 Acta Physiologica Sinica, August 25, 2013, 65(4): 451–460 http://www.actaps.com.cn
Acta Physiologica Sinica, August 25, 2013, 65(4): 451–460 http://www.actaps.com.cn
Review
Role of endogenous cannabinoid system in the gut
Role of endogenous cannabinoid system in the gut
451
LIN Xu-Hong1,2,**, WANG Ya-Qiang2,**, WANG Hui-Chao3, REN Xue-Qun4,*, LI Yong-Yu1,*
1Department of Pathophysiology, Institute of Digestive Disease, School of Medicine, Tongji University, Shanghai 200092, China; 2Department of Clinical Laboratory, Huaihe Hospital Affiliated to Henan University, Kaifeng 475000, China; 3Department of Nephrology, First Affiliated Hospital of Henan University, Kaifeng 475000, China; 4Department of General Surgery, Huaihe Hospital Affiliated to Henan University, Kaifeng 475000, China
Abstract: The plant Cannabis has been used in clinic for centuries, and has been known to be beneficial in a variety of gastrointestinal diseases, such as emesis, diarrhea, inflammatory bowel disease and intestinal pain. In this text, we’ll review the components of the endogenous cannabinoid system as well as its role in the regulation of gastrointestinal activities, thus providing relative information for further study. Moreover, modulation of the endogenous cannabinoid system in gastrointestinal tract may provide a useful therapeutic target for gastrointestinal disorders.
Key words: cannabinoids; endogenous cannabinoid system; cannabinoid receptor; endogenous ligands; gastrointestinal function
1Department of Pathophysiology, Institute of Digestive Disease, School of Medicine, Tongji University, Shanghai 200092, China; 2Department of Clinical Laboratory, Huaihe Hospital Affiliated to Henan University, Kaifeng 475000, China; 3Department of Nephrology, First Affiliated Hospital of Henan University, Kaifeng 475000, China; 4Department of General Surgery, Huaihe Hospital Affiliated to Henan University, Kaifeng 475000, China
Abstract: The plant Cannabis has been used in clinic for centuries, and has been known to be beneficial in a variety of gastrointestinal diseases, such as emesis, diarrhea, inflammatory bowel disease and intestinal pain. In this text, we’ll review the components of the endogenous cannabinoid system as well as its role in the regulation of gastrointestinal activities, thus providing relative information for further study. Moreover, modulation of the endogenous cannabinoid system in gastrointestinal tract may provide a useful therapeutic target for gastrointestinal disorders.
Key words: cannabinoids; endogenous cannabinoid system; cannabinoid receptor; endogenous ligands; gastrointestinal function
内源性大麻素系统在胃肠道的作用
林旭红1,2,**,王亚强2,**,王慧超3,任学群4,*,李永渝1,* 1同济大学医学院病理生理教研室,同济大学消化系疾病研究所,上海 200092;2河南大学淮河医院检验科,开封 475000; 3河南大学一附院肾内科,开封 475000;4河南大学淮河医院普外科,开封 475000
摘 要:植物大麻用于临床已有几千年的历史,在许多胃肠道疾病如呕吐、腹泻、炎症性肠病、肠源性疼痛治疗中发挥重要 作用。本文旨在综述内源性大麻素系统的组成及其在胃肠道活动中的调节作用,为进一步研究提供相关信息,并为通过调 节胃肠道内源性大麻素系统治疗胃肠道疾病提供新靶点。
关键词:大麻素;内源性大麻素系统;大麻素受体;内源性配体;胃肠道功能 中图分类号:R333.3
Received 2013-01-30 Accepted 2013-04-12
This review was supported by the National Natural Science Foundation of China (No. 81141050, 81270477).
**These authors contributed equally to this review.
*Corresponding author. REN Xue-Qun: Tel: +86-378-3906004; E-mail: hhyyrxq@126.com. LI Yong-Yu: Tel: +86-21-65981021;
This review was supported by the National Natural Science Foundation of China (No. 81141050, 81270477).
**These authors contributed equally to this review.
*Corresponding author. REN Xue-Qun: Tel: +86-378-3906004; E-mail: hhyyrxq@126.com. LI Yong-Yu: Tel: +86-21-65981021;
E-mail: liyongyu@tongji.edu.cn
Cannabis sativa has long been used to treat intestinal diseases, and the functions of its extracts, cannabinoids, have been studied extensively in recent years in both physiological and pathophysiolocal processes. Although researches have provided insights to mechanistic basis of treatment, the detailed molecular events involved in the regulation of gut remain unknown. The purpose of this article is to summarize the role of endogenous cannabinoid system in regulating gastrointestine.
1 Endogenous cannabinoid system and its distribution in the gut
The term ‘endogenous cannabinoid system’, first introduced by Di Marzo and Fontana in 1995[1], consists of cannabinoid (CB) receptors, endogenous ligands, enzymes directly responsible for ligand synthesis and metabolism, carriers and transporters that assist in the transit of these hydrophobic mediators, as well as
The term ‘endogenous cannabinoid system’, first introduced by Di Marzo and Fontana in 1995[1], consists of cannabinoid (CB) receptors, endogenous ligands, enzymes directly responsible for ligand synthesis and metabolism, carriers and transporters that assist in the transit of these hydrophobic mediators, as well as
regulatory signal pathways[2–4]. An overview of the endocannabinoid system is shown in Fig. 1.
CB1 receptors are found throughout the gastroin-tes- tinal tract, mainly in myenteric and submucosal plexus- es of the enteric nervous system which represent the major sites of action for cannabinoids in the digestive tract[5]. Double-labelling of immunoreactivity, func- tional studies and co-localization with calbindin all support that CB1 receptors appear to be localized most- ly on motor neurons, interneurons and primary afferent neurons[6,7], and are also expressed in non-neuronal cells such as epithelial cells[8]. CB2 receptors appear to be present in the normal murine colonic epithelium, but not to any extent in the rat and human epithelium[9]. GPR55 is a recently identified CB receptor, and has been found in the gastrointestinal tract, including jejunum, ileum, colon[10], but the distribution of this receptor has not yet been studied in detail. Endocannabinoids, repre- sented as anandamide (AEA) and 2-arachidonoyl glyc- erol (2-AG), vary according to the region in the gut, with 2-AG being higher in the ileum than that in the colon, and AEA being consi-derably higher in the colon than that in the ileum[11,12].
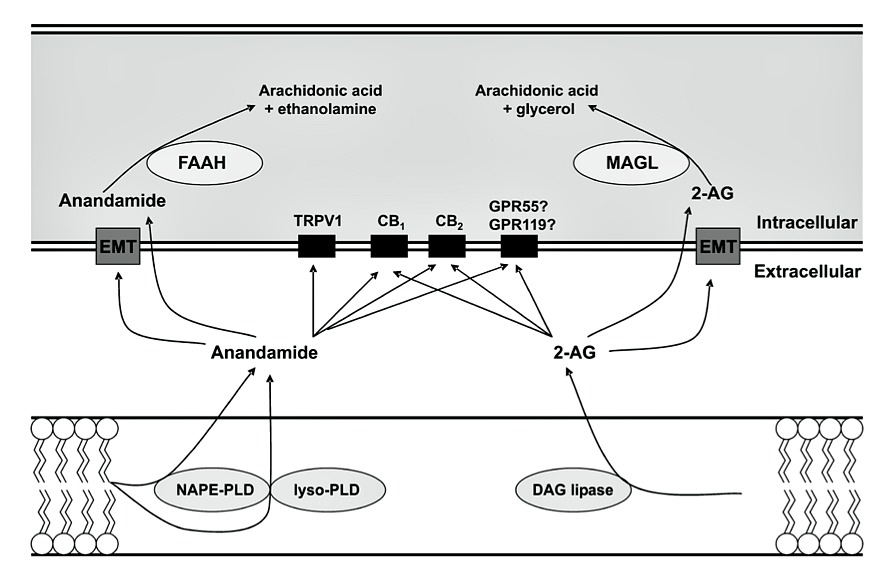
CB1 receptors are found throughout the gastroin-tes- tinal tract, mainly in myenteric and submucosal plexus- es of the enteric nervous system which represent the major sites of action for cannabinoids in the digestive tract[5]. Double-labelling of immunoreactivity, func- tional studies and co-localization with calbindin all support that CB1 receptors appear to be localized most- ly on motor neurons, interneurons and primary afferent neurons[6,7], and are also expressed in non-neuronal cells such as epithelial cells[8]. CB2 receptors appear to be present in the normal murine colonic epithelium, but not to any extent in the rat and human epithelium[9]. GPR55 is a recently identified CB receptor, and has been found in the gastrointestinal tract, including jejunum, ileum, colon[10], but the distribution of this receptor has not yet been studied in detail. Endocannabinoids, repre- sented as anandamide (AEA) and 2-arachidonoyl glyc- erol (2-AG), vary according to the region in the gut, with 2-AG being higher in the ileum than that in the colon, and AEA being consi-derably higher in the colon than that in the ileum[11,12].

Fig. 1. The endocannabinoid system. Biosynthesis of endocannabinoids involves multiple enzymatic steps where endocannabinoids are synthesized from membrane phospholipids. NAPE-PLD: N-acyl-phosphatidylethanolamine; DAG-lipase: diacylglycerol-lipase; 2-AG: 2-arachidonylglycerol; CB: cannabinoid; TRPV1: transient receptor potential vanilloid 1; GPR: G-protein-coupled receptors; EMT: endocannabinoid membrane transporter; FAAH: fatty acid amide hydrolase; MAGL: monoacylglycerol-lipase. This figure was reproduced from reference[66] with permission.
Furthermore, in pathophysiological states, CB1 receptors were found to be over-expressed, and CB agonists were consequently more active in reducing transit compared to control mice, suggesting that the endocannabinoid system is involved in the patho- physiology of motility disorders[19]. Like CB1 receptors, opioid receptors also act as inhibitor of motility, whose effect can not be competitively reversed by CB1 receptors antagonist rimonabant. Meanwhile, anta-go- nists of opioid receptors have no effect on slowing intes- tinal motility induced by cannabinoid WIN55, 212-2[20]. These results demonstrated that cannabinoid receptors and opioid receptors have no interaction in regulating intestinal motility in mice. While under the pathologi- cal state, Salvinorin A as well as the κ-opioid receptors (KOR) agonist U-50488 reduced motility in croton oil- treated mice. The inhibitory effect of both reagents was counteracted by the KOR antagonist nor-binaltor- phimine and the cannabinoid CB1 receptors antagonist rimonabant, however, nor-binaltorphimine and rimona- bant did not counteract the inhibitory effect of salvi- norin A on motility in control mice[21]. This result re- vealed a functional interaction between cannabinoid CB1 receptors and KORs in the inflamed gut in vivo, thus filling gaps in the understanding of relationship between them.
Contrary to CB1 receptors, CB2 receptors-mediated effects on gut motility are less significant under normal conditions, but there is strong evidence that they poten- tially take part in the regulation of motility in pathophysiological states[22]. A recent report demon- strated that the CB1 receptors-mediated reduction of gastrointestinal transit was absent in rats treated with an endotoxic inflammatory agent, and was replaced by CB2 receptors-mediated inhibition of stimulated tran- sit; This inhibition by the CB2 receptors agonist was dose-dependent and reversed by a selective CB2 recep- tors antagonist AM630[23]. Indeed, detailed studies on the isolated rat ileum showed that CB2 receptors ago- nist JWH-133 did not affect the electrically evoked contractions under physiological conditions, whereas it was able to reduce the contractile response in mice treat- ed with lipopolysaccharide (LPS)[22]. By evaluating the inhibition of LPS-enhanced gastrointestinal transit by different agonists, the effects of the CB2 receptors ago- nists on gastrointestinal motility involved intact cy- clooxygenase pathways, as the actions of JWH-133 were reversed by indomethacin; and CB2 receptors ag- onists also acted via inducible nitric oxide synthase as
Contrary to CB1 receptors, CB2 receptors-mediated effects on gut motility are less significant under normal conditions, but there is strong evidence that they poten- tially take part in the regulation of motility in pathophysiological states[22]. A recent report demon- strated that the CB1 receptors-mediated reduction of gastrointestinal transit was absent in rats treated with an endotoxic inflammatory agent, and was replaced by CB2 receptors-mediated inhibition of stimulated tran- sit; This inhibition by the CB2 receptors agonist was dose-dependent and reversed by a selective CB2 recep- tors antagonist AM630[23]. Indeed, detailed studies on the isolated rat ileum showed that CB2 receptors ago- nist JWH-133 did not affect the electrically evoked contractions under physiological conditions, whereas it was able to reduce the contractile response in mice treat- ed with lipopolysaccharide (LPS)[22]. By evaluating the inhibition of LPS-enhanced gastrointestinal transit by different agonists, the effects of the CB2 receptors ago- nists on gastrointestinal motility involved intact cy- clooxygenase pathways, as the actions of JWH-133 were reversed by indomethacin; and CB2 receptors ag- onists also acted via inducible nitric oxide synthase as
well as platelet-activating factor. Thus activation of CB2 receptors in response to LPS represents a mecha- nism for the re-establishment of normal gastrointestinal transit after an inflammatory stimulus. While in other models of altered motility such as croton oil-induced chronic ileitis and acetic acid-induced ileus, the involvement of CB2 receptors has not been demon- strated. Thus it seems that the role for CB2 receptors in the control of gastrointestinal motility confine to partial regions of the gut or under some specific pathophysio- logical states. Further studies exploring the detailed mechanisms are underway.
As to new cannabinoid receptor GPR55, a recent research revealed that O-1602, agonist of GPR55, inhibited the electrical field-induced contractions in the colon strips from wild-type and CB1−/−/CB2−/− mouse in a concentration-dependent manner, suggesting that activation of GPR55 leads to inhibition of neurogenic contractions in the gut and are predominantly prejunc- tional[24]. Results from Lin et al. [25] also confirmed the involvement of GPR55 in gut movement in rodent. While the understanding of the underlying mechanisms needs to be further demonstrated.
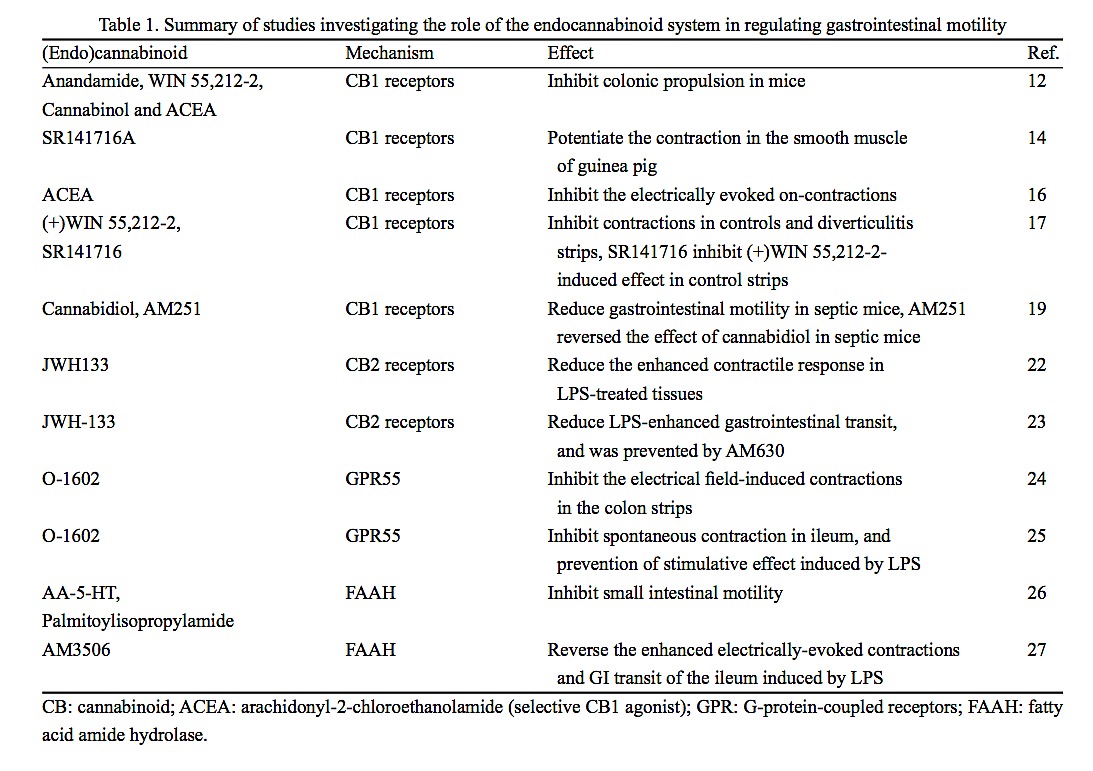
Moreover, studies targeting fatty acid amide hydrolase (FAAH) or an endocannabinoid membrane transporter (EMT) in vivo demonstrate a slowing of gastrointestinal motility after using both inhibitors. These results further support the concept that the endocannabinoid system is tonically active under physiological conditions, once again stressing the important role of the endocannabinoid system in the regulation of motility (Table 1)[26,27].
Similar results have been found in human as compared to rodents. Cannabinoids exhibited function in reducing gastric and intestinal motility in randomized clinical trials[28], which might be helpful for improving diarrhea and constipation. Clinical trials with selective CB receptors agonists and/or antagonists are required to strengthen present knowledge and explore detailed molecular mechanism under the circumstances of avoiding side-effects of related drugs.
2.2 Regulating secretion
Secretion of gastric acid and enteric cavity contribute to digestion, transit, as well as host defense, which needs restrict regulation by neural, humoral, and paracrine factors. At the same time, gastric ulcer formation or abnormal stool happened in the inflammatory bowel disease (IBD) patients mainly attributes to dysfunction of secretion.
As to new cannabinoid receptor GPR55, a recent research revealed that O-1602, agonist of GPR55, inhibited the electrical field-induced contractions in the colon strips from wild-type and CB1−/−/CB2−/− mouse in a concentration-dependent manner, suggesting that activation of GPR55 leads to inhibition of neurogenic contractions in the gut and are predominantly prejunc- tional[24]. Results from Lin et al. [25] also confirmed the involvement of GPR55 in gut movement in rodent. While the understanding of the underlying mechanisms needs to be further demonstrated.
Moreover, studies targeting fatty acid amide hydrolase (FAAH) or an endocannabinoid membrane transporter (EMT) in vivo demonstrate a slowing of gastrointestinal motility after using both inhibitors. These results further support the concept that the endocannabinoid system is tonically active under physiological conditions, once again stressing the important role of the endocannabinoid system in the regulation of motility (Table 1)[26,27].
Similar results have been found in human as compared to rodents. Cannabinoids exhibited function in reducing gastric and intestinal motility in randomized clinical trials[28], which might be helpful for improving diarrhea and constipation. Clinical trials with selective CB receptors agonists and/or antagonists are required to strengthen present knowledge and explore detailed molecular mechanism under the circumstances of avoiding side-effects of related drugs.
2.2 Regulating secretion
Secretion of gastric acid and enteric cavity contribute to digestion, transit, as well as host defense, which needs restrict regulation by neural, humoral, and paracrine factors. At the same time, gastric ulcer formation or abnormal stool happened in the inflammatory bowel disease (IBD) patients mainly attributes to dysfunction of secretion.

No inhibitory effect on gastric acid secretion was observed in response to (−)-WIN55212 or to the CB2 receptors selective agonist JWH-015, but (+)-WIN- 55212 exhibited inhibitory effect, and this effect was antagonized by CB1 receptors antagonists SR141716A and LY320135, but not by CB2 receptors antagonist SR144528[29]. These results strongly suggest that gastric acid secretion is inhibited in response to CB1 receptor activation, although the detailed underlying mechanism has yet to be interpreted. Adami et al.[30] also reple- nished this viewpoint by reporting that CB1 (but not CB2) receptors activation may suppress vagal drive to the gastric mucosa and thereby decrease rat gastric acid secretion induced by 2-deoxy-D-glucose and penta- gastrin. While recent findings revealed the CB1 receptors on human parietal cells and their role in inhibiting acid secretion directly, these results indicated the species differences, and its clinical evaluation needs to be further determined[31].
Under physiological conditions, cannabinoids inhibit intestinal secretion in vitro when administered exogenously[32]. These exogenous agonists play an antisecretory effect on isolated ileum specimen through CB1 receptors[33], as an increased level of AEA and CB1 mRNA has been reported in cholera toxin-induced accumulation of fluid. Furthermore, the increased accumulation of fluid in the ex vivo small intestine in response to cholera toxin is significantly reduced by administration of CB1 receptors agonists, which reduce stimulated ion transport across the mucosa of the intestine, in a rimonibant reversible manner[34]. This was supported by the fact that cannabinoids delayed the onset of caster oil-induced diarrhoea[35].
Under physiological conditions, cannabinoids inhibit intestinal secretion in vitro when administered exogenously[32]. These exogenous agonists play an antisecretory effect on isolated ileum specimen through CB1 receptors[33], as an increased level of AEA and CB1 mRNA has been reported in cholera toxin-induced accumulation of fluid. Furthermore, the increased accumulation of fluid in the ex vivo small intestine in response to cholera toxin is significantly reduced by administration of CB1 receptors agonists, which reduce stimulated ion transport across the mucosa of the intestine, in a rimonibant reversible manner[34]. This was supported by the fact that cannabinoids delayed the onset of caster oil-induced diarrhoea[35].
These results indicate that the endocannabinoids released in the wall of the gut normally limit the degree of secretion. This action may involve intrinsic and extrinsic nerves, rather than a direct action at the epithelium[13].
Though activation of CB1 receptors participates in the intestinal excretion, the detailed mechanism remains unclear, which is probably because that CB1 receptors exert this effect by restriction of cholinergic nerve-mediated secretion. Despite these data, the overall effects of CB1 receptors activation on secretion and absorption in humans is not established, as no clinical studies using orally administered CB1 receptors agonists are available.
Though activation of CB1 receptors participates in the intestinal excretion, the detailed mechanism remains unclear, which is probably because that CB1 receptors exert this effect by restriction of cholinergic nerve-mediated secretion. Despite these data, the overall effects of CB1 receptors activation on secretion and absorption in humans is not established, as no clinical studies using orally administered CB1 receptors agonists are available.
2.3 The involvement of endogenous cannabinoid system in visceral hypersensitivity
Over the past few years, key insights into the relation- ship between endocannabinoids and visceral pain have also been achieved. A large body of evidence from ani- mal models of visceral pain recently supports the con- tention that the endocannabinoid system was involved in pain processing[36, 37]. Cannabinoids have well described analgesic effects in various animal models of acute and chronic pain[38], including the abdominal response to colorectal distention, the acetic acid- induced abdominal stretching and the visceral hyperal- gesia. Mechanisms at the basis of the antino-ciceptive potential of cannabinoids still remain unclear, but at least involve supraspinal, spinal and peripheral CB1 receptors, as well as peripheral CB2 receptors[39–41], although pristine experiments with CB1 and CB2 receptors antagonists have provided conflicting data regarding a potential role for the endocannabinoid sys- tem in nociception. In addition, endogenous com- pounds related to AEA, such as arachidonoyl glycine and palmitoylethanolamide, have antino-ciceptive actions.
The peripheral component of CB1 receptors in pain processing would suggest that local administration of cannabinoids may produce beneficial effects without the problems of unwanted psychotropic effects. After inducing hyperalgaesia by rectal instillation of trinitrobenzene sulphonic acid (TNBS), lower doses of CB receptors agonists were needed to reduce sensitivity to colorectal distention[30,31]. The selective CB1 receptors agonist ACEA has also been demonstrated to possess peripherally mediated effects upon noxious somatosensory processing that are blocked by rimonabant[42]. As CB1 receptors antagonist rimonabant increases hypersensitivity in rectal distention models, a role of the CB1 receptors in the regulation of visceral sensitivity is conceivable.
Many CB2 receptors agonists such as SR144528, AM1241 have antinociceptive effects in a number of models, because they showed no characteristic psychotropic effects[43,44]. But the results of another study demonstrated both the cannabinoid receptor agonist WIN55-212-2 (in a CB1 antagonist-sensitive manner) and the CB2 receptors agonist JWH-015 were active at a lower dose, abolishing the hypersensitivity in TNBS-induced models. When administered alone, the CB1 receptors antagonist rimonabant enhanced colitis-induced hyperalgesia, but the CB2 receptors antagonist SR144528 did not alter thresholds to colorectal distention in TNBS-induced models, suggesting that the endogenous cannabinoid system involved in the inflammatory hyperalgesia and only through CB1 receptors, making a physiological involvement of the CB2 receptors in the regulation of visceral hypersensitivity unlikely[31]. The discrepancy of these researches may lie in the former ones laying particular emphasis on somatic pain, and the role of cannabinoids in the control of visceral nociception has been largely neglected. Fortunately, Hillsley et al.[45] found that the CB2 receptors agonist AM1241 inhibited the bradykinin-evoked visceral afferent response of murine mesenteric afferent nerves, the activation of CB2 receptors as the response to AM1241 was blocked by the selective CB2 receptors antagonist AM630, and what is more, no effect of AM1241 was observed in CB2-deficient mice. The results, which supported by the fact that oral administration of probiotics reduced colorectal distention-induced visceromotor responses in a CB2 receptors antagonist-sensitive manner[46], gave compelling evidence to previous studies (the CB2 receptors agonist HU308 was efficacious in the formalin model in a manner blocked by SR144528[47], which highlights the emerging role of the CB2 receptors in the digestive tract) and collectively implied CB2 receptor-induced analgesic effects of proinfla- mmatory compounds on peripheral endings of visceral afferent nerves. While Guindon and Hohmann[48] identified cannabinoid CB2 receptors as new thera- peutic target for pathological pain states with limited centrally-mediated side effects, and also indicated for us further direction in the study of relationship between endocannabinoid system and hyperalgesia.
Over the past few years, key insights into the relation- ship between endocannabinoids and visceral pain have also been achieved. A large body of evidence from ani- mal models of visceral pain recently supports the con- tention that the endocannabinoid system was involved in pain processing[36, 37]. Cannabinoids have well described analgesic effects in various animal models of acute and chronic pain[38], including the abdominal response to colorectal distention, the acetic acid- induced abdominal stretching and the visceral hyperal- gesia. Mechanisms at the basis of the antino-ciceptive potential of cannabinoids still remain unclear, but at least involve supraspinal, spinal and peripheral CB1 receptors, as well as peripheral CB2 receptors[39–41], although pristine experiments with CB1 and CB2 receptors antagonists have provided conflicting data regarding a potential role for the endocannabinoid sys- tem in nociception. In addition, endogenous com- pounds related to AEA, such as arachidonoyl glycine and palmitoylethanolamide, have antino-ciceptive actions.
The peripheral component of CB1 receptors in pain processing would suggest that local administration of cannabinoids may produce beneficial effects without the problems of unwanted psychotropic effects. After inducing hyperalgaesia by rectal instillation of trinitrobenzene sulphonic acid (TNBS), lower doses of CB receptors agonists were needed to reduce sensitivity to colorectal distention[30,31]. The selective CB1 receptors agonist ACEA has also been demonstrated to possess peripherally mediated effects upon noxious somatosensory processing that are blocked by rimonabant[42]. As CB1 receptors antagonist rimonabant increases hypersensitivity in rectal distention models, a role of the CB1 receptors in the regulation of visceral sensitivity is conceivable.
Many CB2 receptors agonists such as SR144528, AM1241 have antinociceptive effects in a number of models, because they showed no characteristic psychotropic effects[43,44]. But the results of another study demonstrated both the cannabinoid receptor agonist WIN55-212-2 (in a CB1 antagonist-sensitive manner) and the CB2 receptors agonist JWH-015 were active at a lower dose, abolishing the hypersensitivity in TNBS-induced models. When administered alone, the CB1 receptors antagonist rimonabant enhanced colitis-induced hyperalgesia, but the CB2 receptors antagonist SR144528 did not alter thresholds to colorectal distention in TNBS-induced models, suggesting that the endogenous cannabinoid system involved in the inflammatory hyperalgesia and only through CB1 receptors, making a physiological involvement of the CB2 receptors in the regulation of visceral hypersensitivity unlikely[31]. The discrepancy of these researches may lie in the former ones laying particular emphasis on somatic pain, and the role of cannabinoids in the control of visceral nociception has been largely neglected. Fortunately, Hillsley et al.[45] found that the CB2 receptors agonist AM1241 inhibited the bradykinin-evoked visceral afferent response of murine mesenteric afferent nerves, the activation of CB2 receptors as the response to AM1241 was blocked by the selective CB2 receptors antagonist AM630, and what is more, no effect of AM1241 was observed in CB2-deficient mice. The results, which supported by the fact that oral administration of probiotics reduced colorectal distention-induced visceromotor responses in a CB2 receptors antagonist-sensitive manner[46], gave compelling evidence to previous studies (the CB2 receptors agonist HU308 was efficacious in the formalin model in a manner blocked by SR144528[47], which highlights the emerging role of the CB2 receptors in the digestive tract) and collectively implied CB2 receptor-induced analgesic effects of proinfla- mmatory compounds on peripheral endings of visceral afferent nerves. While Guindon and Hohmann[48] identified cannabinoid CB2 receptors as new thera- peutic target for pathological pain states with limited centrally-mediated side effects, and also indicated for us further direction in the study of relationship between endocannabinoid system and hyperalgesia.
It is worth noting that transient receptor potential (TRP) channels that are able to respond to particular changes have been identified. These changes usually were followed by transducting noxious gastrointestinal stimuli into generator currents at molecular level. There is good experimental evidence to suggest that in animal models of colorectal distension, the role of the TRPV1 receptors in visceral hypersensitivity was addressed[49]. The report that locates the TRPV1 immunoreactivity illustrated that TRPV1 has a role in gastrointestinal pain transmission[50] . In stress-induced visceral hyperalgesia model, treatment with WIN 55,212-2 or the TRPV1 antagonist capsazepine prevented the development of visceral hyperalgesia and blocked the upregulation of TRPV1, suggesting that the endocanna- binoid (CB1) and TRP (TRPV1) pathways may play a reciprocal role in stress-induced visceral hyperalgesia, furthermore, Mahmud et al.[51] found the CB1 receptor- mediated analgesic effect is associated with down- regulation of TRPV1. These results above pointed out promising direction for visceral hyperactivity in inflammatory conditions. TRPV4 has roles to a varying degree in gastrointestinal chemo-, thermo- and mechano-nociception[52] and may directly transduce mechanosensation[53]. A recent publication identified that cannabinoids exert analgesic effects in postinfla- mmatory pain models by activation of transient receptor potential A1 (TRPA1) receptors. These findings highlighted the overlap of the endocannabinoid system and the endovanilloid system once again[54]. In addition, as the effects of CB1 receptors activation is just opposite to that of TRPV1 activation, there remains another question, it is not clear whether compounds like AEA which acts on both CB1 and TRPV1 receptors as an agonist, results in analgesic or algesic effects and the concentration required for activation respectively remains unrevealed.
New publications indicated involvement of GPR55 in the control of pain sensitivity. In rodent models of inflammatory and neuropathic pain, GPR55−/− mice were found to lack mechanical hyperalgesia[55], by coincidence, earlier results from Lauckner et al.[56] documented that abundant expression of GPR55 in large dorsal root ganglion neurons in mice and its ability to modulate the activity of these neurons. When N-arachidonoyl (NAGly), an endogenous analog of AEA, was shown to produce antinociceptive effects in a variety of pain models, it was hypothesized to be through an alternative receptor GPR18[57]. Further studies are necessary to explore the potential thera- peutic value of selective GPR55, GPR18 ligands as analgesic agents.
The central role that FAAH plays in regulating AEA signaling in vivo has been exemplified by analysis of modest effects in models of thermal nociception mice, the result showed that lacking FAAH exhibits reduced pain sensitivity in the tail-immersion, hot plate, and formalin tests (both phases)[58]. These changes of FAAH−/− mice were reversed by SR141716A, consistent with elevated AEA levels promoting these behavioral effects by acting on CB1 receptors. Taken together, these data demonstrated that FAAH knockout and FAAH inhibitors block FAAH to prevent the breakdown of AEA, which may be useful in relieving pain, supporting a role for endocannabinoid signaling in the modulation of both acute and chronic visceral pain sensitivity.
The central role that FAAH plays in regulating AEA signaling in vivo has been exemplified by analysis of modest effects in models of thermal nociception mice, the result showed that lacking FAAH exhibits reduced pain sensitivity in the tail-immersion, hot plate, and formalin tests (both phases)[58]. These changes of FAAH−/− mice were reversed by SR141716A, consistent with elevated AEA levels promoting these behavioral effects by acting on CB1 receptors. Taken together, these data demonstrated that FAAH knockout and FAAH inhibitors block FAAH to prevent the breakdown of AEA, which may be useful in relieving pain, supporting a role for endocannabinoid signaling in the modulation of both acute and chronic visceral pain sensitivity.
In summary, these data provided anatomical evidence that, in visceral pain conditions, sensitivity of the cannabinoid system is increased, thus representing a suitable candidate to develop new anti-hyperalgesic drugs.
2.4 The relationship between inflammation and endogenous cannabinoid system
Enhancement of cannabinoid signalling, along with increased expression of CB1/CB2 receptors and/or enhanced endocannabinoid levels have been observed following inflammatory stimuli[59], both in animals and intestinal biopsies of patients with gut inflammatory diseases, including ulcerative colitis, Crohn’s disease, diverticulitis and celiac disease.
Different chemicals were used by Massa et al. [60] to induce bowel inflammation, all these colitis models were more severe in CB1 receptors knockout mice than those in wild type mice, and pretreatment of wild type mice with CB1 receptors antagonists gave rise to up- regulated inflammatory response in various models, while reduced inflammatory response was seen when administered CB1 receptors agonists, which manifested as the reversion of the electrophysiological signs of smooth muscle irritability and reduced tissue myelo- peroxidase activity. These results, at the first time, suggested that cannabinoids exert anti-inflammatory actions in the gut through activation of CB1 receptor, and the mechanism involves inhibition of chemokines and proinflammatory cytokines, mainly from macro- phage and mast cell. Other studies also elucidated that AEA, 2-AG, PEA were found beneficial in the control of inflammation through releasing anti-inflammatory cytokine, inhibiting mast-cell degranulation and reducing extravasation, oedema[61]. In addition, since selective CB1 receptors agonists induced wound closure in HT29 and DLD1 epithelial cells[9], we speculate that cannabinoids can exert protective effect by promoting wound healing via CB1 receptors activation.
More recent data have highlighted the importance of the CB2 receptors in mediating protective effects in the inflamed gut. Kimball et al.[62] found the CB2 receptors immunostaining in mustard oil-induced experimental model of colitis was more marked in infiltrated immune cells, involving most of the epithelium, meanwhile, in dextran sulphate sodium (DSS) colitis, high doses of the CB2-selective receptor agonist JWH-133 improved microscopic and macroscopic scores of inflammation when administered prophylactically. Wright et al.[9] showed that CB2 receptors immunoreactivity was seen in the epithelium of colonic tissue characteristic of IBD and appeared more evident in the epithelium at the crypt fissure where ulceration had occurred. Findings from epithelial cell line demonstrating a number of cannabinoid receptors agonists have been shown to exert an inhibitory effect on TNF-α-induced interl- eukin-release from the human colonic epithelial cell line HT-29 in a CB2 receptors antagonists sensitive way[63]. In a word, apart from inhibiting of chemokines and proinflammatory cytokines, reducing degranu- lation, which share with CB1 receptors, activation of CB2 receptors may regulate gastroenterological inflammation at different levels.
2.4 The relationship between inflammation and endogenous cannabinoid system
Enhancement of cannabinoid signalling, along with increased expression of CB1/CB2 receptors and/or enhanced endocannabinoid levels have been observed following inflammatory stimuli[59], both in animals and intestinal biopsies of patients with gut inflammatory diseases, including ulcerative colitis, Crohn’s disease, diverticulitis and celiac disease.
Different chemicals were used by Massa et al. [60] to induce bowel inflammation, all these colitis models were more severe in CB1 receptors knockout mice than those in wild type mice, and pretreatment of wild type mice with CB1 receptors antagonists gave rise to up- regulated inflammatory response in various models, while reduced inflammatory response was seen when administered CB1 receptors agonists, which manifested as the reversion of the electrophysiological signs of smooth muscle irritability and reduced tissue myelo- peroxidase activity. These results, at the first time, suggested that cannabinoids exert anti-inflammatory actions in the gut through activation of CB1 receptor, and the mechanism involves inhibition of chemokines and proinflammatory cytokines, mainly from macro- phage and mast cell. Other studies also elucidated that AEA, 2-AG, PEA were found beneficial in the control of inflammation through releasing anti-inflammatory cytokine, inhibiting mast-cell degranulation and reducing extravasation, oedema[61]. In addition, since selective CB1 receptors agonists induced wound closure in HT29 and DLD1 epithelial cells[9], we speculate that cannabinoids can exert protective effect by promoting wound healing via CB1 receptors activation.
More recent data have highlighted the importance of the CB2 receptors in mediating protective effects in the inflamed gut. Kimball et al.[62] found the CB2 receptors immunostaining in mustard oil-induced experimental model of colitis was more marked in infiltrated immune cells, involving most of the epithelium, meanwhile, in dextran sulphate sodium (DSS) colitis, high doses of the CB2-selective receptor agonist JWH-133 improved microscopic and macroscopic scores of inflammation when administered prophylactically. Wright et al.[9] showed that CB2 receptors immunoreactivity was seen in the epithelium of colonic tissue characteristic of IBD and appeared more evident in the epithelium at the crypt fissure where ulceration had occurred. Findings from epithelial cell line demonstrating a number of cannabinoid receptors agonists have been shown to exert an inhibitory effect on TNF-α-induced interl- eukin-release from the human colonic epithelial cell line HT-29 in a CB2 receptors antagonists sensitive way[63]. In a word, apart from inhibiting of chemokines and proinflammatory cytokines, reducing degranu- lation, which share with CB1 receptors, activation of CB2 receptors may regulate gastroenterological inflammation at different levels.
Furthermore, significant benefits may be observed when indirectly applied inhibitors of both FAAH and EMT. D’Argenio et al.[64] found notable elevation of AEA levels in the colon of DNBS-treated mice. VDM- 11, the EMT inhibitor, further increased AEA levels and accompanied by abolished inflammation, whereas the FAAH inhibitor, N-arachidonoyl-serotonin (AA-5- HT), did not affect endocannabinoid levels and was less efficacious at attenuating colitis. Treatment with genetic ablation of FAAH resulted in protection against 2,4-dinitrobenzene sulphonic-acid-induced colitis[54], thus paving the way to promising therapeutic targets for the treatment of intestinal disease characterized by excessive inflammatory responses. But we should keep in mind that the effect of FAAH inhibitors may not be entirely due to elevated levels of anadamide, as they also enhance the level of noncannabinoid substrate such as oleoylethanolamide or prodstamides generated via COX-2[65].
3 Summary and prospect
In conclusion, considerable progress has been made in this research field, although further research would still be necessary to fully understand the characteristic and the function of the endocannabinoid system. It is possible to foresee that the complete knowledge about the role of the endocannabinoid system in the gut will facilitate the development of new drugs whose mechanism of action might be based on the modulation of this system, and this might lead to innovative therapeutic strategies to deal with digestive disease.
3 Summary and prospect
In conclusion, considerable progress has been made in this research field, although further research would still be necessary to fully understand the characteristic and the function of the endocannabinoid system. It is possible to foresee that the complete knowledge about the role of the endocannabinoid system in the gut will facilitate the development of new drugs whose mechanism of action might be based on the modulation of this system, and this might lead to innovative therapeutic strategies to deal with digestive disease.
REFERENCES
1 Di Marzo V, Fontana A. Anandamide, an endogenous cannabinomimetic eicosanoid: ‘killing two birds with one stone’. Prostaglandins Leukot Essent Fatty Acids 1995; 53: 1–11.
2 Storr MA, Sharkey KA. The endocannabinoid system and gutbrain signalling. Curr Opin Pharmacol 2007; 7: 575–582. 3 Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The
emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 2006; 27: 73– 100.
4 De Petrocellis L, CascioMG, DiMarzo V. The endocanna- binoid system:a general view and latest additions. Br J Pharmacol 2004; 141: 765–774.
5 Borrelli F, Izzo AA. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract Res Clin Endocrinol Metab 2009; 23: 33–49.
6 Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S. Localization of cannabinoid CB1 receptor immunoreac- tivity in the guinea pig and rat myenteric plexus. J Comp Neurol 2002; 448: 410–422.
7 Yuece B, Sibaev A, Broedl UC, Marsicano G, Göke B, Lutz B, Allescher HD, Storr M. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil 2007; 19: 744– 753.
8 Izzo AA, Camilleri M. Cannabinoids in intestinal inflammation and cancer. Pharmacol Res 2009; 60: 117–125.
9 Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology 2005; 129: 437–453.
10 Brown AJ. Novel cannabinoid receptors. Br J Pharmacol 2007; 152: 567–575.
11 IzzoAA,FezzaF,CapassoR,BisognoT,PintoL,IuvoneT, Esposito G, Mascolo N, Di Marzo V, Capasso F. Canna- binoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol 2001; 134: 563–570.
12 PintoL,IzzoAA,CascioMG,BisognoT,Hospodar-ScottK, Brown DR, Mascolo N, Di Marzo V, Capasso F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 2002; 123: 227–234.
1 Di Marzo V, Fontana A. Anandamide, an endogenous cannabinomimetic eicosanoid: ‘killing two birds with one stone’. Prostaglandins Leukot Essent Fatty Acids 1995; 53: 1–11.
2 Storr MA, Sharkey KA. The endocannabinoid system and gutbrain signalling. Curr Opin Pharmacol 2007; 7: 575–582. 3 Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The
emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 2006; 27: 73– 100.
4 De Petrocellis L, CascioMG, DiMarzo V. The endocanna- binoid system:a general view and latest additions. Br J Pharmacol 2004; 141: 765–774.
5 Borrelli F, Izzo AA. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract Res Clin Endocrinol Metab 2009; 23: 33–49.
6 Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S. Localization of cannabinoid CB1 receptor immunoreac- tivity in the guinea pig and rat myenteric plexus. J Comp Neurol 2002; 448: 410–422.
7 Yuece B, Sibaev A, Broedl UC, Marsicano G, Göke B, Lutz B, Allescher HD, Storr M. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil 2007; 19: 744– 753.
8 Izzo AA, Camilleri M. Cannabinoids in intestinal inflammation and cancer. Pharmacol Res 2009; 60: 117–125.
9 Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology 2005; 129: 437–453.
10 Brown AJ. Novel cannabinoid receptors. Br J Pharmacol 2007; 152: 567–575.
11 IzzoAA,FezzaF,CapassoR,BisognoT,PintoL,IuvoneT, Esposito G, Mascolo N, Di Marzo V, Capasso F. Canna- binoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol 2001; 134: 563–570.
12 PintoL,IzzoAA,CascioMG,BisognoT,Hospodar-ScottK, Brown DR, Mascolo N, Di Marzo V, Capasso F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 2002; 123: 227–234.
- 13 Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol 2004; 141: 1335–1345.
- 14 Pinto L, Capasso R, Di Carlo G, Izzo AA. Endocannabinoids and the gut. Prostaglandins Leukot Essent Fatty Acids 2002; 66: 333–341.
- 15 Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids: an update. Curr Opin Pharmacol 2004; 4: 572–579.
- 16 Hinds NM, Ullrich K, Smid SD. Cannabinoid 1 (CB1) receptors coupled to cholinergic motorneurones inhibit neurogenic circular muscle contractility in the human colon. Br J Pharmacol 2006; 148: 191–199.
- 17 Guagnini F, Valenti M, Mukenge S, Matias I, Bianchetti A, Di Palo S, Ferla G, Di Marzo V, Croci T. Neural contractions in colonic strips from patients with diverticulosis: role of endocannabinoids and substance P. Gut 2006; 55: 946–953.
- 18 Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med 2005; 83: 944–954.
- 19 de Filippis D, Iuvone T, d’amico A, Esposito G, Steardo L, Herman AG, Pelckmans PA, de Winter BY, de Man JG. Effect of cannabidiol on sepsis-induced motility disturbances in mice: involvement of CB receptors and fatty acid amide hydrolase. Neurogastroenterol Motil 2008; 20(8): 919–927.
- 20 Carai MA, Colombo G, Gessa GL, Yalamanchili R, Basavarajppa BS, Hungund BL. Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice. Br J Pharmacol 2006; 148: 1043–1050.
- 21 Capasso R, Borrelli F, Cascio MG, Aviello G, Huben K, Zjawiony JK, Marini P, Romano B, Di Marzo V, Capasso F, Izzo AA. Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between κ-opioid and cannabinoid CB1 receptors. Br J Pharmacol 2008; 155: 681–689.
- 22 Duncan M, Mouihate A, Mackie K, Keenan CM, Buckley NE, Davison JS, Patel KD, Pittman QJ, Sharkey KA. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide- treated rats. Am J Physiol Gastrointest Liver Physiol 2008; 295: G78-G87.
- 23 Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol 2004; 142: 1247–1254.
- 24 Ross GR, Lichtman A, Dewey WL, Akbarali HI. Evidence for the putative cannabinoid receptor (GPR55)-mediated inhibitory effects on intestinal contractility in mice. Pharmacology 2012; 90(1–2): 55–65.
- 25 Lin XH, Yuece B, Li YY, Feng YJ, Feng JY, Yu LY, Li K, Li
YN, Storr M. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol Motil 2011; 23(9): 862–e342.
26 Capasso R, Matias I, Lutz B, Borrelli F, Capasso F, Marsicano G, Mascolo N, Petrosino S, Monory K, Valenti M, Di Marzo V, Izzo AA. Fatty Acid amide hydrolase controls mouse inte- stinal motility in vivo. Gastroenterology 2005; 129: 941–951.
27 Bashashati M, Storr MA, Nikas SP, Wood JT, Godlewski G, Liu J, Ho W, Keenan CM, Zhang H, Alapafuja SO, Cravatt BF, Lutz B, Mackie K, Kunos G, Patel KD, Makriyannis A, Davison JS, Sharkey KA. Inhibiting fatty acid amide hydrolase normalizes endotoxin-induced enhanced gastrointestinal motility in mice. Br J Pharmacol 2012; 165: 1556–1571.
28 Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol 2007; 293: G137–G145.
29 Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut 2001; 48: 859–867.
30 Adami M, Frati P, Bertini S, Kulkarni-Narla A, Brown DR, de Caro G, Coruzzi G, Soldani G.. Gastric antisecretory role and immunohistochemical localization of cannabinoid receptors in the rat stomach. Br J Pharmacol 2002; 135: 1598–1606.
31 Pazos MR, Tolón RM, Benito C, Rodríguez CF, Gorgojo JJ, Nevado M, Alvarez M, Arias F, Almodóvar F, Fernández MT, Lledó JL, González S, Fernández-Ruiz JJ, Romero J. Cannabinoid CB1 receptors are expressed by parietal cells of the human gastric mucosa. J Histochem Cytochem 2008; 56: 511–516.
32 Tyler K, Hillard CJ, Greenwood-Van Meerveld B. Inhibition of small intestinal secretion by cannabinoids is CB1 receptor-mediated in rats. Eur J Pharmacol 2000; 409: 207– 211.
33 MacNaughton WK, Van Sickle MD, Keenan CM, Cushing K, Mackie K, Sharkey KA. Distribution and function of the cannabinoid-1 receptor in the modulation of ion transport in the guinea pig ileum: relationship to capsaicin-sensitive nerves. Am J Physiol Gastrointest Liver Physiol 2004; 286: G863–G871.
34 Izzo AA, Capasso F, Costagliola A, Bsogno T, Marsicano G, Ligresti A, Matias I, Capasso, R, Pinto L, Borrelli F, Cecio A, Lutz B, Mascolo N, Di Marzo V. An endogenous canna- binoid tone attenuates cholera toxin-induced fluid accumu- lation in mice. Gastroenterology 2003; 125: 765–774.
35 Izzo AA, Mascolo N, Pinto L, Capasso R, Capasso F. The role of cannabinoid receptors in intestinalmotility, defae- cation and diarrhoea in rats. Eur J Pharmacol 1999; 384: 37– 42…
…

