Abstract
1. Introduction
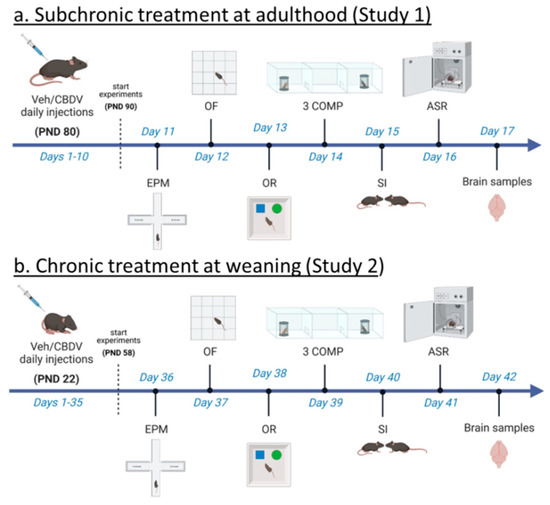
2. Materials and Methods
2.1. Animals
2.2. Experimental Procedures
2.2.1. CBDV Administration at Adulthood (Study 1)
2.2.2. CBDV Administration at Weaning (Study 2)
2.2.3. Behavioral Assessment (Studies 1 and 2)
In all trials, the total distance traveled as well as the time spent in each contact area (20 × 22 cm) containing the stimulus cages was computed using the Ethovision tracking system (version 11, Noldus, The Netherlands). A percentage score was also computed for the last two trials as follows:
2.2.4. Brain Assessment of Inflammatory and Plasticity Markers through RT-qPCR (Studies 1 and 2)
2.3. Statistical Analysis
3. Results
3.1. Study 1: Effects of Subchronic Administration of CBDV at Adulthood
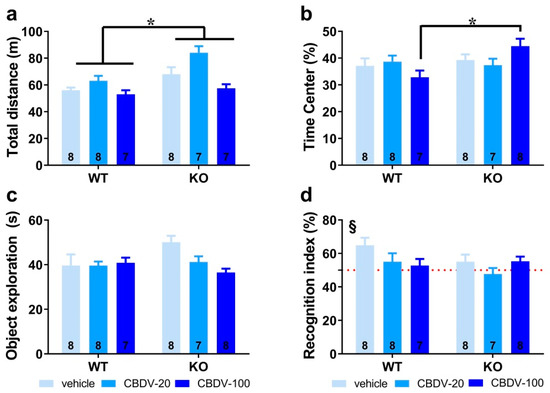
3.1.1. Behavioral Profiling
-
Elevated plus maze
-
Object recognition test
-
Three-compartment test for sociability and social novelty
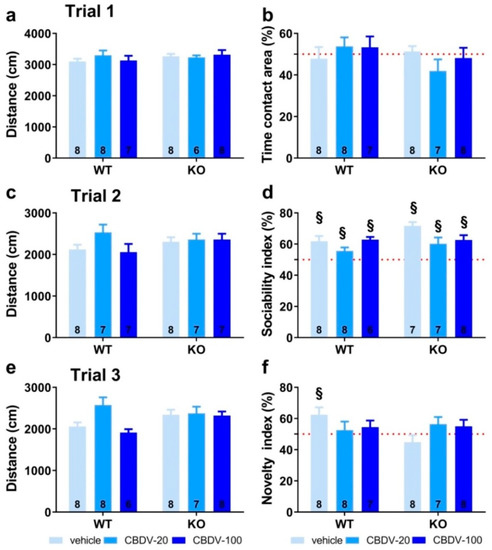
-
Direct social interaction with an adult female
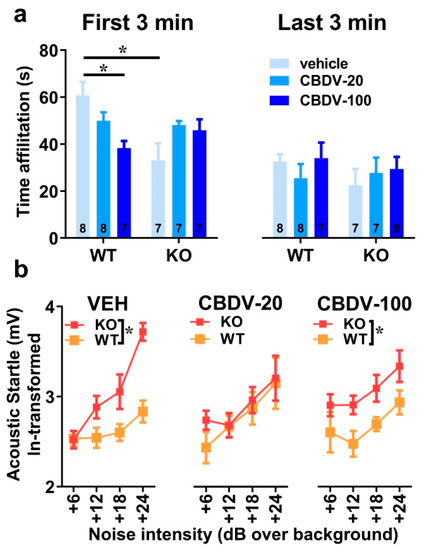
-
Acoustic startle response
3.1.2. Brain Analyses
-
Hippocampus

-
Prefrontal cortex
3.2. Study 2: Effects of Chronic Administration of CBDV at Weaning
3.2.1. Behavioral Profiling
-
Elevated plus maze
-
Object recognition test
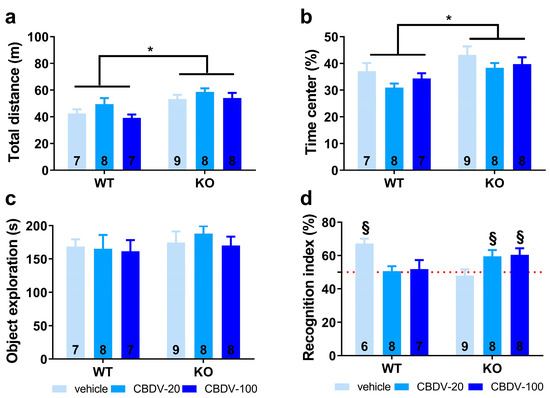
-
Three-compartment test for sociability and social novelty
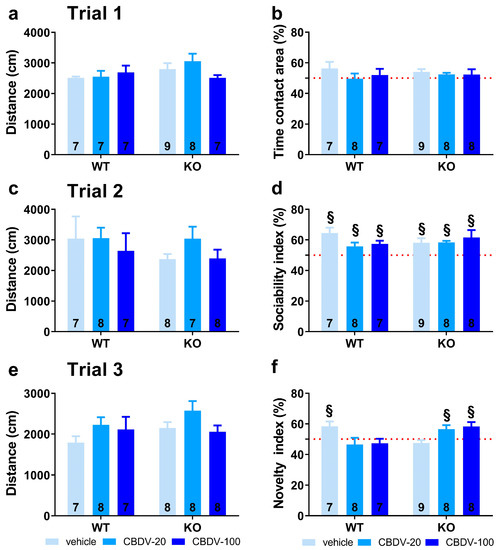
-
Direct social interaction with an adult female
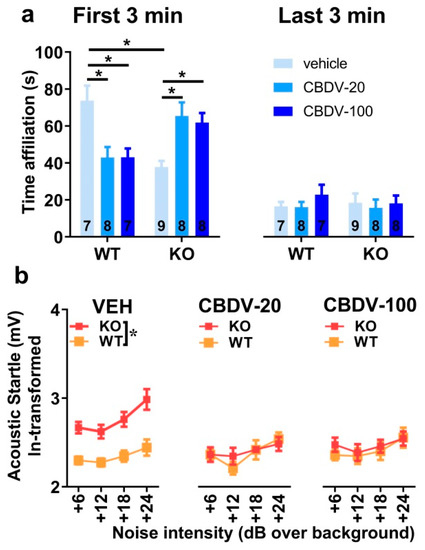
-
Acoustic startle response
3.2.2. Brain Analyses
-
Hippocampus

-
Prefrontal cortex
4. Discussion

5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parolaro, D.; Realini, N.; Vigano, D.; Guidali, C.; Rubino, T. The Endocannabinoid System and Psychiatric Disorders. Exp. Neurol. 2010, 224, 3–14. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar]
- Basavarajappa, B.S.; Nixon, R.A.; Arancio, O. Endocannabinoid System: Emerging Role from Neurodevelopment to Neurodegeneration. Mini Rev. Med. Chem. 2009, 9, 448–462. [Google Scholar] [CrossRef][Green Version]
- Berghuis, P.; Rajnicek, A.M.; Morozov, Y.M.; Ross, R.A.; Mulder, J.; Urban, G.M.; Monory, K.; Marsicano, G.; Matteoli, M.; Canty, A.; et al. Hardwiring the Brain: Endocannabinoids Shape Neuronal Connectivity. Science 2007, 316, 1212–1216. [Google Scholar] [CrossRef][Green Version]
- Monory, K.; Massa, F.; Egertova, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The Endocannabinoid System Controls Key Epileptogenic Circuits in the Hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef][Green Version]
- Vigli, D.; Cosentino, L.; Raggi, C.; Laviola, G.; Woolley-Roberts, M.; De Filippis, B. Chronic Treatment with the Phytocannabinoid Cannabidivarin (Cbdv) Rescues Behavioural Alterations and Brain Atrophy in a Mouse Model of Rett Syndrome. Neuropharmacology 2018, 140, 121–129. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Piscitelli, F.; Brodie, J.S.; Woolley-Roberts, M.; Barbiero, I.; Tramarin, M.; Binelli, G.; Landsberger, N.; Kilstrup-Nielsen, C.; et al. Cannabidivarin Completely Rescues Cognitive Deficits and Delays Neurological and Motor Defects in Male Mecp2 Mutant Mice. J. Psychopharmacol. 2019, 33, 894–907. [Google Scholar] [CrossRef]
- Navarro-Romero, A.; Galera-Lopez, L.; Ortiz-Romero, P.; Llorente-Ovejero, A.; de Los Reyes-Ramirez, L.; de Tena, I.B.; Garcia-Elias, A.; Mas-Stachurska, A.; Reixachs-Sole, M.; Pastor, A.; et al. Cannabinoid Signaling Modulation through Jzl184 Restores Key Phenotypes of a Mouse Model for Williams-Beuren Syndrome. Elife 2022, 11, e72560. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Gomis-Gonzalez, M.; Guegan, T.; Agustin-Pavon, C.; Pastor, A.; Mato, S.; Perez-Samartin, A.; Matute, C.; de la Torre, R.; Dierssen, M.; et al. Targeting the Endocannabinoid System in the Treatment of Fragile X Syndrome. Nat. Med. 2013, 19, 603–607. [Google Scholar] [CrossRef][Green Version]
- Jung, K.M.; Sepers, M.; Henstridge, C.M.; Lassalle, O.; Neuhofer, D.; Martin, H.; Ginger, M.; Frick, A.; DiPatrizio, N.V.; Mackie, K.; et al. Uncoupling of the Endocannabinoid Signalling Complex in a Mouse Model of Fragile X Syndrome. Nat. Commun. 2012, 3, 1080. [Google Scholar] [CrossRef][Green Version]
- Maccarrone, M.; Rossi, S.; Bari, M.; De Chiara, V.; Rapino, C.; Musella, A.; Bernardi, G.; Bagni, C.; Centonze, D. Abnormal Mglu 5 Receptor/Endocannabinoid Coupling in Mice Lacking Fmrp and Bc1 Rna. Neuropsychopharmacology 2010, 35, 1500–1509. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Alger, B.E. Enhanced Endocannabinoid Signaling Elevates Neuronal Excitability in Fragile X Syndrome. J. Neurosci. 2010, 30, 5724–5729. [Google Scholar] [CrossRef][Green Version]
- Pieretti, M.; Zhang, F.P.; Fu, Y.H.; Warren, S.T.; Oostra, B.A.; Caskey, C.T.; Nelson, D.L. Absence of Expression of the Fmr-1 Gene in Fragile X Syndrome. Cell 1991, 66, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.P. Identification of a Gene (Fmr-1) Containing a Cgg Repeat Coincident with a Breakpoint Cluster Region Exhibiting Length Variation in Fragile X Syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The Mglur Theory of Fragile X Mental Retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef]
- Michalon, A.; Bruns, A.; Risterucci, C.; Honer, M.; Ballard, T.M.; Ozmen, L.; Jaeschke, G.; Wettstein, J.G.; von Kienlin, M.; Kunnecke, B.; et al. Chronic Metabotropic Glutamate Receptor 5 Inhibition Corrects Local Alterations of Brain Activity and Improves Cognitive Performance in Fragile X Mice. Biol. Psychiatry 2014, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hoeffer, C.A.; Sanchez, E.; Hagerman, R.J.; Mu, Y.; Nguyen, D.V.; Wong, H.; Whelan, A.M.; Zukin, R.S.; Klann, E.; Tassone, F. Altered Mtor Signaling and Enhanced Cyfip2 Expression Levels in Subjects with Fragile X Syndrome. Genes Brain Behav. 2012, 11, 332–341. [Google Scholar] [CrossRef][Green Version]
- Price, T.J.; Rashid, M.H.; Millecamps, M.; Sanoja, R.; Entrena, J.M.; Cervero, F. Decreased Nociceptive Sensitization in Mice Lacking the Fragile X Mental Retardation Protein: Role of Mglur1/5 and Mtor. J. Neurosci. 2007, 27, 13958–13967. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, A.; Hoeffer, C.A.; Takayasu, Y.; Miyawaki, T.; McBride, S.M.; Klann, E.; Zukin, R.S. Dysregulation of Mtor Signaling in Fragile X Syndrome. J. Neurosci. 2010, 30, 694–702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Varma, N.; Carlson, G.C.; Ledent, C.; Alger, B.E. Metabotropic Glutamate Receptors Drive the Endocannabinoid System in Hippocampus. J. Neurosci. 2001, 21, RC188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puighermanal, E.; Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. Cellular and Intracellular Mechanisms Involved in the Cognitive Impairment of Cannabinoids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3254–3263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puighermanal, E.; Marsicano, G.; Busquets-Garcia, A.; Lutz, B.; Maldonado, R.; Ozaita, A. Cannabinoid Modulation of Hippocampal Long-Term Memory Is Mediated by Mtor Signaling. Nat. Neurosci. 2009, 12, 1152–1158. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Bellocchio, L.; Oddi, D.; D’Amato, F.R.; Marsicano, G.; Crusio, W.E. The Fmr1 Mouse Line as a Model for Autism: The Relevance of the Genetic Background and of the Endocannabinoid System. In Proceedings of the FENS Annual Meeting, Amsterdam, The Netherlands, 3–7 July 2010. [Google Scholar]
- Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. New Insights into the Molecular Pathophysiology of Fragile X Syndrome and Therapeutic Perspectives from the Animal Model. Int. J. Biochem. Cell Biol. 2014, 53, 121–126. [Google Scholar] [CrossRef][Green Version]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the First Sn1-Dag Lipases Points to the Spatial and Temporal Regulation of Endocannabinoid Signaling in the Brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (Cbd) and Its Analogs: A Review of Their Effects on Inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaca, M.V.; Sonego, A.B.; Guimaraes, F.S. Cannabidiol, Neuroprotection and Neuropsychiatric Disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as Therapeutic Agents in Cancer: Current Status and Future Implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef][Green Version]
- Fasinu, P.S.; Phillips, S.; ElSohly, M.A.; Walker, L.A. Current Status and Prospects for Cannabidiol Preparations as New Therapeutic Agents. Pharmacotherapy 2016, 36, 781–796. [Google Scholar] [CrossRef]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as Novel Therapeutic Agents in Cns Disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hill, A.J.; Mercier, M.S.; Hill, T.D.; Glyn, S.E.; Jones, N.A.; Yamasaki, Y.; Futamura, T.; Duncan, M.; Stott, C.G.; Stephens, G.J.; et al. Cannabidivarin Is Anticonvulsant in Mouse and Rat. Br. J. Pharmacol. 2012, 167, 1629–1642. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and Brain Pharmacokinetic Profile of Cannabidiol (Cbd), Cannabidivarine (Cbdv), Delta(9)-Tetrahydrocannabivarin (Thcv) and Cannabigerol (Cbg) in Rats and Mice Following Oral and Intraperitoneal Administration and Cbd Action on Obsessive-Compulsive Behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [PubMed]
- Zamberletti, E.; Gabaglio, M.; Woolley-Roberts, M.; Bingham, S.; Rubino, T.; Parolaro, D. Cannabidivarin Treatment Ameliorates Autism-Like Behaviors and Restores Hippocampal Endocannabinoid System and Glia Alterations Induced by Prenatal Valproic Acid Exposure in Rats. Front. Cell Neurosci. 2019, 13, 367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pietropaolo, S.; Subashi, E. Mouse Models of Fragile X Syndrome. In Behavioral Genetics of the Mouse; Pietropaolo, S., Sluyter, F., Crusio, W.E., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 146–163. [Google Scholar]
- Spear, L.P. The Adolescent Brain and Age-Related Behavioral Manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Hebert, B.; Pietropaolo, S.; Meme, S.; Laudier, B.; Laugeray, A.; Doisne, N.; Quartier, A.; Lefeuvre, S.; Got, L.; Cahard, D.; et al. Rescue of Fragile X Syndrome Phenotypes in Fmr1 Ko Mice by a Bkca Channel Opener Molecule. Orphanet J. Rare Dis. 2014, 9, 124. [Google Scholar] [CrossRef][Green Version]
- Oddi, D.; Subashi, E.; Middei, S.; Bellocchio, L.; Lemaire-Mayo, V.; Guzman, M.; Crusio, W.E.; D’Amato, F.R.; Pietropaolo, S. Early Social Enrichment Rescues Adult Behavioral and Brain Abnormalities in a Mouse Model of Fragile X Syndrome. Neuropsychopharmacology 2015, 40, 1113–1122. [Google Scholar] [CrossRef][Green Version]
- Pietropaolo, S.; Goubran, M.G.; Joffre, C.; Aubert, A.; Lemaire-Mayo, V.; Crusio, W.E.; Laye, S. Dietary Supplementation of Omega-3 Fatty Acids Rescues Fragile X Phenotypes in Fmr1-Ko Mice. Psychoneuroendocrinology 2014, 49, 119–129. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; D’Amato, F.R.; Crusio, W.E. Genetic-Background Modulation of Core and Variable Autistic-Like Symptoms in Fmr1 Knock-out Mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Bonnan, A.; Bony, G.; Ferezou, I.; Pietropaolo, S.; Ginger, M.; Sans, N.; Rossier, J.; Oostra, B.; LeMasson, G.; et al. Dendritic Channelopathies Contribute to Neocortical and Sensory Hyperexcitability in Fmr1(-/Y) Mice. Nat. Neurosci. 2014, 17, 1701–1709. [Google Scholar] [CrossRef]
- Khandjian, E.W. Biology of the Fragile X Mental Retardation Protein, an Rna-Binding Protein. Biochem. Cell Biol. 1999, 77, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Bakker, C.E.; de Diego Otero, Y.; Bontekoe, C.; Raghoe, P.; Luteijn, T.; Hoogeveen, A.T.; Oostra, B.A.; Willemsen, R. Immunocytochemical and Biochemical Characterization of Fmrp, Fxr1p, and Fxr2p in the Mouse. Exp. Cell Res. 2000, 258, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Dutch-Belgian Fragile X Consortium. Fmr1 Knockout Mice: A Model to Study Fragile X Mental Retardation. Cell 1994, 78, 23–33. [Google Scholar]
- Moles, A.; D’Amato, R.F. Ultrasonic Vocalization by Female Mice in the Presence of a Conspecific Carrying Food Cues. Anim. Behav. 2000, 60, 689–694. [Google Scholar] [CrossRef][Green Version]
- Gaudissard, J.; Ginger, M.; Premoli, M.; Memo, M.; Frick, A.; Pietropaolo, S. Behavioral Abnormalities in the Fmr1-Ko2 Mouse Model of Fragile X Syndrome: The Relevance of Early Life Phases. Autism Res. 2017, 10, 1584–1596. [Google Scholar] [CrossRef]
- Gauducheau, M.; Lemaire-Mayo, V.; D’Amato, F.R.; Oddi, D.; Crusio, W.E.; Pietropaolo, S. Age-Specific Autistic-Like Behaviors in Heterozygous Fmr1-Ko Female Mice. Autism Res. 2017, 10, 1067–1078. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Crusio, W.E. Strain-Dependent Changes in Acoustic Startle Response and Its Plasticity across Adolescence in Mice. Behav. Genet. 2009, 39, 623–631. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The Miqe Guidelines: Minimum Information for Publication of Quantitative Real-Time Pcr Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesquille, M.; Baudonnat, M.; Decorte, L.; Louis, C.; Lestage, P.; Beracochea, D. Working Memory Deficits and Related Disinhibition of the Camp/Pka/Creb Are Alleviated by Prefrontal Alpha4beta2 *-Nachrs Stimulation in Aged Mice. Neurobiol. Aging 2013, 34, 1599–1609. [Google Scholar] [CrossRef]
- Kat, R.; Arroyo-Araujo, M.; de Vries, R.B.M.; Koopmans, M.A.; de Boer, S.F.; Kas, M.J.H. Translational Validity and Methodological Underreporting in Animal Research: A Systematic Review and Meta-Analysis of the Fragile X Syndrome (Fmr1 Ko) Rodent Model. Neurosci. Biobehav. Rev. 2022, 139, 104722. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kaphzan, H.; Alvarez-Dieppa, A.C.; Murphy, J.P.; Pierre, P.; Klann, E. Genetic Removal of P70 S6 Kinase 1 Corrects Molecular, Synaptic, and Behavioral Phenotypes in Fragile X Syndrome Mice. Neuron 2012, 76, 325–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dahlhaus, R.; El-Husseini, A. Altered Neuroligin Expression Is Involved in Social Deficits in a Mouse Model of the Fragile X Syndrome. Behav. Brain Res. 2010, 208, 96–105. [Google Scholar] [CrossRef] [PubMed]
- De Diego-Otero, Y.; Romero-Zerbo, Y.; el Bekay, R.; Decara, J.; Sanchez, L.; Fonseca, F.R.-D.; del Arco-Herrera, I. Alpha-Tocopherol Protects against Oxidative Stress in the Fragile X Knockout Mouse: An Experimental Therapeutic Approach for the Fmr1 Deficiency. Neuropsychopharmacology 2009, 34, 1011–1026. [Google Scholar] [CrossRef][Green Version]
- Eadie, B.D.; Zhang, W.N.; Boehme, F.; Gil-Mohapel, J.; Kainer, L.; Simpson, J.M.; Christie, B.R. Fmr1 Knockout Mice Show Reduced Anxiety and Alterations in Neurogenesis That Are Specific to the Ventral Dentate Gyrus. Neurobiol. Dis. 2009, 36, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.L.; Rao, B.S.; Seo, J.S.; Choi, H.S.; Dolan, B.M.; Choi, S.Y.; Chattarji, S.; Tonegawa, S. Inhibition of P21-Activated Kinase Rescues Symptoms of Fragile X Syndrome in Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 11489–11494. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Chuang, D.M.; Smith, C.B. Lithium Ameliorates Phenotypic Deficits in a Mouse Model of Fragile X Syndrome. Int. J. Neuropsychopharmacol. 2011, 14, 1–13. [Google Scholar] [CrossRef][Green Version]
- Mineur, Y.S.; Sluyter, F.; de Wit, S.; Oostra, B.A.; Crusio, W.E. Behavioral and Neuroanatomical Characterization of the Fmr1 Knockout Mouse. Hippocampus 2002, 12, 39–46. [Google Scholar] [CrossRef]
- Olmos-Serrano, J.L.; Corbin, J.G.; Burns, M.P. The Gaba(a) Receptor Agonist Thip Ameliorates Specific Behavioral Deficits in the Mouse Model of Fragile X Syndrome. Dev. Neurosci. 2011, 33, 395–403. [Google Scholar] [CrossRef][Green Version]
- Peier, A.M.; McIlwain, K.L.; Kenneson, A.; Warren, S.T.; Paylor, R.; Nelson, D.L. (Over) Correction of Fmr1 Deficiency with Yac Transgenics: Behavioral and Physical Features. Hum. Mol. Genet. 2000, 9, 1145–1159. [Google Scholar] [CrossRef]
- Restivo, L.; Ferrari, F.; Passino, E.; Sgobio, C.; Bock, J.; Oostra, B.A.; Bagni, C.; Ammassari-Teule, M. Enriched Environment Promotes Behavioral and Morphological Recovery in a Mouse Model for the Fragile X Syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 11557–11562. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Alekseyenko, O.; Hamilton, S.M.; Thomas, A.M.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Modifying Behavioral Phenotypes in Fmr1ko Mice: Genetic Background Differences Reveal Autistic-Like Responses. Autism Res. 2011, 4, 40–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spencer, C.M.; Alekseyenko, O.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Altered Anxiety-Related and Social Behaviors in the Fmr1 Knockout Mouse Model of Fragile X Syndrome. Genes Brain Behav. 2005, 4, 420–430. [Google Scholar] [CrossRef]
- Thomas, A.M.; Bui, N.; Graham, D.; Perkins, J.R.; Yuva-Paylor, L.A.; Paylor, R. Genetic Reduction of Group 1 Metabotropic Glutamate Receptors Alters Select Behaviors in a Mouse Model for Fragile X Syndrome. Behav. Brain Res. 2011, 223, 310–321. [Google Scholar] [CrossRef][Green Version]
- Uutela, M.; Lindholm, J.; Louhivuori, V.; Wei, H.; Louhivuori, L.M.; Pertovaara, A.; Akerman, K.; Castren, E.; Castren, M.L. Reduction of Bdnf Expression in Fmr1 Knockout Mice Worsens Cognitive Deficits but Improves Hyperactivity and Sensorimotor Deficits. Genes Brain Behav. 2012, 11, 513–523. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Huynh, L.X.; Crusio, W.E. Social Behavior Deficits in the Fmr1 Mutant Mouse. Behav. Brain Res. 2006, 168, 172–175. [Google Scholar] [CrossRef]
- Heitzer, A.M.; Roth, A.K.; Nawrocki, L.; Wrenn, C.C.; Valdovinos, M.G. Brief Report: Altered Social Behavior in Isolation-Reared Fmr1 Knockout Mice. J. Autism Dev. Disord 2012, 43, 1452–1458. [Google Scholar] [CrossRef]
- Mines, M.A.; Yuskaitis, C.J.; King, M.K.; Beurel, E.; Jope, R.S. Gsk3 Influences Social Preference and Anxiety-Related Behaviors During Social Interaction in a Mouse Model of Fragile X Syndrome and Autism. PLoS ONE 2010, 5, e9706. [Google Scholar] [CrossRef][Green Version]
- Michalon, A.; Sidorov, M.; Ballard, T.M.; Ozmen, L.; Spooren, W.; Wettstein, J.G.; Jaeschke, G.; Bear, M.F.; Lindemann, L. Chronic Pharmacological Mglu5 Inhibition Corrects Fragile X in Adult Mice. Neuron 2012, 74, 49–56. [Google Scholar] [CrossRef]
- Ventura, R.; Pascucci, T.; Catania, M.V.; Musumeci, S.A.; Puglisi-Allegra, S. Object Recognition Impairment in Fmr1 Knockout Mice Is Reversed by Amphetamine: Involvement of Dopamine in the Medial Prefrontal Cortex. Behav. Pharmacol. 2004, 15, 433–442. [Google Scholar] [CrossRef]
- Nielsen, D.M.; Derber, W.J.; McClellan, D.A.; Crnic, L.S. Alterations in the Auditory Startle Response in Fmr1 Targeted Mutant Mouse Models of Fragile X Syndrome. Brain Res. 2002, 927, 8–17. [Google Scholar] [CrossRef]
- Veeraragavan, S.; Graham, D.; Bui, N.; Yuva-Paylor, L.A.; Wess, J.; Paylor, R. Genetic Reduction of Muscarinic M4 Receptor Modulates Analgesic Response and Acoustic Startle Response in a Mouse Model of Fragile X Syndrome (Fxs). Behav. Brain Res. 2011, 228, 1–8. [Google Scholar] [CrossRef][Green Version]
- Heulens, I.; D’Hulst, C.; Van Dam, D.; De Deyn, P.P.; Kooy, R.F. Pharmacological Treatment of Fragile X Syndrome with Gabaergic Drugs in a Knockout Mouse Model. Behav. Brain Res. 2012, 229, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. Adolescent Brain Development and Animal Models. Ann. N. Y. Acad. Sci. 2004, 1021, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P.; Brake, S.C. Periadolescence: Age-Dependent Behavior and Psychopharmacological Responsivity in Rats. Dev. Psychobiol. 1983, 16, 83–109. [Google Scholar] [CrossRef]
- Gantois, I.; Khoutorsky, A.; Popic, J.; Aguilar-Valles, A.; Freemantle, E.; Cao, R.; Sharma, V.; Pooters, T.; Nagpal, A.; Skalecka, A.; et al. Metformin Ameliorates Core Deficits in a Mouse Model of Fragile X Syndrome. Nat. Med. 2017, 23, 674–677. [Google Scholar] [CrossRef][Green Version]
- Lemaire-Mayo, V.; Piquemal, M.; Crusio, W.E.; Louette, E.; Pietropaolo, S. Therapeutic Effects of Chlorzoxazone, a Bkca Channel Agonist, in a Mouse Model of Fragile X Syndrome. bioRxiv 2020. bioRxiv:2020.12.11.389569. [Google Scholar]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic Cannabidiol Treatment Improves Social and Object Recognition in Double Transgenic Appswe/Ps1e9 Mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef]
- Zupan, B.; Toth, M. Wild-Type Male Offspring of Fmr-1+/− Mothers Exhibit Characteristics of the Fragile X Phenotype. Neuropsychopharmacology 2008, 33, 2667–2675. [Google Scholar] [CrossRef]
- Lemaire-Mayo, V.; ESubashi; Henkous, N.; Beracochea, D.; Pietropaolo, S. Behavioral Effects of Chronic Stress in the Fmr1 Mouse Model for Fragile X Syndrome. Behav. Brain Res. 2017, 320, 128–135. [Google Scholar] [CrossRef]
- Markham, J.A.; Beckel-Mitchener, A.C.; Estrada, C.M.; Greenough, W.T. Corticosterone Response to Acute Stress in a Mouse Model of Fragile X Syndrome. Psychoneuroendocrinology 2006, 31, 781–785. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Memo, M.; Lemaire, V.; Pietropaolo, S. Long-Term Behavioral Effects of Prenatal Stress in the Fmr1-Knock-out Mouse Model for Fragile X Syndrome. Front. Cell Neurosci. 2022, 16, 917183. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Wohr, M.; Crusio, W.E.; Lemaire, V.; Pietropaolo, S. Autistic-Like Behavioral Effects of Prenatal Stress in Juvenile Fmr1 Mice: The Relevance of Sex Differences and Gene-Environment Interactions. Sci. Rep. 2022, 12, 7269. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Xia, Z.; Huang, T.; Smith, C.B. Effects of Chronic Immobilization Stress on Anxiety-Like Behavior and Basolateral Amygdala Morphology in Fmr1 Knockout Mice. Neuroscience 2011, 194, 282–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daskalakis, N.P.; De Kloet, E.R.; Yehuda, R.; Malaspina, D.; Kranz, T.M. Early Life Stress Effects on Glucocorticoid-Bdnf Interplay in the Hippocampus. Front. Mol. Neurosci. 2015, 8, 68. [Google Scholar] [CrossRef][Green Version]
- Nowacka, M.; Obuchowicz, E. Bdnf and Vegf in the Pathogenesis of Stress-Induced Affective Diseases: An Insight from Experimental Studies. Pharmacol. Rep. 2013, 65, 535–546. [Google Scholar] [CrossRef]
- Pardon, M.C. Role of Neurotrophic Factors in Behavioral Processes: Implications for the Treatment of Psychiatric and Neurodegenerative Disorders. Vitam. Horm. 2010, 82, 185–200. [Google Scholar]
- Schaaf, M.J.; De Kloet, E.R.; Vreugdenhil, E. Corticosterone Effects on Bdnf Expression in the Hippocampus. Implications for Memory Formation. Stress 2000, 3, 201–208. [Google Scholar] [CrossRef]
- Smith, M.A. Hippocampal Vulnerability to Stress and Aging: Possible Role of Neurotrophic Factors. Behav. Brain Res. 1996, 78, 25–36. [Google Scholar] [CrossRef][Green Version]
- Suri, D.; Vaidya, V.A. Glucocorticoid Regulation of Brain-Derived Neurotrophic Factor: Relevance to Hippocampal Structural and Functional Plasticity. Neuroscience 2013, 239, 196–213. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Long, J.M.; Ingram, D.K.; Mattson, M.P. Dietary Restriction Increases the Number of Newly Generated Neural Cells, and Induces Bdnf Expression, in the Dentate Gyrus of Rats. J. Mol. Neurosci. 2000, 15, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Gronli, J.; Bramham, C.; Murison, R.; Kanhema, T.; Fiske, E.; Bjorvatn, B.; Ursin, R.; Portas, C.M. Chronic Mild Stress Inhibits Bdnf Protein Expression and Creb Activation in the Dentate Gyrus but Not in the Hippocampus Proper. Pharmacol. Biochem. Behav. 2006, 85, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and Glucocorticoids Affect the Expression of Brain-Derived Neurotrophic Factor and Neurotrophin-3 Mrnas in the Hippocampus. J. Neurosci. 1995, 15 Pt 1, 1768–1777. [Google Scholar] [CrossRef][Green Version]
- Onishchenko, N.; Karpova, N.; Sabri, F.; Castren, E.; Ceccatelli, S. Long-Lasting Depression-Like Behavior and Epigenetic Changes of Bdnf Gene Expression Induced by Perinatal Exposure to Methylmercury. J. Neurochem. 2008, 106, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ounallah-Saad, H.; Renbaum, P.; Zalzstein, Y.; Ben-Hur, T.; Levy-Lahad, E.; Yirmiya, R. A Dual Role for Interleukin-1 in Hippocampal-Dependent Memory Processes. Psychoneuroendocrinology 2007, 32, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef][Green Version]
- Schaefer, T.L.; Davenport, M.H.; Grainger, L.M.; Robinson, C.K.; Earnheart, A.T.; Stegman, M.S.; Lang, A.L.; Ashworth, A.A.; Molinaro, G.; Huber, K.M.; et al. Acamprosate in a Mouse Model of Fragile X Syndrome: Modulation of Spontaneous Cortical Activity, Erk1/2 Activation, Locomotor Behavior, and Anxiety. J. Neurodev. Disord. 2017, 9, 6. [Google Scholar] [CrossRef][Green Version]
- Rotschafer, S.E.; Trujillo, M.S.; Dansie, L.E.; Ethell, I.M.; Razak, K.A. Minocycline Treatment Reverses Ultrasonic Vocalization Production Deficit in a Mouse Model of Fragile X Syndrome. Brain Res. 2012, 1439, 7–14. [Google Scholar] [CrossRef]
- Toledo, M.A.; TWen, H.; Binder, D.K.; Ethell, I.M.; Razak, K.A. Reversal of Ultrasonic Vocalization Deficits in a Mouse Model of Fragile X Syndrome with Minocycline Treatment or Genetic Reduction of Mmp-9. Behav. Brain Res. 2019, 372, 112068. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar]
- Pietropaolo, S.; Provenzano, G. Editorial: Targeting Excitation-Inhibition Imbalance in Neurodevelopmental and Autism Spectrum Disorders. Front. Neurosci. 2022, 16, 968115. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
Share and Cite
Premoli, M.; Fyke, W.; Bellocchio, L.; Lemaire, V.; Wolley-Roberts, M.; Bontempi, B.; Pietropaolo, S. Early Administration of the Phytocannabinoid Cannabidivarin Prevents the Neurobehavioral Abnormalities Associated with the Fmr1-KO Mouse Model of Fragile X Syndrome. Cells 2023, 12, 1927. https://doi.org/10.3390/cells12151927
Premoli M, Fyke W, Bellocchio L, Lemaire V, Wolley-Roberts M, Bontempi B, Pietropaolo S. Early Administration of the Phytocannabinoid Cannabidivarin Prevents the Neurobehavioral Abnormalities Associated with the Fmr1-KO Mouse Model of Fragile X Syndrome. Cells. 2023; 12(15):1927. https://doi.org/10.3390/cells12151927Chicago/Turabian Style
Premoli, Marika, William Fyke, Luigi Bellocchio, Valerie Lemaire, Marie Wolley-Roberts, Bruno Bontempi, and Susanna Pietropaolo. 2023. “Early Administration of the Phytocannabinoid Cannabidivarin Prevents the Neurobehavioral Abnormalities Associated with the Fmr1-KO Mouse Model of Fragile X Syndrome” Cells 12, no. 15: 1927. https://doi.org/10.3390/cells12151927
Find Other Styles
Article Metrics
Citations
Article Access Statistics
For more information on the journal statistics, click here.


