Learn more: PMC Disclaimer | PMC Copyright Notice
. 2024 Sep 14;7(1):206–212. doi: 10.1159/000541034
Abstract
Introduction
Diabetes mellitus (DM) is a common endocrinopathy in felines. Treatment is based on glycemic control and management of clinical signs by insulin administration coupled with a low-carbohydrate and high-protein content diet. However, achieving adequate remission or glycemia control is not always possible. Effects of cannabinoids on the regulation of glucose uptake and the incidence of diabetes have been observed in experimental models. Nevertheless, little is known about their possible relevance in controlling this condition in veterinary and human medicine.
Case Presentation
This is a case study of an 18-year-old, neutered, mixed-breed female domestic longhair cat diagnosed with type 2 DM. She was treated with long-acting glargine (3–5 IU/12 h), and her diet changed to ultra-processed commercial food for diabetic cats. Three months after the start of the treatment with insulin, cannabidiol (CBD)-enriched extract in handmade olive oil, tetrahydrocannabinol: CBD ratio = 1:24, was incorporated. The route of administration was oromucosal. After 3 months, the glycemia was reduced. The patient decreased the polyuria/polydipsia, recovered sleep cycles, remained attentive to all movements, and increased her physical activity.
Conclusion
This report provides evidence that using a CBD-rich extract was effective as a co-adjuvant in alleviating clinical signs of DM and concurrent disorders, allowing for the reduction of insulin intake.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder in which the body’s ability to produce or respond to the hormone insulin is impaired. This results in abnormal carbohydrate metabolism and elevated glucose levels in the blood and urine. DM is one of the most common and prominent metabolic diseases diagnosed in canine and feline families after human beings. The clinical features are rarely observed in other large domestic animals such as horses, cattle, buffalo, swine, and other small ruminants [1].
DM in felines presents a prevalence ranging from 0.25% to 1% depending on the region and the feline breeds analyzed [2, 3]. Risk factors bring together genetic and environmental determinants. The latter include obesity, low physical activity, age, male sex, neutering, indoor confinement, and a specific type of medication [4]. The classification of DM in veterinary medicine is based on the human counterpart. The common and general forms of DM are known to be insulin-dependent (ID) type 1 and non-IDDM type 2. Type 3 DM has been identified as a complication of insulin antagonisms, occurring due to pancreatic islet damage by pancreatic necrosis, tumor progression, and pancreatitis [1]. Type 2 DM is the most common in cats, characterized by insulin resistance and β-cell failure to maintain euglycemia [2].
The endocannabinoid system (ECS) is conserved in many animal species, from invertebrates to mammals [5, 6]. It comprises ligands, receptors, and enzymes that control the synthesis and degradation of ligands. Cannabinoid receptors 1 (CB1R) and 2 (CB2R) are the classical transmembrane receptors coupled to the G protein [7]. Moreover, other proteins like ionic channels or intracellular receptors are distributed in different organs, conforming with a diversity of ligands, the endocannabinoidome, a term which would better reflect the multisystem influence of cannabinoids and ECS modulators [8]. ECS plays an essential role in regulating body weight, food intake, the development of hyperglycemia, insulin resistance, and dyslipidemia [9, 10]. Experimental models indicated that peripheral overactivation of the ECS, mainly the CB1R signaling, plays a significant role in the progression of insulin resistance, diabetes (especially type 2), and its age-related comorbidities such as atherosclerosis, nephropathy, neuropathy, and retinopathy [6].
Like in humans, predisposing factors for type 2 DM in cats are overweight, sedentarism, and aging [11]. Managing DM in cats represents a challenge for guardians and the veterinary care team to prevent/delay deterioration in the patient’s quality of life. Treatment of diabetic cats focuses on maximizing the chances of remission through tight control of blood glucose (72 to <180 mg/dL) [2]. For long-term diabetic cats, clinical management usually includes optimizing body weight by feeding an appropriate diet to avoid clinical hypoglycemia [2, 12, 13]. Incorporating cannabinoids in managing DM and the possible beneficial and adverse effects have little scientific evidence. For example, among other cannabinoids, cannabidiol (CBD) exhibits anti-inflammatory and antioxidant properties and modulates several cellular processes in which ECS is involved [14]. Their properties in controlling clinical signs of several human diseases encouraged people to suggest cannabinoids to veterinarians for treatment pathologies in pets [15]. However, more extensive information is necessary to consider cannabinoid use for animals. This work aimed to evaluate whether applying a CBD-rich extract induces changes in symptoms associated with type 2 DM in a feline patient as a co-adjuvant treatment.
Case Presentation
A female feline patient, mixed-breed, 18 years old, spayed, weighing 5.2 kg, was treated. Anamnesis was accompanied by biochemical analysis to confirm the feline type 2 DM diagnosis. Fructosamine levels were 464 μmol/L (reference value felines <340 μmol/L). Measurement of glycosuria was carried out by visually reading a reagent strip. Treatment started with long-acting basal insulin glargine, commercially known as Toujeo® (300 U/mL), from the SANOFI laboratory, administered 3–5 IU every 12 h (BID) for 3 months. The diet changed to ultra-processed commercial food for diabetic cats. The brand was Royal Canin (Diabetic Feline) with the following analytical constituents: protein: 46.0% – fat content: 12.0% – crude ash: 6.4% – crude fibers: 3.8% – starch: 19% – total sugars: 1.5% – essential fatty acids: 2.88%.
Due to the difficulty in stabilizing the glycemia and the associated clinical signs, we started cannabinoid administration added to insulin treatment. We utilized a full-spectrum CBD-enriched cannabis extract from Chemotype 3 of Cannabis sativa Blue Shark strain (high proportion of CBD, determined by the genetics of the plant). This extract was provided by a private cultivation in Rosario, Santa Fe, Argentina. The extract contained olive oil as the vehicle, and the tetrahydrocannabinol (THC): CBD ratio was 1:24. Determination of cannabinoids in the extract was performed through third-party services using high-performance liquid chromatography (Agilent Tec). The extraction method used was Rick Simpson Oil. The limit of detection for cannabinoids was 0.001 mg/mL, and the limit of quantification was 0.005 mg/mL. The data were analyzed using open Chemstation software. Three months after starting treatment with insulin glargine, CBD-enriched extract was incorporated and sustained for 3 months. The CBD-enriched extract was administered via oromucosal, 1 h after the meal, every 12 h (BID), separated from insulin application. Table 1 summarizes the characteristics of the extract and doses received expressed as mg/kg/day.
Table 1.
Extract characteristics and dosage
| CBD-enriched extract | |
|---|---|
| Chemotype of the plant | 3 |
| CBD in the extract, mg/mL | 6.4 |
| THC in the extract, mg/mL | 0.2 |
| Drops/day during the treatment | 8 |
| CBD dose expressed in mg/kg/day | 0.49 |
| THC dose expressed in mg/kg/day | 0.002 |
Concentration of the main cannabinoids, CBD and THC, expressed in mg/mL, present in the extract obtained from Cannabis sp Blue Shark strain. The number of drops administered via oromucosal and doses of CBD and THC received in mg/kg/day during the treatment are indicated.
CBD, cannabidiol; THC, tetrahydrocannabinol.
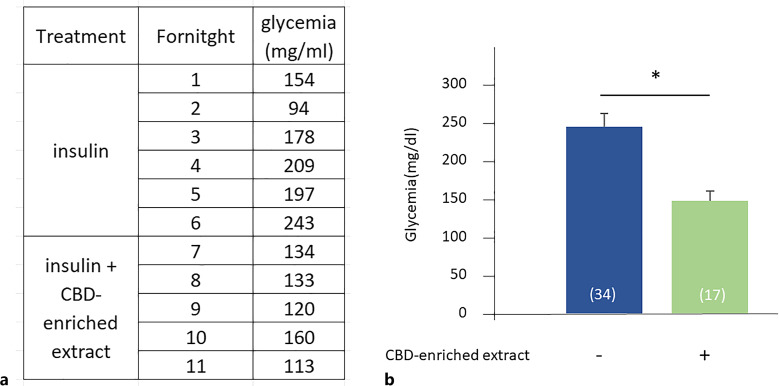
Treatment with CBD-enriched extract produced a significant decrease in glycemia, with average values from 244.47 ± 17.07 mg/dL (n = 34) to 147.88 ± 13.04 mg/dL (n = 17), presenting sporadic hyperglycemia peaks (250–350 mg/dL) associated with stressful situations (otitis, pain due to infection in dental pieces, presence of maintenance personnel in the house). Despite these specific events, cannabinoid treatment allowed the reduction of insulin doses from 5 to 4 IU. Figure 1 shows the variation of glycemia throughout the treatment and average values before and after 3 months of CBD-enriched extract administration, in addition to the insulin. Values related to the stressful situations mentioned above were excluded.
Fig. 1.
In conjunction with improved blood glucose regulation, polyuria/polydipsia was reduced, and the patient recovered sleep cycles, remained attentive to all movements, and began to play. Table 2 displays hepatic, lipid, and renal function indicators measured before and after 3 months of treatment with the extract. These parameters remained constant. Only a slight increment of ALP and glucose levels was detected, and they also remained below the reference levels.
Table 2.
Hepatic, lipid, and renal profile before and 3 months after the addition of a CBD-enriched extract to insulin treatment
| Analyte | Value before oil treatment | Three months after oil treatment | Normal range in felines |
|---|---|---|---|
| Hepatic profile | |||
| GPT (ALT) alanine aminotrasferase or glutamic pyruvic transaminase | 57 | 58 | <80 U/L |
| GOT (AST) aspartate aminotransferase or glutamic-oxaloacetic transaminase | 20 | 20 | <70 U/L |
| GGT gamma-glutamyltransferase | 5 | – | 0–10 U/L |
| Total protein | 7.2 | 8.3 | 5.6–7.8 g/dL |
| Albumin | 3.58 | 3.7 | 2.1–3.6 g/dL |
| Globulin | – | 4.6 | 3–4 g/dL |
| Albumin/globulin ratio | 0.9 | – | 0.5–1.3 |
| ALP (alkaline phosphatase) | 62 | 87 | <100 U/L |
| Total bilirubin | 0.14 | 0.01 | 0–0.6 mg/dL |
| Direct bilirubin | 0.00 | 0.00 | 0–0.3 mg/dL |
| Indirect bilirubin | 0.14 | – | |
| Glucose | 76 | 83 | 70–110 mg/dL |
| Lipid profile | |||
| Cholesterol | 184 | 289 | 0–150 mg/dL |
| Triglyceride levels | 47 | 100 | 50–100 mg/dL |
| HDL cholesterol | – | 200 | >100 mg/dL |
| LDL cholesterol | – | 69 | <60 mg/mL |
| Renal profile | |||
| Creatinine | 2.34 | 2.9 | <1.5 mg/dL |
| Urea | 66 | 114 | 30–60 mg/dL |
| Phosphorus | 4.6 | 4.8 | 2.6–6 mg/dL |
All parameters remained below the reference levels, suggesting that CBD-dominant extract administration via oromusosal, each 12 h (BID) during this period, was safe.
Discussion
This case report shows the beneficial effects of a CBD-dominant extract in a feline patient with type 2 DM. As described before, age, overweight, and genetics associated with cat breeds are comorbidity factors [4]. Tonkinese, Norwegian Forest, and Burmese cats had increased odds of DM compared to crossbred cats [16]. In this case, being overweight, neutering, and aging were relevant to developing type 2 DM in this cat. CBD-dominant extract, administered as a co-adjuvant treatment to insulin application, showed improved glycemia accompanied by reduced polyuria/polydipsia and glycosuria.
There is little information from studies in animal models about the use of full-spectrum preparations for treating insulin resistance and diabetes progression. In obese rats, cannabis extracts showed protective effects on β-cell survival and function, probably due to the control of pro-inflammatory pathways [17]. Other reports indicated that CBD improved metabolic dysfunction by incrementing insulin levels and reducing glycemia, fructosamine lipid, and total cholesterol [18]. Researchers showed that, CBD and Δ 9-tetrahydrocannabivarin (Δ9-THCV), both non-psychotropic cannabinoids, contributed to regulating glucose and lipid metabolism [14].
Despite this evidence in animal models, there is little information about the clinical impact of cannabinoids in diabetic cats. This case report constitutes a precedent of the use of CBD-enriched extract as a co-adjuvant to insulin treatment. Doses, frequency, and the way of administration of CBD or CBD-enriched extracts are relevant issues that need to be considered for medical use. For example, the administration of 100 mg twice daily of CBD in patients with type 2 DM had no effect compared with placebo, only a decreased polypeptidic hormone resistin and increased glucose-dependent insulinotropic peptide have been detected [19]. In humans, 0.18 mg/kg/day of CBD oil did not affect glycemia [20]. Herein, the dose of CBD, the main cannabinoid, was 0.49 mg/kg/day, administered twice a day (BID), transmucosal. This dose, smaller than other reports, was enough to control glycemia. A probable cause of the effectiveness might be the combination of an adequate dose of CBD with other cannabinoids and terpenes in this full-spectrum preparation. The protective impact of α-humulene on the function and survival of β-cells in diabetic rats has been reported [21]. β-caryophyllene, in conjunction with glibenclamide, a standard hypoglycemic drug, significantly reduced glycemia in rats with induced type 2 DM [22].
Another aspect to consider is the occurrence of adverse effects of cannabis administration. Although animals may well tolerate CBD, and it may have few side effects, we need to be careful with products with excess THC or toxins, such as heavy metals or pesticides that may cause harm. The most common signs of side effects are neurological, gastrointestinal, heart rate variation, and temperature control [15].
In the context of type 2 DM, information about side effects related to cannabinoid consumption is diverse. A report on a human diabetic patient shows that the replacement of insulin degludec for CBD oil did not produce secondary effects [20]. Contrarily, rimonabant, a synthetic CB1R inverse agonist, has effectively reduced food intake and body weight, two factors associated with type 2 DM. Although rimonabant was also effective in improving glucose homeostasis, it was not considered a plausible treatment due to numerous side effects [23]. In contrast, CBD and tetrahydrocannabivarin (THCV) act as antagonistic of CB1R and do not present adverse side effects such as psychoactive effects, depression, or anxiety [6]. On the other hand, the phytocannabinoid Δ9-THC is a stimulant of appetite and increases food intake, added to the psychotropic effect, not resulting in an indicated compound to treat diabetes [24]. Natural full-spectrum oil with 24:1 CBD:THC ratio used here shows positive results without secondary effects.
Herein, hepatic enzymes remain unaltered. Only the ALP exhibited a slight increment among the analyzed parameters, although it remained below the reference level. A study about the effects of long-term oral supplementation of healthy cats with 4 mg/kg of CBD extract did not show variation in hepatic parameters. They found an alanine aminotransferase increment 4 weeks after starting the study. In the long term, values did not show statistical differences compared with the placebo group [25]. It has been observed that the consumption of high therapeutic doses of CBD (more than 20 mg/kg/day) in both human patients and animal models increases the probability of hepatotoxicity, even more so when combined with other drugs, as in the case of certain antiepileptics. However, there is a lower risk of adverse hepatic effects at lower concentrations of cannabinoids contained in homemade extracts, dietary supplements, and food products [26]. More studies are needed to determine the doses at which hepatotoxic effects occur. In the same way, glucose exhibited a slight increment in the patient, which was not statistically significant compared to the treatment before CBD-enriched extract. As reported, glucose levels in cats present high variability under multiple situations. Stress hyperglycemia is frequent in sick cats or cats displaying signs of fear, including the stress of visits to medical facilities. Ambiental factors are challenging for managing DM [27].
Finally, a cannabinoid extract was conducted to reduce neuropathic pain, possibly associated with the control of oxidative stress [28, 29]. Such could explain why sleep regulation and activity state during wakefulness improved in this patient. The question remains about the effects of other compounds in extracts obtained from Cannabis sp that could potentiate the effect of cannabinoids. As we mentioned before, terpenes and flavonoids, among others, constitute a therapeutic potential in complications associated with DM [6, 30].
In summary, the present report constitutes a precedent showing that treatment with a CBD-rich extract improved the clinical condition of this feline patient with type 2 DM. However, further studies will be necessary to determine the mechanisms of action of phytocannabinoids. For example, other cannabinoid receptors beyond CB1R and CB2R are involved in the pathogenesis of DM, such as PPARs [31]. Further studies are needed to elucidate the mechanisms of action of cannabinoids on ECS and their role in DM.
Our results contribute to evaluating the relevance of the ECS in the development and progression of diabetes and the potential use of phytocannabinoids as part of the treatment. This case report constitutes a precedent for the medical use of phytocannabinoids in veterinary medicine. Information about the ECS and the potential of cannabinoids as contributors to DM treatment should be explored deeply.
Conclusion
This case report shows the beneficial effects of a CBD-enriched phytocannabinoid extract in a feline patient with type 2 DM. Added to glycemia control, indicators measured showed an improvement in the patient’s quality of life. Moreover, neurological and behavioral aspects associated with DM and aging improved. No secondary effects were observed.
Acknowledgments
We thank Melin for leaving this knowledge as a legacy and her tutor for her disposition and willingness to comply with the treatment throughout this time. Also our thanks to the RACME, Argentinean cannabic veterinarians, CONICET, and the UNLP for providing the academic context to carry out this treatment.
Statement of Ethics
This study protocol was reviewed and approved by the Research Ethics Committee from Veterinarios Cannábicos Argentinos (VCA), Approval No. [Exp 03/2022]. Written informed consent was obtained from the tutor of the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
J.I.M. and G.P. management of case, and collection of data. P.F. writing and editing of the manuscript, figure preparation, analysis of data, and review of final submission. J.I.M. review of final submission. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
All data are included in the manuscript. Further inquiries can be directed to the corresponding author.
References
- 1. Niaz K, Maqbool F, Khan F, Hassan FI, Momtaz S, Abdollahi M. Comparative occurrence of diabetes in canine, feline, and few wild animals and their association with pancreatic diseases and ketoacidosis with therapeutic approach. Vet World. 2018;11(4):410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gottlieb S, Rand J. Managing feline diabetes: current perspectives. Vet Med. 2018;9:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xenoulis PG, Fracassi F. Feline Comorbidities: clinical perspective on diabetes mellitus and pancreatitis. J Feline Med Surg. 2022;24(7):651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Öhlund M, Egenvall A, Fall T, Hansson-Hamlin H, Röcklinsberg H, Holst BS. Environmental risk factors for diabetes mellitus in cats. J Vet Intern Med. 2017;31(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silver RJ. The endocannabinoid system of animals. Animals. 2019;9:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghasemi-Gojani E, Kovalchuk I, Kovalchuk O. Cannabinoids and terpenes for diabetes mellitus and its complications: from mechanisms to new therapies. Trends Endocrinol Metab. 2022;33(12):828–49. [DOI] [PubMed] [Google Scholar]
- 7. Joshi N, Onaivi ES. Endocannabinoid system components: overview and tissue distribution. Advances in experimental medicine and biology; 2019. [DOI] [PubMed] [Google Scholar]
- 8. Davis MP. Overview of the endocannabinoid system and endocannabinoidome. In: Cannabis and cannabinoid-based medicines in cancer care; 2022; p. 1–40. [Google Scholar]
- 9. Nogueiras R, Rohner-Jeanrenaud F, Woods SC, Tschöp MH. The endocannabinoid system and the control of glucose homeostasis. Journal of neuroendocrinology; 2008. [DOI] [PubMed] [Google Scholar]
- 10. Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91(8):3171–80. [DOI] [PubMed] [Google Scholar]
- 11. Gostelow R, Hazuchova K. Pathophysiology of prediabetes, diabetes, and diabetic remission in cats. Vet Clin North Am Small Anim Pract. 2023;53(3):511–29. [DOI] [PubMed] [Google Scholar]
- 12. Marshall RD, Rand JS, Morton JM. Treatment of newly diagnosed diabetic cats with glargine insulin improves glycaemic control and results in higher probability of remission than protamine zinc and lente insulins. J Feline Med Surg. 2009;11(8):683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zini E, Hafner M, Osto M, Franchini M, Ackermann M, Lutz TA, et al. Predictors of clinical remission in cats with diabetes mellitus. J Vet Intern Med. 2010;24(6):1314–21. [DOI] [PubMed] [Google Scholar]
- 14. Lowe H, Toyang N, Steele B, Bryant J, Ngwa W. The endocannabinoid system: a potential target for the treatment of various diseases. Int J Mol Sci. 2021;22(17):9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Briyne N, Holmes D, Sandler I, Stiles E, Szymanski D, Moody S, et al. Cannabis, cannabidiol oils and tetrahydrocannabinol-what do veterinarians need to know? Animals. 2021;11(3):892–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Neill DG, Gostelow R, Orme C, Church DB, Niessen SJM, Verheyen K, et al. Epidemiology of diabetes mellitus among 193,435 cats attending primary-care veterinary practices in england. J Vet Intern Med. 2016;30(4):964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levendal RA, Schumann D, Donath M, Frost CL. Cannabis exposure associated with weight reduction and β-cell protection in an obese rat model. Phytomedicine. 2012;19(7):575–82. [DOI] [PubMed] [Google Scholar]
- 18. Zorzenon MRT, Santiago AN, Mori MA, Piovan S, Jansen CA, Perina Padilha ME, et al. Cannabidiol improves metabolic dysfunction in middle-aged diabetic rats submitted to a chronic cerebral hypoperfusion. Chem Biol Interact. 2019;312:108819. [DOI] [PubMed] [Google Scholar]
- 19. Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39(10):1777–86. [DOI] [PubMed] [Google Scholar]
- 20. Mattes RG, Espinosa ML, Oh SS, Anatrella EM, Urteaga EM. Cannabidiol (CBD) use in type 2 diabetes: a case report. Diabetes Spectr. 2021;34(2):198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunawan IWG, Bawa Putra AA, Widihati IAG. The response to oxidative stress α-humulene compounds Hibiscus manihot L leaf on the activity of 8-hydroxy-2-deoksiquanosin levels pancreatic β-cells in diabetic rats. Biomed Pharmacol J. 2016;9(2):433–41. [Google Scholar]
- 22. Basha RH, Sankaranarayanan C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014;116(8):1469–79. [DOI] [PubMed] [Google Scholar]
- 23. Wiciński M, Fajkiel-Madajczyk A, Kurant Z, Gryczka K, Kurant D, Szambelan M, et al. The use of cannabidiol in metabolic syndrome—an opportunity to improve the patient’s health or much ado about nothing? J Clin Med. 2023;12(14):4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirkham TC. Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry. 2009;21(2):163–71. [DOI] [PubMed] [Google Scholar]
- 25. Coltherd JC, Bednall R, Bakke AM, Ellerby Z, Newman C, Watson P, et al. Healthy cats tolerate long-term daily feeding of Cannabidiol. Front Vet Sci. 2023;10:1324622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stohs S, Ray S. Is cannabidiol hepatotoxic or hepatoprotective: a review. Toxicol Res Appl. 2020;4:239784732092294. [Google Scholar]
- 27. Rand JS, Kinnaird E, Baglioni A, Blackshaw J, Priest J. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med. 2002;16(2):123–32. [DOI] [PubMed] [Google Scholar]
- 28. Comelli F, Bettoni I, Colleoni M, Giagnoni G, Costa B. Beneficial effects of a Cannabis sativa extract treatment on diabetes-induced neuropathy and oxidative stress. Phytother Res. 2009;23(12):1678–84. [DOI] [PubMed] [Google Scholar]
- 29. Arora A, Taliyan R, Sharma PL. Ameliorative potential of Cannabis sativa extract on diabetes induced neurophatic pain in rats. 2010. Available from: www.ijpsr.com
- 30. Hashiesh HM, Meeran MFN, Sharma C, Sadek B, KaabiOjha JSK, Ojha SK. Therapeutic potential of β-caryophyllene: a dietary cannabinoid in diabetes and associated complications. Nutrients. 2020;12(10):2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holm LJ, Mønsted MØ, Haupt-Jorgensen M, Buschard K. PPARs and the development of type 1 diabetes. PPAR Research. 2020;2020:6198628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the manuscript. Further inquiries can be directed to the corresponding author.