory effect on the genetic expression of fibrotic markers, specifically toll‐like receptor 4, TGF‐β1, α‐SMA, and microRNA‐155.
Additionally, the administration of AM1241 has demonstrated improvements in the histopathological changes observed in rats treated with thioacetamide. The rats that received AM1241 treatment exhibited limited necrotic changes, portal inflammation, fibroplasia, cytoplasmic vacuolation, and pseudolobulation of hepatic parenchyma. The histological examination revealed that the rats treated with AM1241 exhibited mild focal degeneration and portal inflammation, which were notably less severe compared to the rats treated with thioacetamide. The latter group displayed an almost normal histological appearance. The fibrotic extent was assessed using the Ishak scoring system. The group treated with AM1241 exhibited the lowest fibrosis score, suggesting a minimal presence of fibroplasia and indicating a restricted proliferation of fibrotic tissue.50AM1241 exerts its influence on hepatic fibrosis by modulating the expression of PDGF and impeding its activity, thereby leading to a decline in the synthesis of ECM.51Similar results were reported previously, confirming the antifibrotic role of AM1241 in chronically CCL4‐treated rats.52In genetic analysis, we examined the connection between allelic variants (Q63R and PNPLA3 variant) in the CB2 receptor gene. Our findings unveiled a significant correlation between the genetic variation and the severity of NAFLD in a group of children who were diagnosed with NAFLD through liver biopsy.59The data demonstrated the activation of CB2 receptors and their effects on reducing inflammation and fibrosis, thereby mitigating the impact of fibrogenic stimuli.
4. CB2 AGONISTS IN LUNG FIBROSIS
The respiratory passages exhibit a susceptibility to inflammatory responses triggered by various environmental irritants and stimulants. The primary objective in managing respiratory disease is to mitigate the inflammatory response and its potential for becoming chronic, which could potentially heighten the susceptibility to developing pulmonary fibrosis. Given the correlation observed between pathological states and the ECS, numerous therapeutic strategies rely on the manipulation of constituents of this system, particularly by exerting influence on its receptor entities.60
The immunomodulatory impact of cannabinoids is ascribed to their interaction with immune cells and the subsequent release of cytokines from these inflammatory cells. Human eosinophils have been found to express a discernible quantity of CB2 receptors, suggesting a notable level of responsivity of these cells to cannabinoid agonists. CB2 receptors are additionally involved in regulating the functioning of dendritic cells through the inhibition of their movement, which is accompanied by a significant decrease in the production of MMP.61
Pulmonary fibrosis is classified as an interstitial lung disease characterized by the concurrent presence of inflammation and fibrosis within the pulmonary tissue. Given that CB2 receptors are predominantly found on immune cells within the lung, their stimulation leads to the initiation of programmed cell death known as apoptosis. This process effectively hinders the growth and reproduction of cells while also reducing the production and release of pro‐inflammatory signaling molecules called cytokines. Consequently, the activation of CB2 receptors is recognized for its ability to exhibit anti‐inflammatory, immunosuppressive, and anti‐fibrotic effects.62In a model of allergic asthma induced by ovalbumin, the administration of cannabidiol has demonstrated the ability to counteract the heightened presence of collagen fibers within the alveolar septa. In this study, AM630 (a selective CB2 antagonist) was administered in combination with cannabidiol. This concurrent administration conferred reversed reduction in collagen deposition in airways. Therefore, it can be considered that CBD antifibrotic effect is possibly attributed to CB2 receptor activation.63
In a comparable manner, the compound WIN 55212‐2, which serves as an analog for endocannabinoids, has exhibited the ability to inhibit the manifestation of various fibrotic indicators. Additionally, it has shown the capacity to augment the expression and stimulation of M2 macrophages, thereby adopting a defensive strategy against inflammation. This defensive mechanism is further supported by an elevation in the release of anti‐inflammatory cytokines. Interleukin‐10 (IL‐10) is a cytokine that plays a crucial role.64A recent investigation has successfully identified a newly discovered synthetic CB2 agonist, known as YX‐2102, through the utilization of organocatalytic methodologies. This compound has subsequently been subjected to systematic analysis in order to evaluate its potential therapeutic applications. The administration of YX‐2102 has demonstrated an improvement in the extent of lung fibrosis induced by bleomycin, resulting in reduced lung coefficients. The application of the Szapiel score revealed a reduction in fibrotic lesions and a decrease in the accumulation of ECM proteins following the administration of the treatment.65
The additional findings highlighted the observed protective properties exhibited by CB2 agonists. The administration of JWH 133 has demonstrated a mitigating effect on the pathological alterations that were observed in conjunction with the fibrotic condition induced by nicotine. After the administration of JWH 133, an observation was made of a typical structure characterized by a slender septum, the absence of excessive blood flow, and reduced collagen accumulation compared to the other experimental groups.66The investigation also encompassed the examination of the protective effect of JWH 133 in a model of lung fibrosis induced by bleomycin. The co‐administration of JWH 133 and bleomycin resulted in a notable reduction in induced fibrosis. The computed tomography images revealed a notable decrease in pulmonary density within the upper, central, and lower regions of the lung in mice treated with JWH 133. Moreover, the administration of JWH 133 has exhibited regulatory effects on the progressive fibrotic mechanism, resulting in a reduction of intralobular opacity and fibrosis stranding that is typically induced by bleomycin.67
Additional compounds were examined within the context of a bleomycin‐induced lung fibrosis model. In the study conducted by Parlar et al. it was observed that AM1241 exhibited a comparable impact on bleomycin‐induced pulmonary fibrosis,68which was evidenced by a reduction in the accumulation of collagen fibers and the content of hydroxyproline, as indicated by staining techniques. Additionally, AM1241 demonstrated concurrent anti‐inflammatory and antioxidant properties, further contributing to its therapeutic potential in combating pulmonary fibrosis. The protective mechanisms of AM1241 and other CB2 agonists are shown in Table 3.
TABLE 3.
The protective role of different cannabinoids in the lungs.
| Compound | Dose, duration, and route of administration | Mechanisms of protection | Reference |
|---|---|---|---|
| Cannabidiol | 5 or 10 mg/kg i.p. for 8 days to Balb/c mice | ↓Collagen, ↓IL‐4, IL‐5, IL‐13 | [63] |
| WIN 55212–2 | 0.2–1 mg/kg, i.p. for 28 days to C57BL/6 mice | ↓TGF‐β, α‐SMA, PDGFRa, fibronectin, and collagen I, ↓TNF‐ α and IL‐6, ↑CD206, CD163 ↑IL‐10
↓CCR7 + cells (M1) |
[64] |
| YX‐2102 | 25 mg/kg, i.p. for 21 days to Sprague–Dawley rats | ↓fibronectin and α‐SMA, ↑E‐cadherin
↓myeloperoxidase, CD68, ↓IL‐6, MCP‐1, IL‐1β, ↑CD206 |
[65] |
| JWH 133 | 1 mg/kg, i.p. for 14 days to Swiss mice | ↓Hyperemia
↓hemorrhage ↓leucocytes infiltration ↓hyperplasia ↓collagen |
[66] |
| JWH133 | 2.5 mg/kg, i.p. for 21 days to C57BL/6 mice | ↓Hydroxyproline
↓collagen I, ↓α‐SMA, ↓TGF‐β/Smad, ↓PDGF |
[67] |
| AM1241 | 1, 3 mg/kg, i.p. for 28 days to Wistar rats. | ↓IL‐6 and TNF‐α
↓MDA, ↑GSH |
[68] |
| EHP‐101 | 5, 10, and 25 mg/kg, p.o. for 4 weeks to BALB/c mice | ↑vessels perimeter
↓myofibroblast ↓collagen |
[69] |
| Pirfenidone | 300 mg/kg, p.o. for 15 days to C57BL/6 mice | ↓collagen, ↓TNF‐α, IL‐1β, and IL‐6 | [70] |
| Lenabasum | 0.1, 0.3, 1, 3, 10,
and 30 μM to monocyte‐derived macrophages |
↓IL‐8, TNF‐α, and IL‐13 | [71] |
| Ajulemic acid | 1 mg or 5 mg/kg, p.o. for 21 days to DBA/2 mice | ↓HO‐proline, ↓TGF‐β1, ↑PPAR‐γ, ↓pSMAD2/3, ↓CTGF, ↓α‐SMA | [72] |
The evaluation of the anti‐fibrotic effect of EHP‐101 was conducted in a study involving bleomycin‐induced fibrosis. The researchers assessed the impact of EHP‐101 on collagen deposition by analyzing the Ascroft score and conducting picrosirius red staining. The results revealed a significant reduction in collagen deposition in mice treated with EHP‐101 compared to those in the bleomycin‐only group.69The antifibrotic effects of pirfenidone have been investigated in order to elucidate their reliance on the activation of CB2 receptors. The findings demonstrated a notable elevation in the protein abundance of CB2 receptors in mice that were co‐administered with Pirfenidone, as compared to the group of mice treated solely with bleomycin. The lung sections obtained from mice subjected to pirfenidone treatment exhibited a notable reduction in collagen accumulation and diminished fibrosis progression upon staining with Masson’s trichrome.70
Synthetic compounds are subjected to evaluation and subsequent reporting regarding their therapeutic potential in the context of targeting and mitigating lung fibrosis. Lenabasum, alternatively referred to as ajulemic acid, is a synthetic compound that acts as a selective CB2 agonist. It is an analog of THC and has demonstrated notable anti‐inflammatory and antifibrotic properties. Treating macrophages derived from pediatric individuals with cardiac fibrosis resulted in a diminished polarization of macrophages, characterized by a decreased proportion of CD80+ M1 macrophages, alongside an enhanced phagocytic activity.71Additionally, it has been observed that Ajulemic acid exhibits a protective effect against the development of lung fibrosis in mice induced by bleomycin. The utilization of ajulemic acid treatment in a preventative manner exhibited a mitigating effect on the inflammation that was induced in the sub‐pleural regions. Furthermore, it provided protection against the extension of damage to the parenchymal tissue and impairment of the alveolar units. This was evidenced by a reduction in collagen deposition as observed in the histological analysis of the lung sections.72
5. CB2 AGONISTS IN RENAL FIBROSIS
The maintenance of body homeostasis is achieved by the kidneys, which play a crucial role in the clearance of toxins, regulation of body volume, and preservation of hemodynamic balance. Hence, it is imperative to comprehend the fundamental pathways that undergo modifications leading to diverse manifestations of renal impairment and can be harnessed to develop efficacious therapeutic strategies for managing renal disorders. Cannabinoid receptors can be found within the typical kidney, exhibiting a notably abundant presence of CB2 receptors in glomeruli, podocytes, and proximal tubule cells.
Studies conducted on animal models have demonstrated that the activation of CB2 receptors has a positive impact on conditions such as type 1 diabetes mellitus, renal fibrosis, and drug‐induced tubular injury. The presence of endocannabinoids in the renal tissue suggests significant biological importance and serves as evidence for the direct impact of the ECS on the kidney. This impact is observed through the regulation of blood flow and the reabsorption of sodium in the kidney tubules.73
Given that kidney fibrosis is widely recognized as the primary consequence of chronic kidney diseases, we conducted a unilateral ureteral obstruction experiment to assess the nephroprotective effects of SMM‐295 against the development of induced renal fibrosis. The administration of SMM‐295 exhibited a protective effect against tubulointerstitial fibrosis, thereby resulting in a notable reduction in DNA damage by approximately 50% when compared to the mice treated with the vehicle.74
In a different investigation, the compound HU‐910 was assessed for its potential protective properties in the context of renal fibrosis following bile duct ligation, which is an extraneous complication. The findings revealed a noteworthy reduction in kidney injury, characterized by diminished tubular dilatation, cellular necrosis, infiltration of leukocytes, and collagenous tissue. The administration of HU‐910 also resulted in the restoration of microvascular flow, as well as the inhibition of vascular inflammation and endothelial activation.75Various compounds that activate the CB2 receptor have demonstrated the ability to induce an antifibrotic response in diverse models of experimentally induced renal fibrosis. The (+)‐enantiomer of the naturally occurring (−)‐cannabidiol, along with its derivative (+)‐CBD hydroxypentylester ((+)‐CBD‐HPE), have been shown to exhibit pharmacological properties in reducing the severity of nephropathy, a diabetic complication, in mice induced with streptozotocin. Functional assays demonstrated an enhanced CB2 receptor binding affinity of (+)‐CBD‐HPE with agonist function compared to the original cannabidiol. The administration of (+)‐CBD‐HPE resulted in a 1.95‐fold decrease in the presence of interstitial fibrotic lesions. Various staining techniques revealed a notable improvement in fibrosis within the renal glomeruli and interstitium after administering (+)‐CBD‐HPE treatment to mice, as compared to mice treated with a control substance.76
In another approach, celastrol, a natural compound, demonstrated an antifibrotic effect through the upregulation of CB2 receptors and the reversal of the induced downregulation of CB2 receptors that is a result of the unilateral ureteral obstruction and therefore blunting the development of fibrosis. In vitro results also exhibited similar results with reversed effect on the TGF‐β1‐induced downregulation of CB2 receptors. Same study demonstrated that the administration of a CB2R antagonist SR144528 augmented the unilateral ureteral obstruction‐induced fibrosis and prevented celastrol‐mediated protective effect, indicating that the protective effect of celastrol is dependent on increasing the expression of CB2 receptor.77Additionally, treatment with AM1241 helped reducing the accumulation of collagen and the expression of TGF‐β in the kidney tissue of high‐fat diet‐fed rats.78The anti‐fibrotic effect of CB2 agonists in kidney is presented in Table 4.
TABLE 4.
The protective role of different cannabinoids in the kidneys.
| Compound | Dose, duration, and route of administration | Mechanisms of protection | Reference |
|---|---|---|---|
| SMM‐295 | 12 mg/kg, i.p. for 7 days to mice | ↓α‐SMA, ↓γ‐H2AX
↓fibronectin |
[74] |
| HU‐910 | 10 mg/kg, i.p. for 14 days C57BL/6J male mice | ↓KIM‐1, OPN and NGAL, ↓Col1a1, Col3a1, Tgfβ mRNA
↓CD68 and CD11b ↓ICAM‐1, SOD1, ↑Catalase, SOD2, ↓4‐HNE, MDA, 3‐NT |
[75] |
| (+)‐CBD‐HPE | 10 mg/kg, i.p. for 7 days to C57BL6J mice | ↓CD3+, ↓ECM proteins | [76] |
| Celastrol | 1 mg/kg, i.p. for 7 days to BALB/C mice | ↓fibronectin, ↑CB2, collagen I, α‐SMA
↓F4/80+, Ly‐6G+, CD3+, ↓Smad3 |
[77] |
| AM1241 | 3 mg/kg, i.p. for 6 weeks to Sprague–Dawley rats | ↓ collagen, ↓TGF‐β
↓VEGF |
[78] |
6. CB2 AGONISTS IN SKIN FIBROSIS
Fibrosis is a prevalent pathological dermal condition characterized by the disrupted metabolic processes of the connective tissue. The process of fibroblast activation and subsequent aberrant synthesis of ECM proteins is commonly referred to as cutaneous fibrosis or fibrosing connective tissue disorder. Skin fibrotic disorders are recognized to be facilitated by an immune response that triggers the generation of autoantibodies, resulting in damage to endothelial cells and an inflammatory process that leads to impaired functionality of dermal fibroblasts and an excessive accumulation of collagen, thereby contributing to the development of fibrosis. Endocannabinoid receptors are distributed within the suprabasal layers of the epidermis, as well as in hair follicles and other occasional regions of the basal layer, such as nerve fibers, hair follicles, and sebaceous glands, specifically within the keratinocytes.79
Various physiological functions of the ECS and the activation of CB2 receptors have been documented in various dermatological conditions. Cannabinoids demonstrate anti‐inflammatory and immunosuppressive effects, which are the primary underlying mechanisms observed in the majority of skin pathologies.80Additionally, there have been reports indicating that the activation of CB2 receptors leads to a decrease in mast cell degranulation, which serves as an additional origin of profibrotic factors.81The antifibrotic effect of CB2 agonists is additionally ascribed to the downregulation of adhesion molecules, decreased activation of endothelial cells, and the induction of apoptosis in activated fibroblasts.80, 82
Bleomycin has been observed to induce an augmentation in the vascular wall’s thickness, which is a morphometric characteristic commonly observed in individuals with scleroderma. This condition is characterized by an excessive deposition of collagen surrounding the blood vessels. The observed histological alterations have been demonstrated to exhibit recovery upon administration of EHP‐101. EHP‐101 effectively restored the expression levels of activated myofibroblasts and tenascin in the treated mice, thereby bringing them back to a state comparable to that of the mice challenged with bleomycin. The characterization involved examining the impact of bleomycin induction and the ameliorating effects of EHP‐101 on gene expression at a transcriptomic level. The expression of genes involved in inflammatory and fibrotic pathways, such as the epithelial–mesenchymal transition, was downregulated upon administration of EHP‐101. Conversely, the same genes were upregulated in response to bleomycin treatment.69
The VCE‐004.3 derivative, which is derived from cannabidiol, has been found to possess antifibrotic properties. It has the capacity to decrease the accumulation of collagen, suppress the release of inflammatory cytokines, and inhibit the degranulation of mast cells.83Its efficacy was further proven based on an in vitro and in vivo analysis. The in vitro findings demonstrated that VCE‐004.8 exerted an inhibitory impact on the transcriptional process by targeting the collagen gene promoter. Consequently, this hindered collagen synthesis and impeded gene transcription in response to TGFβ1 stimulation. This is in addition to its efficacy in the murine model of experimentally induced skin scleroderma. VCE‐004.8 exhibited a mitigating effect on the consequent fibrosis of the skin by reducing the thickness of the dermal layer, while also preserving the content of subcutaneous adipose tissue. Additionally, it hindered the triggered release of granules from mast cells.84Table 5 demonstrates the protective role of CB2 receptor activation in alleviating skin fibrosis.
TABLE 5.
The protective role of different cannabinoids in skin fibrosis.
| Compound | Dose, duration, and route of administration | Mechanisms of protection | Reference |
|---|---|---|---|
| JWH‐133 | 1,1.5, 2, 2.5, 3, 4 mg/kg, i.p. for 6 weeks to BALB/c
Mice |
↓collagen
↓dermal thickness |
[85] |
| JWH‐133 | 2.5 mg/kg, i.p. for 4 weeks to C57BL mice | ↓dermal thickness | [86] |
| JWH‐133 | 3 μM for 18 hours to human fibroblasts | ↓collagen, ↓α‐SMA | [87] |
| EHP‐101 | 5, 10, and 25 mg/kg, p.o. for 3 weeks to BALB/c mice | ↓collagen, ↓Tenascin, ↓VCAM, ↓Vimentin | [69] |
| VCE‐004.3 | 20 mg/kg, i.p. for 6 weeks to BALB/C mice | ↓collagen, ↓F4/80+
↓SMAD2/SMAD3 ↓ERK1/2, ↓CD3+, ↓ macrophages |
[83] |
| VCE‐004.8 | 10, 20 mg/kg, i.p. for 3 weeks to BALB/c mice | ↓Smad2/Smad3
↓F4/80+, ↓Col3A1, Col1A2, IL‐1β and IL‐13 |
[84] |
| GP1a | 3 mg/kg, i.p. BALB/c mic | ↓MCP‐1, SDF‐1,
TNF‐α and TGF‐β1 ↓L‐6, IL‐1β, and VEGF, ↓collagen |
[88] |
| GP1a | 3 mg/kg/day, i.p. BALB/c mice | ↓collagen I
↓TGF‐β1, P‐Smad3 |
[89] |
| WIN55,212‐2 | 1 mg/kg/day, s.c. for 21 days to DBA/2 J mice | ↓hydroxyproline
↓α‐SMA, ↓PDGF and ↓TGFβ |
[90] |
| WIN55,212‐2 | 1 and 10 M for 24 days to dermal fibroblasts | ↓ collagen I
↓TGF‐ β and CTGF ↓p‐ERK‐1/2 |
[91] |
Similarly, the effect of JWH‐133 on the progression of dermal fibrosis in mice subjected to hypochlorous acid (HOCl) challenges was examined. The experimental findings demonstrated that the administration of JWH‐133 to the mice resulted in a notable decrease in the thickness of the skin and the accumulation of collagen. JWH‐133 exhibited protective effects against dermal fibrosis through the inhibition of fibroblast proliferation, as observed both in vitro and in vivo. The study revealed that mice lacking the CB2 gene (CB2−/−) exhibit a higher susceptibility to developing augmented fibrosis compared to mice with intact CB2 genes (CB2+/+). The histopathological analysis revealed an observed increase in dermal thickness accompanied by heightened skin fibrosis in CB2−/− individuals following exposure to HOCl.85
Recently, it has been demonstrated that the activation of CB2 receptors plays a role in the differentiation of human fibroblasts and the synthesis of collagen. The administration of JWH‐133 to human fibroblasts stimulated by TGF‐β resulted in a notable decrease in the synthesis of α‐SMA and collagen production.86, 87Gp1a, a highly selective CB2 agonist, has been discovered to exhibit efficacy in the reduction of inflammation and promotion of wound healing. This effect is observed specifically in incised skin wound healing, achieved by diminishing the infiltration of M1 macrophages.92Previous studies have reported that Gp1a has the potential to improve the simultaneous development of fibrogenesis that arises from skin wounds. The fibrotic markers, which are produced by cells that release fibrotic factors, consist of fibroblasts (positive for pro‐collagen I), myofibroblasts (positive for pro‐collagen I and α‐smooth muscle actin), and fibrocytes (positive for CD45 and pro‐collagen I). In the treated mice, these markers were observed at a reduced level compared to the mice treated with the vehicle. Additionally, the ratio of fibroblast‐to‐myofibroblast differentiation was low in the treated mice.88
In another study, the involvement of GP1a was further elucidated in a simulated scenario of cutaneous wound healing. The observations revealed a reduction in the amount of collagen deposited and thinner fibers when compared to mice treated with the control substance. In contrast, the vehicle‐treated mice displayed a higher presence of polymorphonuclear cells and an accumulation of fibroblasts with a spindle‐like shape in the injured wounded area.89Furthermore, the compound WIN55,212‐2 exhibited a suppressive impact on the increase in dermal thickness caused by bleomycin, while maintaining the presence of subcutaneous adipose tissue in the perivascular spaces and reducing the infiltration of inflammatory cells in the subcutaneous layer. Additionally, it was observed that the administration of WIN55,212‐2 resulted in a suppression of PDGF and connective tissue growth factor expression. Furthermore, there was a decrease in the phosphorylation of p‐SMAD2/3 in WIN55,212‐2‐treated mice compared to mice challenged with bleomycin.90
In an in vitro study, the effectiveness of WIN55,212‐2 was observed by its ability to hinder the process of profibrotic fibroblast transdifferentiation. Fibroblasts obtained from patients with diffuse cutaneous systemic sclerosis exhibited a significantly elevated protein expression of α‐SMA in comparison to fibroblasts derived from individuals without the condition. The fibroblasts exhibited a decrease in protein expression subsequent to the administration of WIN55,212‐2 treatment.91The antifibrotic effects of CB2 receptors were further investigated and confirmed by utilizing CB2 receptor knockout mice. In this investigation, the mice lacking CB2 receptors displayed an increased vulnerability to the initiation of dermal fibrosis compared to the mice with intact CB2 receptors. The pharmacological suppression of CB2 receptors in CB2+/+ mice using AM‐630 led to a significant increase in the development of fibrosis and thickening of the skin. On the contrary, the administration of JWH‐133 resulted in the activation of CB2 receptors, leading to a notable improvement in the measured dermal thickness.86
7. CONCLUSIONS
This review provides an outline showcasing the antifibrogenic effect exhibited by the CB2 agonists. The pharmacological manipulation of these therapeutic properties holds potential for their application in the treatment of fibrosis and preservation of organ histology. The observed elevated rates of mortality in relation to fibrosis indicate a pressing requirement for the exploration and identification of pharmacotherapeutic interventions possessing antifibrogenic properties, to address this incapacitating ailment.
The existing evidence supports the utilization of CB2 ligands to stimulate CB2 receptors or alternative methods to enhance the expression of these receptors in order to protect against organ fibrosis. Convincing number of studies are currently being conducted on discovering antifibrogenic therapies. The therapeutic potential of the ECS in the treatment of fibrosis has been observed to be quite intriguing. The significance of CB2 agonism arises from its ability to induce anti‐inflammatory responses, thereby aiding in the mitigation of fibrosis progression.
The present review emphasizes the significant importance of the potential of activated CB2 receptors to suppress the production of pro‐inflammatory cytokines and impede the activity of diverse transcriptional factors that contribute to the amplification of the inflammatory process. The antifibrotic effect of CB2 agonists is hypothesized to arise from their ability to impede the differentiation process of fibroblasts, thereby preventing their transition into cells that secrete fibrotic proteins. The established correlation between inflammation and fibrosis has been extensively documented in scientific literature.
Nevertheless, to uncover additional molecular mechanisms that contribute to the antifibrotic effect, further comprehensive investigations are necessary. The extensive data presented in this study regarding the biological activities of CB2 agonists hold great promise for their potential clinical applications. However, it is also advised to carry out pharmacokinetic studies to unravel the kinetic properties of these diverse ligands, thereby ensuring the secure and precise extrapolation of these compounds in forthcoming clinical investigations.
8. NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,93, 94and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.95
AUTHOR CONTRIBUTIONS
Conceptualization, S.O.; Methodology, L.B.E.; and M.F.N.M.; Formal analysis, and investigation, L.B.E.; Writing‐original draft preparation, L.B.E.; Writing‐review and editing, L.B.E., S.O., and S.B.S.; Validation, S.B.S.; Visualization, N.K.J.; Supervision, S.O.; Funding acquisition, S.O. All authors have read and agreed to the published version of the manuscript. Funding: The authors are thankful to the United Arab Emirates University for the research grants and open‐access support for this publication. The authors are also thankful to the College of Graduate Studies, UAE University, Al Ain, UAE, for the Graduate scholarship to Ms. Lujain.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
INFORMED CONSENT STATEMENT
Not applicable.
ACKNOWLEDGMENT
The authors are thankful to the United Arab Emirates University for the award of the research grants (# 12R121 and 12R104 to Shreesh Ojha).
DATA AVAILABILITY STATEMENT
This is a review and the majority of the articles referred to are cited appropriately in the manuscript.
REFERENCES
- 1. Horowitz JC, Thannickal VJ. Mechanisms for the resolution of organ fibrosis. Phys Ther. 2018;34:43‐55. doi: 10.1152/physiol.00033.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galliot B, Crescenzi M, Jacinto A, Tajbakhsh S. Trends in tissue repair and regeneration. Development. 2017;144:357‐364. doi: 10.1242/dev.144279 [DOI] [PubMed] [Google Scholar]
- 3. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199‐210. doi: 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suthahar N, Meijers WC, Silljé HHW, de Boer RA. From inflammation to fibrosis‐molecular and cellular mechanisms of myocardial tissue Remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep. 2017;14:235‐250. doi: 10.1007/s11897-017-0343-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fonseca BM, Costa MA, Almada M, Correia‐da‐Silva G, Teixeira NA. Endogenous cannabinoids revisited: a biochemistry perspective. Prostaglandins Other Lipid Mediat. 2013;102‐103:13‐30. doi: 10.1016/j.prostaglandins.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 6. Munro S, Thomas KL, Abu‐Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61‐65. doi: 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- 7. Galiègue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54‐61. doi: 10.1111/j.1432-1033.1995.tb20780.x [DOI] [PubMed] [Google Scholar]
- 8. Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516‐525. doi: 10.1016/j.biopsych.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steffens S, Pacher P. Targeting cannabinoid receptor CB2 in cardiovascular disorders: promises and controversies. Br J Pharmacol. 2012;167:313‐323. doi: 10.1111/j.1476-5381.2012.02042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strisciuglio C, Creoli M, Tortora C, et al. Increased expression of CB2 receptor in the intestinal biopsies of children with inflammatory bowel disease. Pediatr Res. 2022;93:1‐6. doi: 10.1038/s41390-022-02109-5 [DOI] [PubMed] [Google Scholar]
- 11. Lotersztajn S, Teixeira‐Clerc F, Julien B, et al. CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol. 2008;153:286‐289. doi: 10.1038/sj.bjp.0707511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ofek O, Karsak M, Leclerc N, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103:696‐701. doi: 10.1073/pnas.0504187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walker OLS, Holloway AC, Raha S. The role of the endocannabinoid system in female reproductive tissues. J Ovarian Res. 2019;12:3. doi: 10.1186/s13048-018-0478-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agirregoitia E, Carracedo A, Subirán N, et al. The CB2 cannabinoid receptor regulates human sperm cell motility. Fertil Steril. 2010;93:1378‐1387. doi: 10.1016/j.fertnstert.2009.01.153 [DOI] [PubMed] [Google Scholar]
- 15. Graham ES, Angel CE, Schwarcz LE, Dunbar PR, Glass M. Detailed characterisation of CB2 receptor protein expression in peripheral blood immune cells from healthy human volunteers using flow cytometry. Int J Immunopathol Pharmacol. 2010;23:25‐34. doi: 10.1177/039463201002300103 [DOI] [PubMed] [Google Scholar]
- 16. Bort A, Alvarado‐Vazquez PA, Moracho‐Vilrriales C, et al. Effects of JWH015 in cytokine secretion in primary human keratinocytes and fibroblasts and its suitability for topical/transdermal delivery. Mol Pain. 2017;13:1744806916688220. doi: 10.1177/1744806916688220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simard M, Rakotoarivelo V, Di Marzo V, Flamand N. Expression and functions of the CB2 receptor in human leukocytes. Front Pharmacol. 2022;13:826400. doi: 10.3389/fphar.2022.826400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parlar A, Arslan SO, Doğan MF, et al. The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp Ther Med. 2018;16:4900‐4908. doi: 10.3892/etm.2018.6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pryimak N, Zaiachuk M, Kovalchuk O, Kovalchuk I. The potential use of cannabis in tissue fibrosis. Front Cell Dev Biol. 2021;9:715380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873‐884. doi: 10.1038/nrn1247 [DOI] [PubMed] [Google Scholar]
- 21. Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzmán M, Galve‐Roperh I. Non‐psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405‐2407. doi: 10.1096/fj.06-6164fje [DOI] [PubMed] [Google Scholar]
- 22. Chen D‐j, Gao M, Gao F‐f, Su Q‐x, Wu J. Brain cannabinoid receptor 2: expression, function and modulation. Acta Pharmacol Sin. 2017;38:312‐316. doi: 10.1038/aps.2016.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bie B, Wu J, Foss JF, Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol. 2018;31:31‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muñoz‐Luque J, Ros J, Fernández‐Varo G, et al. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther. 2008;324:475‐483. doi: 10.1124/jpet.107.131896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amin MR, Ali DW. Pharmacology of medical cannabis. Adv Exp Med Biol. 2019;1162:151‐165. doi: 10.1007/978-3-030-21737-2_8 [DOI] [PubMed] [Google Scholar]
- 26. Sunda F, Arowolo A. A molecular basis for the anti‐inflammatory and anti‐fibrosis properties of cannabidiol. FASEB J. 2020;34:14083‐14092. doi: 10.1096/fj.202000975R [DOI] [PubMed] [Google Scholar]
- 27. Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis. Circ Res. 2016;118:1021‐1040. doi: 10.1161/CIRCRESAHA.115.306565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Navarrete C, Garcia‐Martin A, DeMesa J, Muñoz E. Cannabinoids in metabolic syndrome and cardiac fibrosis. Curr Hypertens Rep. 2020;22:98. doi: 10.1007/s11906-020-01112-7 [DOI] [PubMed] [Google Scholar]
- 29. Li X, Han D, Tian Z, et al. Activation of cannabinoid receptor type II by AM1241 ameliorates myocardial fibrosis via Nrf2‐mediated inhibition of TGF‐β1/Smad3 pathway in myocardial infarction mice. Cell Physiol Biochem. 2016;39:1521‐1536. doi: 10.1159/000447855 [DOI] [PubMed] [Google Scholar]
- 30. Han D, Li X, Fan WS, et al. Activation of cannabinoid receptor type II by AM1241 protects adipose‐derived mesenchymal stem cells from oxidative damage and enhances their therapeutic efficacy in myocardial infarction mice via Stat3 activation. Oncotarget. 2017;8:64853‐64866. doi: 10.18632/oncotarget.17614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu D, Xu C, Xue X, et al. Activation of cannabinoid receptor 2 attenuates angiotensin II‐induced atrial fibrillation via a potential NOX/CaMKII mechanism. Front Cardiovasc Med. 2022;9:968014. doi: 10.3389/fcvm.2022.968014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Ma S, Wang Q, et al. Effects of cannabinoid receptor type 2 on endogenous myocardial regeneration by activating cardiac progenitor cells in mouse infarcted heart. Sci China Life Sci. 2014;57:201‐208. doi: 10.1007/s11427-013-4604-z [DOI] [PubMed] [Google Scholar]
- 33. García‐Martín A, Navarrete C, Garrido‐Rodríguez M, et al. EHP‐101 alleviates angiotensin II‐induced fibrosis and inflammation in mice. Biomed Pharmacother. 2021;142:112007. doi: 10.1016/j.biopha.2021.112007 [DOI] [PubMed] [Google Scholar]
- 34. Defer N, Wan J, Souktani R, et al. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion‐induced cardiomyopathy. FASEB J. 2009;23:2120‐2130. doi: 10.1096/fj.09-129478 [DOI] [PubMed] [Google Scholar]
- 35. Li X, Peng Z, Zhou Y, et al. Quetiapine induces myocardial necroptotic cell death through bidirectional regulation of cannabinoid receptors. Toxicol Lett. 2019;313:77‐90. doi: 10.1016/j.toxlet.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 36. Chen Z, Zheng L, Chen G. 2‐Arachidonoylglycerol attenuates myocardial fibrosis in diabetic mice via the TGF‐β1/Smad pathway. Cardiovasc Drugs Ther. 2022;37:647‐654. doi: 10.1007/s10557-021-07307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matyas C, Erdelyi K, Trojnar E, et al. Interplay of liver‐heart inflammatory Axis and cannabinoid 2 receptor signaling in an experimental model of hepatic cardiomyopathy. Hepatology. 2020;71:1391‐1407. doi: 10.1002/hep.30916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ambrosio ALB, Dias SMG, Polikarpov I, Zurier RB, Burstein SH, Garratt RC. Ajulemic acid, a synthetic nonpsychoactive cannabinoid acid, bound to the ligand binding domain of the human peroxisome proliferator‐activated receptor γ*. J Biol Chem. 2007;282:18625‐18633. doi: 10.1074/jbc.M702538200 [DOI] [PubMed] [Google Scholar]
- 39. Horckmans M, Bianchini M, Santovito D, et al. Pericardial adipose tissue regulates Granulopoiesis, fibrosis, and cardiac function after myocardial infarction. Circulation. 2018;137:948‐960. doi: 10.1161/circulationaha.117.028833 [DOI] [PubMed] [Google Scholar]
- 40. Duerr GD, Heinemann JC, Kley J, et al. Myocardial maladaptation to pressure overload in CB2 receptor‐deficient mice. J Mol Cell Cardiol. 2019;133:86‐98. doi: 10.1016/j.yjmcc.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 41. Duerr GD, Heinemann JC, Suchan G, et al. The endocannabinoid‐CB2 receptor axis protects the ischemic heart at the early stage of cardiomyopathy. Basic Res Cardiol. 2014;109:425. doi: 10.1007/s00395-014-0425-x [DOI] [PubMed] [Google Scholar]
- 42. Mallat A, Teixeira‐Clerc F, Deveaux V, Manin S, Lotersztajn S. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br J Pharmacol. 2011;163:1432‐1440. doi: 10.1111/j.1476-5381.2011.01397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siegmund SV, Schwabe RF. Endocannabinoids and liver disease. II. Endocannabinoids in the pathogenesis and treatment of liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G357‐G362. doi: 10.1152/ajpgi.00456.2007 [DOI] [PubMed] [Google Scholar]
- 44. Julien B, Grenard P, Teixeira‐Clerc F, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742‐755. doi: 10.1053/j.gastro.2004.12.050 [DOI] [PubMed] [Google Scholar]
- 45. Carmona‐Hidalgo B, González‐Mariscal I, García‐Martín A, et al. Δ9‐Tetrahydrocannabinolic acid markedly alleviates liver fibrosis and inflammation in mice. Phytomedicine. 2021;81:153426. doi: 10.1016/j.phymed.2020.153426 [DOI] [PubMed] [Google Scholar]
- 46. Aljobaily N, Krutsinger K, Viereckl MJ, et al. Low‐dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with methionine/choline deficient diet‐induced NASH model via modulation of cannabinoid receptor. Nutrients. 2022;15(1):178. doi: 10.3390/nu15010178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang HQ, Wan Z, Zhang Q, et al. Schisandrin B targets cannabinoid 2 receptor in Kupffer cell to ameliorate CCl(4)‐induced liver fibrosis by suppressing NF‐κB and p38 MAPK pathway. Phytomedicine. 2022;98:153960. doi: 10.1016/j.phymed.2022.153960 [DOI] [PubMed] [Google Scholar]
- 48. Wu HM, Kim TH, Kim A, Koo JH, Joo MS, Kim SG. Liver X receptor α‐induced cannabinoid receptor 2 inhibits ubiquitin‐specific peptidase 4 through miR‐27b, protecting hepatocytes from TGF‐β. Hepatol Commun. 2019;3:1373‐1387. doi: 10.1002/hep4.1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mahmoud MF, Swefy SE, Hasan RA, Ibrahim A. Role of cannabinoid receptors in hepatic fibrosis and apoptosis associated with bile duct ligation in rats. Eur J Pharmacol. 2014;742:118‐124. doi: 10.1016/j.ejphar.2014.08.021 [DOI] [PubMed] [Google Scholar]
- 50. Ali AM, El‐Tawil OS, Al‐Mokaddem AK, Abd El‐Rahman SS. Promoted inhibition of TLR4/miR‐155/ NF(k)B p65 signaling by cannabinoid receptor 2 agonist (AM1241), aborts inflammation and progress of hepatic fibrosis induced by thioacetamide. Chem Biol Interact. 2021;336:109398. doi: 10.1016/j.cbi.2021.109398 [DOI] [PubMed] [Google Scholar]
- 51. He P, Wu YF, Yang HY, Cheng ML, Liang YD, Wang YP. Effect of cannabinoid receptor‐2 agonist AM1241 on platelet‐derived growth factor expression in the liver tissue of mice with hepatic fibrosis. Zhonghua Gan Zang Bing Za Zhi. 2017;25:841‐846. doi: 10.3760/cma.j.issn.1007-3418.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 52. Reichenbach V, Ros J, Fernández‐Varo G, et al. Prevention of fibrosis progression in CCl4‐treated rats: role of the hepatic endocannabinoid and apelin systems. J Pharmacol Exp Ther. 2012;340:629‐637. doi: 10.1124/jpet.111.188078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Long C, Xie N, Shu Y, et al. Knockout of the cannabinoid receptor 2 gene promotes inflammation and hepatic stellate cell activation by promoting A20/nuclear factor‐κB (NF‐κB) expression in mice with carbon tetrachloride‐induced liver fibrosis. Med Sci Monit. 2021;27:e931236. doi: 10.12659/msm.931236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guillot A, Hamdaoui N, Bizy A, et al. Cannabinoid receptor 2 counteracts interleukin‐17‐induced immune and fibrogenic responses in mouse liver. Hepatology. 2014;59:296‐306. doi: 10.1002/hep.26598 [DOI] [PubMed] [Google Scholar]
- 55. Trebicka J, Racz I, Siegmund SV, et al. Role of cannabinoid receptors in alcoholic hepatic injury: steatosis and fibrogenesis are increased in CB2 receptor‐deficient mice and decreased in CB1 receptor knockouts. Liver Int. 2011;31:860‐870. doi: 10.1111/j.1478-3231.2011.02496.x [DOI] [PubMed] [Google Scholar]
- 56. Liu H, Gao X, Duan R, et al. Endocannabinoids anandamide and its cannabinoid receptors in liver fibrosis after murine schistosomiasis. J Huazhong Univ Sci Technolog Med Sci. 2009;29:182‐186. doi: 10.1007/s11596-009-0209-y [DOI] [PubMed] [Google Scholar]
- 57. Zhang Z, Guo Y, Zhang S, et al. Curcumin modulates cannabinoid receptors in liver fibrosis in vivo and inhibits extracellular matrix expression in hepatic stellate cells by suppressing cannabinoid receptor type‐1 in vitro. Eur J Pharmacol. 2013;721:133‐140. doi: 10.1016/j.ejphar.2013.09.042 [DOI] [PubMed] [Google Scholar]
- 58. Huang SS, Chen DZ, Wu H, et al. Cannabinoid receptors are involved in the protective effect of a novel curcumin derivative C66 against CCl4‐induced liver fibrosis. Eur J Pharmacol. 2016;779:22‐30. doi: 10.1016/j.ejphar.2016.02.067 [DOI] [PubMed] [Google Scholar]
- 59. Rossi F, Bellini G, Alisi A, et al. Cannabinoid receptor type 2 functional variant influences liver damage in children with non‐alcoholic fatty liver disease. PLoS One. 2012;7:e42259. doi: 10.1371/journal.pone.0042259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bozkurt TE. Endocannabinoid system in the airways. Molecules. 2019;24:24. doi: 10.3390/molecules24244626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Turcotte C, Blanchet MR, Laviolette M, Flamand N. Impact of cannabis, cannabinoids, and endocannabinoids in the lungs. Front Pharmacol. 2016;7:317. doi: 10.3389/fphar.2016.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kicman A, Pędzińska‐Betiuk A, Kozłowska H. The potential of cannabinoids and inhibitors of endocannabinoid degradation in respiratory diseases. Eur J Pharmacol. 2021;911:174560. doi: 10.1016/j.ejphar.2021.174560 [DOI] [PubMed] [Google Scholar]
- 63. Vuolo F, Abreu SC, Michels M, et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur J Pharmacol. 2019;843:251‐259. doi: 10.1016/j.ejphar.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 64. He Q, Zhang W, Zhang J, Deng Y. Cannabinoid analogue WIN 55212‐2 protects Paraquat‐induced lung injury and enhances macrophage M2 polarization. Inflammation. 2022;45:2256‐2267. doi: 10.1007/s10753-022-01688-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu T, Gu J, Yuan Y, et al. Discovery of a pyrano[2,3‐b]pyridine derivative YX‐2102 as a cannabinoid receptor 2 agonist for alleviating lung fibrosis. J Transl Med. 2022;20:565. doi: 10.1186/s12967-022-03773-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wawryk‐Gawda E, Chłapek K, Zarobkiewicz MK, et al. CB2R agonist prevents nicotine induced lung fibrosis. Exp Lung Res. 2018;44:344‐351. doi: 10.1080/01902148.2018.1543368 [DOI] [PubMed] [Google Scholar]
- 67. Fu Q, Zheng Y, Dong X, Wang L, Jiang CG. Activation of cannabinoid receptor type 2 by JWH133 alleviates bleomycin‐induced pulmonary fibrosis in mice. Oncotarget. 2017;8:103486‐103498. doi: 10.18632/oncotarget.21975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Parlar A, Arslan SO, Yumrutas O, et al. Effects of cannabinoid receptor 2 synthetic agonist, AM1241, on bleomycin induced pulmonary fibrosis. Biotech Histochem. 2021;96:48‐59. doi: 10.1080/10520295.2020.1758343 [DOI] [PubMed] [Google Scholar]
- 69. García‐Martín A, Garrido‐Rodríguez M, Navarrete C, et al. EHP‐101, an oral formulation of the cannabidiol aminoquinone VCE‐004.8, alleviates bleomycin‐induced skin and lung fibrosis. Biochem Pharmacol. 2018;157:304‐313. doi: 10.1016/j.bcp.2018.07.047 [DOI] [PubMed] [Google Scholar]
- 70. Liu J, Shi G. Pirfenidone activates cannabinoid receptor 2 in a mouse model of bleomycin‐induced pulmonary fibrosis. Exp Ther Med. 2019;18:4241‐4248. doi: 10.3892/etm.2019.8045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tarique AA, Evron T, Zhang G, et al. Anti‐inflammatory effects of lenabasum, a cannabinoid receptor type 2 agonist, on macrophages from cystic fibrosis. J Cyst Fibros. 2020;19:823‐829. doi: 10.1016/j.jcf.2020.03.015 [DOI] [PubMed] [Google Scholar]
- 72. Lucattelli M, Fineschi S, Selvi E, et al. Ajulemic acid exerts potent anti‐fibrotic effect during the fibrogenic phase of bleomycin lung. Respir Res. 2016;17:49. doi: 10.1186/s12931-016-0373-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Barutta F, Bruno G, Mastrocola R, Bellini S, Gruden G. The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int. 2018;94:252‐258. doi: 10.1016/j.kint.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 74. Swanson ML, Regner KR, Moore BM 2nd, Park F. Cannabinoid type 2 receptor activation reduces the progression of kidney fibrosis using a mouse model of unilateral ureteral obstruction. Cannabis Cannabinoid Res. 2022;7:790‐803. doi: 10.1089/can.2021.0127 [DOI] [PubMed] [Google Scholar]
- 75. Trojnar E, Erdelyi K, Matyas C, et al. Cannabinoid‐2 receptor activation ameliorates hepatorenal syndrome. Free Radic Biol Med. 2020;152:540‐550. doi: 10.1016/j.freeradbiomed.2019.11.027 [DOI] [PubMed] [Google Scholar]
- 76. González‐Mariscal I, Carmona‐Hidalgo B, Winkler M, et al. (+)‐trans‐Cannabidiol‐2‐hydroxy pentyl is a dual CB1R antagonist/CB2R agonist that prevents diabetic nephropathy in mice. Pharmacol Res. 2021;169:105492. doi: 10.1016/j.phrs.2021.105492 [DOI] [PubMed] [Google Scholar]
- 77. Tang M, Cao X, Zhang K, et al. Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death Dis. 2018;9:601. doi: 10.1038/s41419-018-0666-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jenkin KA, O’Keefe L, Simcocks AC, et al. Renal effects of chronic pharmacological manipulation of CB2 receptors in rats with diet‐induced obesity. Br J Pharmacol. 2016;173:1128‐1142. doi: 10.1111/bph.13056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Canady J, Karrer S, Fleck M, Bosserhoff AK. Fibrosing connective tissue disorders of the skin: molecular similarities and distinctions. J Dermatol Sci. 2013;70:151‐158. doi: 10.1016/j.jdermsci.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 80. Río Cd, Millán E, García V, Appendino G, DeMesa J, Muñoz E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem Pharmacol. 2018;157:122‐133. doi: 10.1016/j.bcp.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 81. Jonsson K‐O, Persson E, Fowler CJ. The cannabinoid CB2 receptor selective agonist JWH133 reduces mast cell oedema in response to compound 48/80 in vivo but not the release of β‐hexosaminidase from skin slices in vitro. Life Sci. 2006;78:598‐606. doi: 10.1016/j.lfs.2005.05.059 [DOI] [PubMed] [Google Scholar]
- 82. Cintosun A, Lara‐Corrales I, Pope E. Mechanisms of cannabinoids and potential applicability to skin diseases. Clin Drug Investig. 2020;40:293‐304. doi: 10.1007/s40261-020-00894-7 [DOI] [PubMed] [Google Scholar]
- 83. Del Rio C, Cantarero I, Palomares B, et al. VCE‐004.3, a cannabidiol aminoquinone derivative, prevents bleomycin‐induced skin fibrosis and inflammation through PPARγ‐ and CB(2) receptor‐dependent pathways. Br J Pharmacol. 2018;175:3813‐3831. doi: 10.1111/bph.14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. del Río C, Navarrete C, Collado JA, et al. The cannabinoid quinol VCE‐004.8 alleviates bleomycin‐induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator‐activated receptor‐γ and CB2 pathways. Sci Rep. 2016;6:21703. doi: 10.1038/srep21703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Servettaz A, Kavian N, Nicco C, et al. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am J Pathol. 2010;177:187‐196. doi: 10.2353/ajpath.2010.090763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Akhmetshina A, Dees C, Busch N, et al. The cannabinoid receptor CB2 exerts antifibrotic effects in experimental dermal fibrosis. Arthritis Rheum. 2009;60:1129‐1136. doi: 10.1002/art.24395 [DOI] [PubMed] [Google Scholar]
- 87. Correia‐Sá I, Carvalho C, A Machado V, et al. Targeting cannabinoid receptor 2 (CB2) limits collagen production‐an in vitro study in a primary culture of human fibroblasts. Fundam Clin Pharmacol. 2022;36:89‐99. doi: 10.1111/fcp.12716 [DOI] [PubMed] [Google Scholar]
- 88. Wang LL, Zhao R, Li JY, et al. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re‐epithelialization during skin wound healing. Eur J Pharmacol. 2016;786:128‐136. doi: 10.1016/j.ejphar.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 89. Li SS, Wang LL, Liu M, et al. Cannabinoid CB₂ receptors are involved in the regulation of fibrogenesis during skin wound repair in mice. Mol Med Rep. 2016;13:3441‐3450. doi: 10.3892/mmr.2016.4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Balistreri E, Garcia‐Gonzalez E, Selvi E, et al. The cannabinoid WIN55, 212‐2 abrogates dermal fibrosis in scleroderma bleomycin model. Ann Rheum Dis. 2011;70:695‐699. doi: 10.1136/ard.2010.137539 [DOI] [PubMed] [Google Scholar]
- 91. Garcia‐Gonzalez E, Selvi E, Balistreri E, et al. Cannabinoids inhibit fibrogenesis in diffuse systemic sclerosis fibroblasts. Rheumatology (Oxford). 2009;48:1050‐1056. doi: 10.1093/rheumatology/kep189 [DOI] [PubMed] [Google Scholar]
- 92. Du Y, Ren P, Wang Q, et al. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J Inflamm. 2018;15:25. doi: 10.1186/s12950-018-0201-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Res. 2018;46:D1091‐D1106. doi: 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Alexander SPH, Christopoulos A, Davenport AP, et al. The Concise guide to PHARMACOLOGY 2023/24: G protein‐coupled receptors. Br J Pharmacol. 2023;180:S23‐S144. doi: 10.1111/bph.16177 [DOI] [PubMed] [Google Scholar]
- 95. Alexander SPH, Christopoulos A, Davenport AP, et al. The concise guide to pharmacology 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019;176(Suppl 1):S21‐s141. doi: 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review and the majority of the articles referred to are cited appropriately in the manuscript.



