Abstract
Aim: Memory improving and anti-inflammatory properties of cannabidiol (CBD) were investigated in an experimental model of lipopolysaccharide (LPS)-induced inflammation.
Materials and methods: Male Wistar rats were randomly divided into 4 groups: control, LPS control, LPS + CBD 5 mg/kg bw, and LPS + CBD 10 mg/kg bw. Animals were treated with CBD 14 days before LPS administration and throughout the experiment. Step-through passive avoidance task, Y-maze, and novel object recognition test (NORT) were used to assess the memory functions. The following parameters were recorded: latency time, spontaneous alternations percentage (SA%) and recognition index (RI). IL-10, IL-6, TNF-α, and IL-1β serum levels were measured to evaluate the immunomodulatory properties of CBD.
Results: LPS led to significant decrease of the recorded parameters in all memory tasks. This demonstrated the memory-impairing effect of LPS-induced inflammation. In the Y-maze and NORT tests, both doses of CBD increased SA% and RI, respectively. Significant difference was found in comparison with the LPS controls. Rats from the CBD treated groups showed increased latency in the step-through passive avoidance task. In the short-term memory test, both CBD doses significantly increased this parameter when compared with both control groups (p<0.05 and p<0.001, respectively), whereas in the long-term memory test, statistical significance was reached only in comparison with the LPS controls (p<0.01). CBD treatment failed to reduce TNF-α and IL-6 serum levels. The lower studied dose significantly decreased IL-10 and IL-1β concentrations compared to LPS controls (p<0.01 and p<0.05, respectively).
Conclusions: CBD improved spatial working and recognition memory in rats with LPS-induced inflammation. Suppression of IL-1β production could be attributed to the observed effect.
Keywords
cannabidiol, cytokines, immunomodulatory, memory neuroinflammation
Introduction
Cannabis sativa is a plant that has been cultivated by humans and utilized in medicine since ancient times.[1] Cannabidiol (CBD) is one of the most important Cannabis-derived molecules, accounting for approximately 40% of the plant extract and lacking the properties of an addictive drug.[2,3]
CBD was isolated from the plant for the first time in the late 1930s, but its chemical structure was not fully understood until 1963. Given that CBD exerts multiple mechanisms of action, it has attracted the attention of researchers for its therapeutic potential in a wide range of neuropsychiatric disorders, including epilepsy, Alzheimer’s disease (AD), Parkinson’s disease (PD), mood disorders, anxiety disorders, and schizophrenia.[1] Several preclinical studies have shown that chronic or acute CBD application improved working memory, object and social recognition, spatial learning and memory in different neurodegenerative models including the model of AD.[4]
The neuroprotective effect of CBD is associated with its antioxidant and anti-inflammatory activities and the modulation of a wide range of brain biological targets involved in the development and progression of neurodegenerative diseases. CBD exerts its anti-inflammatory action by modulating the release of proinflammatory cytokines and interacting with transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ) and nuclear factor κB (NF-κB).[5]
Bacterial lipopolysaccharide (LPS) is a molecule found in the outer membrane of Gram-negative bacteria. Its administration (intraperitoneal or intracerebroventricular) is a commonly used model of neuroinflammation associated with neurodegenerative disorders.[6] LPS stimulates the production of tumor necrosis factor-α (TNF-α) and other pro-inflammatory molecules through binding to toll-like receptor 4 (TLR 4).[7] These molecules play a role in both inflammation and memory functions. TNF-α is one of the most potent pro-inflammatory cytokines. Systemic inflammatory diseases such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease are known to increase the risk of AD. Treatment with TNF-α blocking agents has been associated with reduced risk.[8] Interleukin-6 (IL-6) is another cytokine associated with the worsening of memory functions. The main source of IL-6 in the CNS are astrocytes, but it is also produced by microglia and neurons. Its synthesis can be induced by a large number of factors causing CNS injury or increased neuronal activity. High levels of IL-6 are observed in disorders associated with disrupted cognition and behavior.[9] Overexpression of IL-6 in the hippocampus of animals with experimental AD results in memory impairment.[10] IL-6 deficiency improves long-term reference memory.[11] Clinical studies demonstrated that in patients with AD, the serum levels of this pro-inflammatory molecule are elevated and may correlate with those in cerebrospinal fluid.[12] Experimental data showed that cannabidiol interfere with pro-inflammatory cytokine production. Low levels of TNF-α and IL-6 were measured in the homogenized brains of the CBD treated experimental animals.[13] In experimental study using a model of mice inoculated with amyloid beta (Aβ) in the right dorsal hippocampus, CBD suppressed the expression of interleukin-1β (IL-1β), a cytokine associated with neurodegenerative processes.[14] Elevated levels of this inflammatory cytokine are also implicated in the pathogenesis of memory disturbances observed during inflammation as well as stress- and aging-induced memory impairment.[15]
IL-10 is an immunoregulatory molecule which plays a crucial role in the suppression of inflammatory response and protection from unrestricted inflammation. In the brain, LPS stimulates IL-10 production from activated microglia and this is important for prevention of neuronal loss and inhibition of inflammation. The protective role of IL-10 is associated with its capability of inhibiting expression of pro-inflammatory molecules (IL-1beta, TNF-α, iNOS) and production of reactive oxygen species.[16] Thus, IL-10 is critical for the termination of inflammation not only in the peripheral tissues but also in the CNS.[17] This molecule reduces the increased permeability of the blood-brain barrier (BBB) in settings of inflammation.[18] As a result, the access of peripherally produced pro-inflammatory cytokines to the brain may be reduced. Therefore, stimulation of IL-10 production might have beneficial effect on memory functions.
Aim
The aim of the present study was to investigate the effect of CBD on learning and memory processes and markers of systemic inflammation in LPS-challenged rats.
Materials and methods
Ethical statement
The experiments have been approved by the Animal Health and Welfare Directorate of the Bulgarian Food Safety Agency (No. 257/2019) and by the Ethics Committee at the Medical University of Plovdiv (No. 8/5.11.2020).
Experimental design
Male Wistar rats (200±20 g bw) were used in the experiments. The animals were housed under standard laboratory conditions – 12-h light/dark cycle, food and water ad libitum. Rats were randomly divided into four groups (n=8) as follows: control, LPS control, LPS + CBD 5 mg/kg bw per os, and LPS + CBD 10 mg/kg bw per os. The animals were treated with CBD 14 days before the administration of LPS and throughout the experiment. Control groups received per os olive oil in a dose of 0.1 ml/kg bw. Inflammation was induced by intraperitoneal injection of LPS from E. coli O55:B5 (250 µg/kg/day) for 5 consecutive days starting from day 15. Beginning at day 20 of the experiment, participants completed behavioral tasks to measure their learning and memory. At the end of the study, blood samples for immunological assays were collected 4 hours after a booster dose of LPS (250 µg/kg) .
Behavioral tests
Y-maze
Y-maze and spontaneous alternations are commonly used to evaluate spatial working memory in rodents. It is made of black acrylic glass and consists of three arms interconnected at 120°. The arms have identical dimensions (50×10×30 cm) and were randomly labeled А, В, and С. The spontaneous alternation test was performed in two consecutive days: a training session (on day 1) and a memory retention test (on day 2). The animal was placed in the middle of the maze and allowed to investigate the arms for five minutes. An alternation is any consecutive entry into the three different arms of the maze. For example ABC, BCA, CAB, CBA, etc. Spontaneous alternations % (SA%) were determined by the following formula:
SA%=Number of alterationsTotal number of entries−2×100SA%=Number of alterationsTotal number of entries−2×100
Novel object recognition test (NORT)
NORT is widely used to assess exploratory behavior and recognition memory in rodents. The experiment was conducted in two consecutive days in an open black acrylic glass box (60×60×40 cm). The study protocol included 3 phases: habituation, exploration (investigation), and testing. All rats were left to settle in the test box for 5 minutes without the presence of objects. During the exploration phase, the rats were allowed to investigate two identical objects for 5 minutes. The testing phase was performed on the second day. One of the objects used in the exploration phase was replaced with a novel one and the rats were allowed to investigate them for 5 minutes. We detected the time during which the animal explored the novel and the familiar object. The following formula was used for calculation of the recognition index (RI):
RI=NN+F×100��=��+�×100
where N is the time for exploring the novel object, and F is the time for exploring the familiar object.
Step-through “passive” avoidance test
The step-through “passive” avoidance apparatus (UgoBasile, Italy) represents a box with two-compartments: one black and one white, brightly illuminated, which are connected by a sliding automatic door. Each animal was placed into the bright compartment and had access to the dark one following a door delay of 7 seconds. When the animal entered the black compartment, the door closed and the animal was subjected to a short-lasting aversive stimulus (an electrical foot shock for 9 seconds with intensity of 0.4 mA). The time (in seconds) spent in the bright compartment was recorded. The learning session consisted of two consecutive days. Short- and long-term memory retention tests were performed on days 3 and 9, respectively. Three trials were performed each day with a 30-min pause between them. On the memory retention days of the experiment, no shock was delivered to the animal. The maximum stay in the illuminated chamber was 178 seconds (cut-off time).
Samples collection
Pyrogen and endotoxin-free collecting tubes were used. The blood samples were centrifuged for 10 minutes following careful clotting and serum removal. The collected serum was aliquoted and frozen at −70°C.
Immunological assay
The IL-10, IL-6, TNF-α, and IL-1β levels were detected in rats’ serum. Solid-phase ELISA was used following the manufacturer’s directions (Diaclone). Absorbance reading was performed at 450 nm and a standard curve was plotted to calculate the serum cytokine concentration.
Statistical analysis
Statistical analysis was performed using IMB SPSS 19.0. One way ANOVA was used to compare differences between groups followed by Tukey’s post hoc test. Data were presented as the mean ± standard error of mean (x̄±SEM). p<0.05 was considered statistically significant.
Results
Effects of CBD treatment on LPS-induced memory impairment
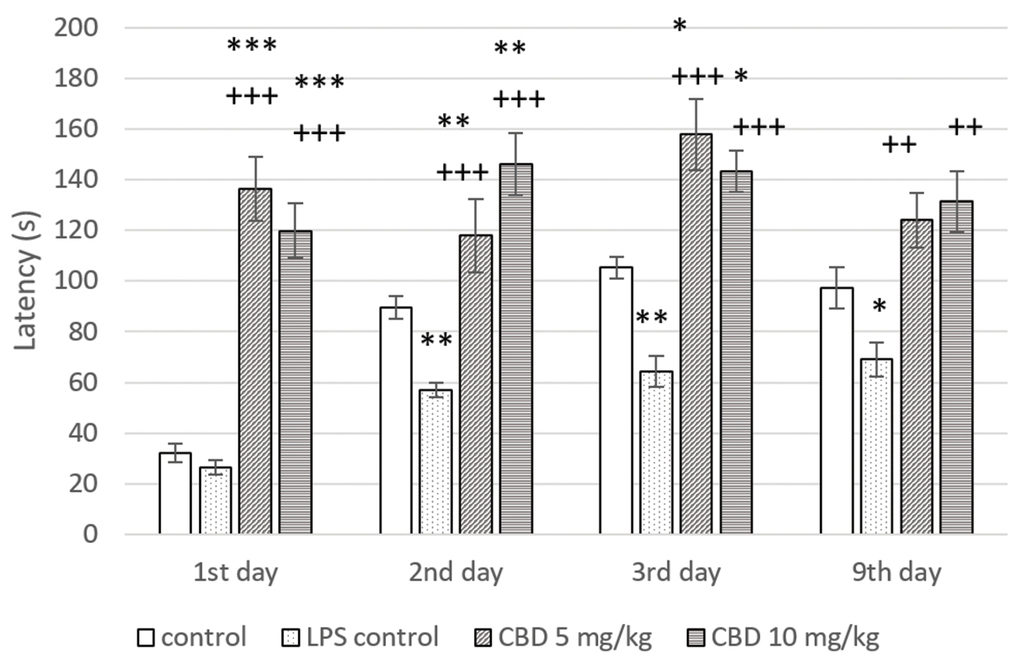
Step-through passive avoidance test
А non-significant difference was found in latency between control groups in the first day of the training session. In animals from LPS challenged control, a significant decrease in latency was registered on the second day of the training session (p<0.01) and in the short- (p<0.01) and long-term (p<0.05) memory tests compared to controls. These results demonstrated the memory-impairing effect of LPS administration. Rats in both CBD treated groups showed a significant prolongation of latency during the two-day learning session in comparison to control and LPS control (p<0.001 on day 1; p<0.01 and p<0.001, respectively, on day 2). In the short-term memory study, both tested doses of CBD significantly increased latency relative to controls without and with LPS-induced memory impairment (p<0.05 and p<0.001, respectively). When animals were tested for long-term memory on day 9, CBD administration led to a significant increase in this parameter only in comparison with LPS challenged control group (p<0.01) (Fig. 1).
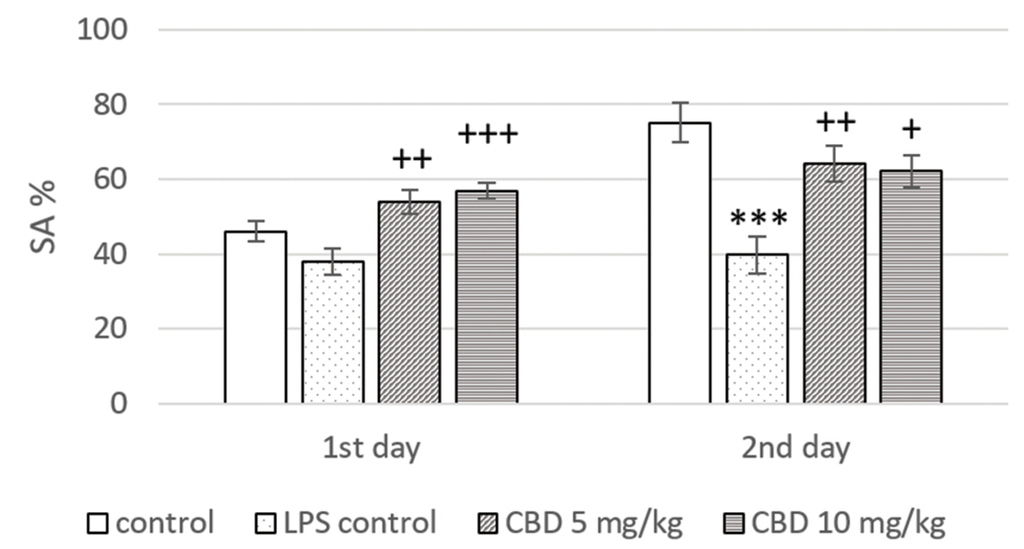
Y-maze
In the memory retention test (day 2), rats from the LPS treated control had a significant reduction in SA% when compared to the control group (p<0.001). Animals treated with CBD at a dose of 5 mg/kg and 10 mg/kg showed a significant increase in SA% in the training (p<0.01 and p<0.001, respectively) and the memory (p<0.01 and p<0.05, respectively) session only in comparison to LPS control (Fig. 2).
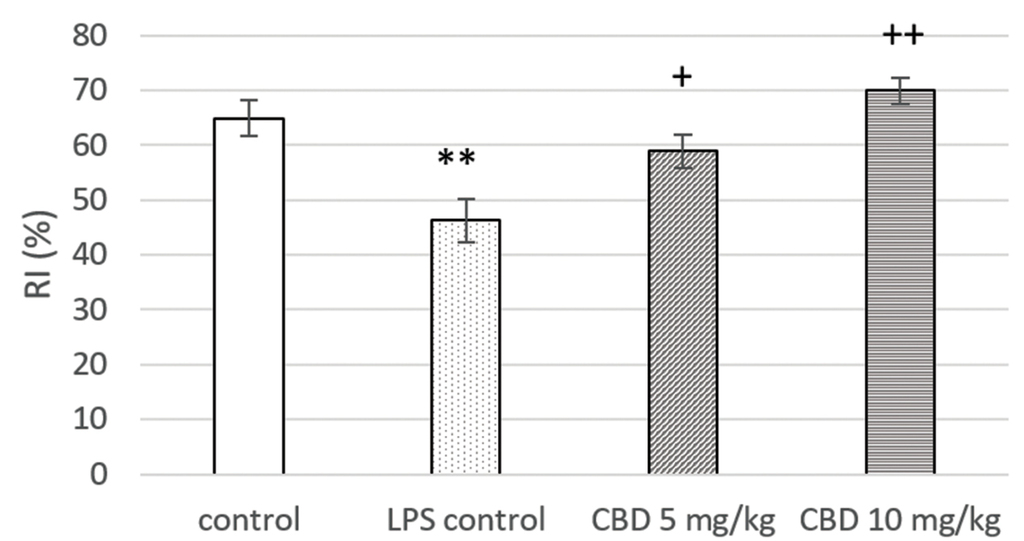
NORT
LPS administration led to a significant decrease in RI when compared to controls (p<0.01). CBD treatment increased this parameter in comparison with the LPS controls (p<0.05 and p<0.01, respectively for 5 mg/kg and 10 mg/kg) (Fig. 3).
Effects of CBD treatment on LPS-induced changes in serum levels of pro- and anti-inflammatory cytokines
TNF-α
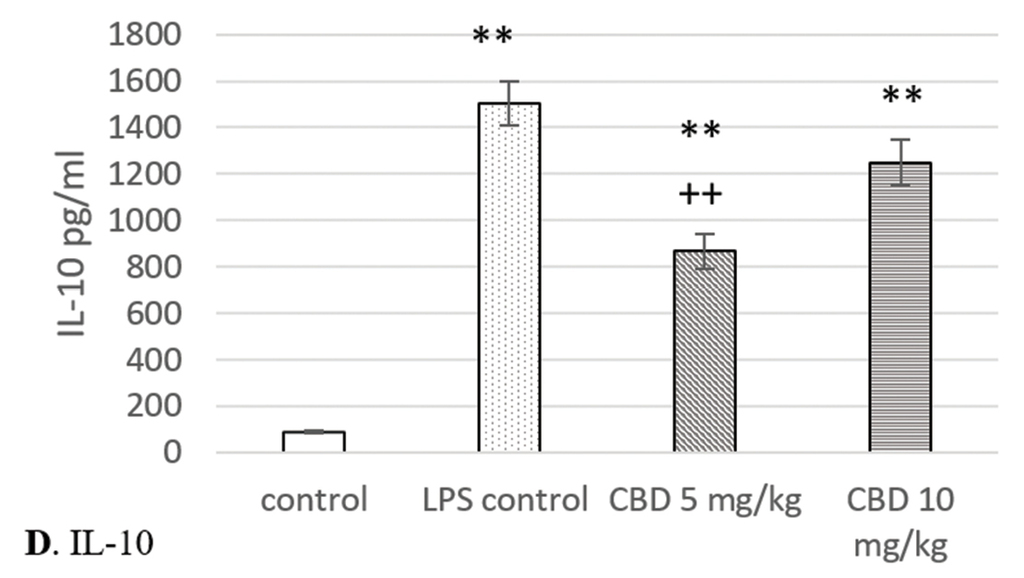
A significant increase in the serum TNF-α levels was observed in rats from the LPS challenged control group (p<0.001). Cannabidiol administration slightly and insignificantly reduced TNF-α concentrations. In animals from both cannabidiol treated groups, serum levels of this inflammatory molecule were statistically significantly elevated in comparison to controls (p<0.01) (Fig. 4A).
Effects of cannabidiol on serum levels of TNF-α, IL-6, IL-10, and IL-1β in rats with LPS-induced model of systemic and neuro-inflammation. A. TNF-α; B. IL-6; C. IL-1β; D. IL-10. *p<0.05 compared to control; **p<0.01 compared to control; ***p<0.001 compared to control; +p<0.05 compared to LPS control; ++p<0.01 compared to LPS control.
IL-6
Cannabidiol treatment failed to reduce the IL-6 serum levels. They were significantly higher in rats from LPS control and cannabidiol treated groups when compared with control (p<0.01) (Fig. 4B).
IL-1β
LPS challenge significantly increased the IL-1β serum concentration in comparison to controls. The highest levels were reached in LPS controls (p<0.01), whereas the elevation was lower in rats treated with 5 mg/kg and 10 mg/kg CBD (p<0.05 and p<0.01, respectively). IL-1β was the only pro-inflammatory molecule whose serum levels were significantly reduced by cannabidiol. Its administration resulted in a decrease of IL-1β concentrations when compared to LPS controls. This was statistically significant at a dose of 5 mg/kg (p<0.05) (Fig. 4C).
IL-10
In rats from both cannabidiol treated groups and LPS control, a significant increase in IL-10 serum levels was registered in comparison to control (p<0.01 and p<0.001, respectively). Cannabidiol administration led to reduction in concentrations of this cytokine. Its levels were lower in animals from cannabidiol treated groups when compared with the LPS challenged control, but significance was reached only at the lower dose (p<0.01) (Fig. 4D).
Discussion
The findings of the current study suggest that cannabidiol improves memory impairment in rats with systemic and neuroinflammation caused by bacterial LPS. To our knowledge, this is the first study that has demonstrated the ameliorating effect of cannabidiol on both spatial working and recognition memory in an experimental model of neuroinflammation, neurodegeneration, and memory decline induced by peripheral injection of LPS. Our results are consistent with some previous data that cannabidiol prevents memory impairment in other animal models of neurodegenerative disorders.[19] The memory improving effect of cannabidiol can be related to its neuroprotective properties. It has been demonstrated that cannabidiol indirectly interacts with cannabinoid receptors by preventing degradation of endocannabinoids. This may result in reduced neuronal excitotoxicity and enhanced neurite outgrowth.[20] In addition, cannabidiol inhibits apoptosis and exerts antioxidant effect.[3] The present study proposes to examine the effect of cannabidiol on memory functions in the setting of neuroinflammation and to seek to determine whether the obtained results could be attributed to the immunomodulatory effect of this agent.
The design of the current research included three different behavior-based tests used for the assessment of different aspects of memory functions. In all tasks, rats from the LPS challenged control group showed worsening of memory functions. Peripherally administered LPS reaches the brain by different routes. In the central nervous system, it activates microglia and astrocytes which leads to inflammatory response with excessive production of pro-inflammatory molecules, oxidative stress, mitochondrial dysfunction, increased cyclooxygenase-2 expression, etc. Finally, this causes neuronal loss and memory decline.[6] Our results showed that cannabidiol treatment had beneficial effect and prevented the memory impairing effect of LPS.
The step-through passive avoidance paradigm is based on the aversive associative learning. When the animal enters the dark compartment during the learning session, a foot shock is received. Prolonged time spent in the illuminated chamber is considered a criterion for memory retention. Increased latency was observed in cannabidiol treated rats during the short- and long-term memory testing (on days 3 and 9, respectively) in comparison with LPS-challenged control. The behavioral response in this task involves the hippocampus.[21] Therefore, we might speculate that this brain structure plays a role in the observed memory improving effect of cannabidiol. NORT is used for the assessment of recognition memory which requires discrimination, identification, and comparison of the characteristics of current and previously experienced events. The hippocampus, the prefrontal cortex and different parahippocampal regions are the neurobiological base of this type of memory.[22] Cannabidiol treatment restored recognition memory deficit in LPS challenged rats. These results are in accordance with the findings of recent studies demonstrating the effect of cannabidiol on recognition memory in a transgenic model of AD.[23] Based on the results from NORT in the present study, we can assume that not only the hippocampus, but also other brain structures are implicated in the beneficial effect of this agent on memory functions. This was confirmed in the Y-maze test where cannabidiol increased %SA. High values of this parameter showed good working memory and required normal functioning of the hippocampus and frontal cortex.[24]
Pro-inflammatory cytokines have an important role in the development of neuroinflammation and, hence, impairment of memory functions. By contrast, molecules that suppress inflammatory processes such as IL-10 may improve cognition. We further investigated the immunomodulatory effect of CBD, at doses that improve memory functions, by measuring the serum TNF-α, IL-6, IL-10, and IL-1β levels.
Besides its role in inflammation and immune response, TNF-α is important for the functioning of the CNS in both physiological and pathological conditions. Basal levels of TNF-α are involved in the regulation of essential physiological processes such as synaptic transmission and plasticity, whereas overexpression potentiates excitotoxicity and stimulates neuroinflammation.[25] Peripherally produced TNF-α may cross even the intact blood-brain barrier. On the other hand, peripheral TNF-α-producing immune cells are able to migrate across this barrier into the brain and cerebrospinal fluid.[26] Therefore, inflammatory response induced by intraperitoneal injection of LPS may result in neuro-inflammation driven by TNF-α. This is followed by a decline in memory function. The current study demonstrated the memory impairing effect of systemic LPS administration. This might be related to elevated TNF-α concentrations. Trivedi et al.[27] found that CBD decreases the serum levels of TNF-α in rats with systemic inflammatory response, but their doses were much higher than those used in the current study. Our results showed that cannabidiol did not significantly decrease serum levels of this inflammatory molecule. This is probably due to the lower doses used in the present study. These doses have been chosen based on previous studies[19,28] about the memory improving effect of CBD in other experimental models of cognitive impairment. Thus, the observed beneficial effect of cannabidiol on memory functions cannot be attributed to its effect on TNF-α production.
Peripheral inflammation could also be a source for IL-6 in the CNS as a saturable transport mechanism for this cytokine is found in the blood-brain barrier.[29] Moreover, elevated brain IL-6 levels may affect its serum concentration.[9] Systemic administration of LPS causes an increase of serum IL-6 levels as well as its concentration in brain structures as cerebral cortex, cerebellum, and hippocampus.[30] Our results are in consistence with previous findings about the stimulatory effect of intraperitoneal LPS administration on serum IL-6 levels. LPS increases synthesis of this pro-inflammatory molecule by binding to TLR 4. Cannabidiol suppresses IL-6 production from peripheral blood monocytes activated by bacterial LPS[31] and in in-vivo models of systemic inflammation[27]. Our findings did not confirm this observation, probably due to the lower doses we used in the current research. There was no decrease in the IL-6 serum concentrations in rats treated with cannabidiol in comparison with the non-treated group with LPS-induced inflammation.
IL-1β is not only an inflammatory molecule, but it is also involved in the modulation of learning and memory processes. While physiological IL-1β levels are required for long-term potentiation in the hippocampus, overexpression of this cytokine in the presence of inflammation is associated with memory impairment. Experimental studies showed that exogenous administration or transgenic overexpression of IL-1β has an adverse effect on hippocampal-dependent memory, whereas in memory tasks that are independent of hippocampal function, this is not observed.[15] Our results showed that systemic administration of LPS resulted in significantly higher IL-1β serum levels in LPS challenged control. Since this cytokine could penetrate the blood-brain barrier[29], we can suggest that the observed memory impairment in hippocampal-dependent tasks was mediated by it. Li et al.[32] demonstrated that in mice, peripheral administration of LPS leads to elevated IL-1β levels in the hippocampus. IL-1β knock-down in the dentate gyrus of the hippocampus significantly reduces oxidative stress in this brain structure and attenuates memory deficit following LPS injection. The immune modulating properties of cannabidiol are at least partially related to its ability to suppress IL-1β production. It was shown that cannabidiol inhibits the production of this cytokine from monocytes after activation through almost all toll-like receptors.[31] The results of the current research demonstrated that cannabidiol treatment decreases serum IL-1β levels in LPS challenged rats. This could be attributed to the memory improving effect of this compound in the settings of inflammation.
IL-10 synthesis is stimulated by different factors, including bacterial LPS.[33] MicroRNA-98 inhibits IL-10 production and its down-regulation following LPS exposure is important for adequate IL-10 synthesis.[34] The results from the current study showed that systemic LPS administration significantly increased IL-10 serum levels. The available data about the effect of cannabidiol on IL-10 production are controversial. In an experimental model of bronchial asthma, cannabidiol treatment decreases serum levels of Th2 cytokines, including IL-10.[35] Other studies demonstrated that cannabidiol increases colonic IL-10 production in a murine model of experimental colitis[36] and serum level of this cytokine in mice with LPS-induced inflammation.[37] IL-10 reduces the increased permeability of the BBB in settings of inflammation.[18] Therefore, the access of peripherally produced pro-inflammatory cytokines to the brain may be reduced. In our study cannabidiol treated rats had higher IL-10 serum levels when compared with non-treated control but lower in comparison to the LPS challenged control group. It is difficult to determine whether the increase in serum IL-10 levels is due to cannabidiol treatment or whether it is LPS-induced. The decrease in IL-10 concentrations upon CBD treatment might be related to the lower intensity of inflammatory response due to the inhibition of IL-1β production.
Conclusions
CBD improved spatial working and recognition memory in rats with LPS-induced inflammation. This effect is probably hippocampal-dependent, although other brain structures such as the medial frontal cortex may also be involved. The immunomodulatory effect of CBD treatment in the settings of LPS-induced inflammation was demonstrated by suppressing the IL-1β production and that could attribute to the observed memory improving effect.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgements
This research was financially supported by a grant DPDP-No. 09/2020 from the Medical University of Plovdiv.
References
- 1. Elsaid S, Kloiber S, Le Foll B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog Mol Biol Transl Sci 2019; 167:25–75.
- 2. Austrich-Olivares A, Garcia-Gutierrez MS, Illescas L, et al. Cannabidiol CB1 receptor involvement in the actions of CBD on anxiety and coping behaviors in mice. Pharmaceuticals (Basel) 2022; 15(4):473.
- 3. Da Silva VK, De Freitas BS, Garcia RCL, et al. Antiapoptotic effects of cannabidiol in an experimental model of cognitive decline induced by brain iron overload. Transl Psychiatry 2018; 8:176.
- 4. Kozela E, Krawczyk M, Kos T, et al. Cannabidiol improves cognitive impairment and reverse cortical transcriptional changes induced by ketamine, in schizophrenia-like model in rats. Mol Neurobiol 2020; 57:1733–47.
- 5. Silvestro S, Schepici G, Bramanti P, et al. Molecular targets of cannabidiol in experimental models of neurological disease. Molecules 2020; 25(21):5186.
- 6. Zakaria R, Wan Yaacob WM, Othman Z, et al. Lipopolysaccharide-induced memory impairment in rats: a model of Alzheimer’s disease. Physiol Res 2017; 66(4):553–65.
- 7. Mazgaeen L, Gurung P. Recent advances in lipopolysaccharide recognition systems. Int J Mol Sci 2020; 21(2):379.
- 8. Torres-Acosta N, O’Keefe JH, O’Keefe EL, et al. Therapeutic potential of TNF-α inhibition for Alzheimer’s disease prevention. J Alzheimers Dis 2020; 78(2):619–626.
- 9. Gruol DL. IL-6 regulation of synaptic function in the CNS. Neuropharmacology 2015; 96(Pt A):42–54.
- 10. Lyra E Silva NM, Gonçalves RA, Pascoal TA, et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl Psychiatry 2021; 11(1):251.
- 11. Bialuk I, Winnicka MM. Facilitatory effect of IL-6 deficiency on long-term spatial memory in young adult mice. Behav Genet 2018; 48(3):236–46.
- 12. Park JC, Han SH, Mook-Jung I. Peripheral inflammatory biomarkers in Alzheimer’s disease: a brief review. BMB Rep 2020; 53(1):10–9.
- 13. Martin-Moreno AM, Reigada D, Ramirez BG, et al. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol 2011; 79(6):964–73.
- 14. Esposito G, Scuderi C, Savani C, et al. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1 beta and iNOS expression. Br J Pharmacol 2007; 151(8):1272–9.
- 15. Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25(2):181–213.
- 16. Park KW, Lee HG, Jin BK, et al. Interleukin-10 endogenously expressed in microglia prevents lipopolysaccharide-induced neurodegeneration in the rat cerebral cortex in vivo. Exp Mol Med 2007; 39(6):812–9.
- 17. Burmeister AR, Marriott I. The interleukin-10 family of cytokines and their role in the CNS. Front Cell Neurosci 2018; 12:458.
- 18. Lin R, Chen F, Wen S, et al. Interleukin-10 attenuates impairment of the blood-brain barrier in a severe acute pancreatitis rat model. J Inflamm (Lond) 2018; 15:4.
- 19. Fagherazzi EV, Garcia VA, Maurmann N, et al. Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology (Berl) 2012; 219(4):1133–40.
- 20. Li H, Liu Y, Tian D, et al. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur J Med Chem 2020; 192:112163.
- 21. Baarendse PJ, Van Grootheest G, Jansen RF, et al. Differential involvement of the dorsal hippocampus in passive avoidance in C57bl/6J and DBA/2J mice. Hippocampus 2008; 18(1):11–9.
- 22. Pitsikas N. The role of nitric oxide in the object recognition memory. Behav Brain Res 2015; 285:200–7.
- 23. Coles M, Watt G, Kreilaus F, et al. Medium-dose chronic cannabidiol treatment reverses object recognition memory deficits of APPSwe/PS1ΔE9 transgenic female mice. Front Pharmacol 2020; 11:587604.
- 24. Kraeuter AK, Guest PC, Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 2019; 1916:105–11.
- 25. Olmos G, Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014; 2014:861231.
- 26. Decourt B, Lahiri DK, Sabbagh MN. Targeting tumor necrosis factor alpha for Alzheimer’s disease. Curr Alzheimer Res 2017; 14(4):412–25.
- 27. Trivedi MK, Mondal S, Gangwar M, et al. Anti-inflammatory potential of cannabidiol (CBD) on combination of caecal slurry, LPS, and E. coli-induced systemic inflammatory response syndrome (SIRS) in Sprague Dawley rats. Inflammopharmacology 2022; 30(1):225–32.
- 28. Osborne AL, Solowij N, Babic I, et al. Improved social interaction, recognition and working memory with cannabidiol treatment in a prenatal infection (poly I:C) rat model. Neuropsychopharmacology 2017; 42(7):1447–57.
- 29. Threlkeld SW, Lynch JL, Lynch KM, et al. Ovine proinflammatory cytokines cross the murine blood-brain barrier by a common saturable transport mechanism. Neuroimmunomodulation 2010; 17(6):405–10.
- 30. Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation 2009; 6:9.
- 31. Sermet S, Li J, Bach A, et al. Cannabidiol selectively modulates interleukin (IL)-1β and IL-6 production in toll-like receptor activated human peripheral blood monocytes. Toxicology 2021; 464:153016.
- 32. Li M, Li C, Yu H, et al. Lentivirus-mediated interleukin-1β (IL-1β) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J Neuroinflammation 2017; 14(1):190.
- 33. Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy – review of a new approach. Pharmacol Rev 2003; 55(2):241–69.
- 34. Liu Y, Chen Q, Song Y, et al. MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett 2011; 585(12):1963–8.
- 35. Vuolo F, Petronilho F, Sonai B, et al. Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediators Inflamm 2015;2015:538670.
- 36. Borrelli F, Aviello G, Romano B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl) 2009; 87(11):1111–21.
- 37. Verrico CD, Wesson S, Konduri V, et al. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine